Abstract
Acquired abnormalities in platelets, endothelium, and their interaction occur in sepsis, immune heparin-induced thrombocytopenia (HIT), and the antiphospholipid syndrome. Although of distinct pathogeneses, these three disorders have several clinical features in common, including thrombocytopenia and the potential for life- and limb-threatening thrombotic events, ranging from microvascular (sepsis > antiphospholipid > HIT) to macrovascular (HIT > antiphospholipid > sepsis) thrombosis, both venous and arterial.
In Section I, Dr. William Aird reviews basic aspects of endothelial-platelet interactions as a springboard to considering the common problem of thrombocytopenia (and its mechanism) in sepsis. The relationship between thrombocytopenia and other aspects of the host response in sepsis, including activation of coagulation/inflammation pathways and the development of organ dysfunction, is discussed. Practical issues of platelet count triggers and targeted use of activated protein C concentrates are reviewed.
In Section II, Dr. Theodore Warkentin describes HIT as a clinicopathologic syndrome, i.e., the diagnosis should be based on the concurrence of an appropriate clinical picture together with detection of platelet-activating and/or platelet factor 4-dependent antibodies (usually in high levels). HIT is a profound prothrombotic state (odds ratio for thrombosis, 20–40), and the risk for thrombosis persists for a time even when heparin is stopped. Thus, pharmacologic control of thrombin (or its generation), and postponing oral anticoagulation pending substantial resolution of thrombocytopenia, is appropriate. Indeed, coumarin-associated protein C depletion during uncontrolled thrombin generation of HIT can explain limb loss (coumarin-associated venous limb gangrene) or skin necrosis syndromes in some patients.
In Section III, Dr. Jacob Rand presents the most recent concepts on the mechanisms of thrombosis in the antiphospholipid syndrome, and focuses on the role of β2-glycoprotein I as a major antigenic target in this condition. Diagnosis of the syndrome is often complicated because the clinical laboratory tests to identify this condition have been empirically derived. Dr. Rand addresses the practical aspects of current testing for the syndrome and current recommendations for treating patients with thrombosis and with spontaneous pregnancy losses.
I. Endothelial-Platelet Interplay in Sepsis
William C. Aird, MD*
Beth Israel Deaconess Medical Center, 330 Brookline Ave., RW-663, Boston MA 02215
This section will review the role of platelet-endothelial cell interactions in sepsis. The goals are to: 1) underscore the importance of platelet–endothelial cell dialogue in both health and disease, 2) consider thrombocytopenia in the context of the overall host response to sepsis, 3) highlight the potential for co-existence of thrombocytopenia and a hypercoagulable state, and 4) argue that optimal treatment for thrombocytopenia in sepsis is to target the underlying host response, and not the platelets per se.
Platelet-Endothelial Cell Interactions
Most hematologists are intimately familiar with the circulating platelet, but poorly informed about the endothelium. This comes as no surprise. Platelets are readily assayed by relatively simple protocols, including the complete blood count, peripheral smear, bleeding time, and platelet function studies. Moreover, there are well-established correlations between platelet number (and to a lesser degree platelet function) and clinical phenotypes. On the other hand, the endothelium is tethered to the wall of the blood vessel and therefore poorly accessible for study. Although assays do exist for circulating markers of activated endothelium, these are indirect measures of endothelial function and provide little in the way of useful information. Pathological specimens of the endothelium are not routinely available and even if they were, the findings would not necessarily correlate with function. For all intents and purposes, the endothelium remains a hidden, enigmatic, and under-appreciated cell layer.
In truth, endothelial cells are every bit as active and interactive as the circulating platelet, if not more so. The endothelium is a truly pervasive organ; the human body contains approximately 1012–13 endothelial cells, weighing 1 kg and covering a surface area of 4000–7000 m2.1 Among other functions, the endothelium mediates vasomotor tone, regulates cellular and nutrient trafficking, maintains blood fluidity, contributes to the local balance in proinflammatory and antiinflammatory mediators, participates in generation of new blood vessels, and undergoes programmed cell death.2 Importantly, each of these activities is differentially regulated in space and time (a phenomenon that has been variably termed endothelial cell heterogeneity or vascular diversity).3
Although platelets and endothelial cells differ in important ways, they also have some features in common (Table 1 ). For example, both cell types are derived from a common bone marrow–derived progenitor cell.4 Endothelial cells and megakaryocytes share certain transcriptional networks and gene expression programs (e.g., GATA-2, von Willebrand factor, multimerin, P-selectin). Endothelial cells and platelets each store bioactive materials in their cytoplasmic granules. Finally, while the endothelium may be considered sedentary in its ways, endothelial cells and their precursors have been shown to circulate in the blood.5
Platelets and endothelial cells are no strangers to one another. The average platelet survives only 7 to 9 days. Provided it does not spend excessive time in the splenic pool, the platelet makes approximately 10,000 trips around the circulatory loop in its lifetime. If one were to survey the travelogue of the platelet, one would undoubtedly find a rich history of cell–cell interactions, both with other circulating cells and with the underlying endothelium. The platelet may be thought of as a mobile node, constantly picking up new information (e.g., via endocytosis) and periodically imparting information (e.g., via secretion) to neighboring cells.
From a mechanistic standpoint, platelets and endothelial cells communicate on multiple levels. Cross talk may occur over a distance (paracrine signaling), via transient interactions (“give-and-go” mechanism), or through receptor-mediated cell–cell adhesion. Platelets may release or transfer substances that impact on endothelial cell function, and vice versa (Figure 1; see Appendix, page 603). In one direction, platelets release interleukin-1β (IL-1β), transforming growth factor-β (TGF-β), platelet derived growth factor (PDGF), and vascular endothelial growth factor (VEGF), each of which may trigger signal transduction pathways in the endothelium. In the other direction, endothelial cells express cell surface receptors or soluble mediators that either inhibit platelet function (e.g., nucleoside triphosphate diphosphohydrolases, prostacyclin, nitric oxide) or promote platelet activation (e.g., platelet-activating factor). Recent studies have demonstrated a critical role for the CD40-CD40ligand (CD40L) system in mediating reciprocal interactions between platelets and endothelial cells (Figure 2 ).
Miscommunication (usually in the way of excessive dialogue) between platelets and the endothelium underlies several disease states, either as a causative factor and/or as a consequence of the disease process. As will be discussed below and elsewhere in this Education Program session, sepsis, heparin-induced thrombocytopenia (HIT), and the antiphospholipid antibody syndrome each result in the concomitant activation of platelets and the endothelium, increased interaction between these two cell types, and a secondary procoagulant or hypercoagulable state.
Sepsis Pathophysiology—Building a Conceptual Framework
Normally, biological systems approach steady state or homeostasis. One way to understand the inner workings of a biological system (whether an organism, an organ, or a cell type) is to observe how it behaves under stress. Sepsis represents a powerful model for exploring the outer limits of the stress response in humans. The sepsis syndrome represents a continuum in clinical and pathophysiological severity. However, it is a continuum with definable, albeit arbitrary, phases that characterize patients at risk for morbidity and mortality.6 Infection is defined as the invasion of normally sterile tissues with microorganisms. Sepsis constitutes the systemic inflammatory response to infection; severe sepsis is sepsis complicated by dysfunction of one or more organs. There are more than 750,000 cases of severe sepsis per year in the United States. Severe sepsis is associated with a mortality rate of 30%–50%.7 It follows from these data that an important goal in critical care medicine is to develop novel therapeutic strategies for improving survival in this patient population.
When considering the pathophysiology of severe sepsis, several important themes emerge: 1) it is the host response, rather than the nature of the pathogen, that is the primary determinant of patient outcome; 2) sepsis invariably activates the coagulation and inflammatory pathways; 3) the monocyte/tissue macrophage is in the driver’s seat, initiating the host response to infection; and 4) the endothelial cell, far from an innocent bystander, is an active participant, serving to amplify the host response. The monocyte and endothelial cell work together to protect the host against pathogens, but in the process may inflict collateral damage (e.g., organ dysfunction), which is not diffuse but remarkably focal in its distribution. An interesting question in sepsis research is when does the host response cross the line from function (adaptive response) to dysfunction (maladaptive response)?
The basic pathophysiology of the septic response is summarized in Figure 3 . According to this scheme, the monocyte/tissue macrophage binds lipopolysaccharide (or some other component of the bacterial wall) via unique pattern recognition receptors on the surface of the cell, resulting in the activation of inflammatory and coagulation cascades. Once activated, these pathways communicate with one another to further amplify the host response. At center stage is the monocyte/tissue macrophage, to some extent the neutrophil, and some would argue the endothelial cell, the so-called “foot soldiers” of the innate immune response, serving to divide the world into self and nonself based on physical properties. This is a highly evolutionarily conserved mechanism, which in many ways is hard wired into our systems. That is both good and bad: good because it is fast, reliable, and durable; bad because, like every great weapon, it can ultimately turn on its bearer.
The release of inflammatory mediators results in additional activation of monocytes and endothelial cells. These autocrine and paracrine pathways create a positive feedback loop, leading to increased expression of tissue factor on monocytes/tissue macrophages (and possibly some subsets of endothelial cells), as well as a procoagulant and pro-adhesive endothelial surface. In addition to generating fibrin, activated clotting factors interact with protease-activated receptors on the surface of monocytes, platelets, and endothelial cells, resulting in increased inflammatory response. In short, the host response to sepsis is a highly orchestrated process that involves a plethora of interdependent, nonlinear interactions between many cell types and soluble factors. As a result, any consideration of the role of a single cell or mediator must be viewed in the context of the larger response. These principles will be applied to an understanding of the pathogenesis and treatment of sepsis-associated thrombocytopenia in the last two sections.
A Traditional Approach to Thrombocytopenia in the Septic Patient
Before discussing new perspectives, it is helpful to consider the more traditional approach to the patient with sepsis-associated thrombocytopenia—one that not only resonates with our clinical training and experience but also serves as a diagnostic and therapeutic foundation on which to build.
Approach to Thrombocytopenia
In general terms, thrombocytopenia may arise from decreased platelet production, increased destruction, and/or sequestration in the spleen. A more practical approach to thrombocytopenia is to consider the clinical setting (Table 2 ).8 In the intensive care unit (ICU), thrombocytopenia occurs in up to 20% of medical and 35% of surgical admissions.9 While there are many causes of thrombocytopenia in this setting, sepsis is a clear risk factor, with an estimated incidence of 35%–59%.9 In addition, there is an inverse relationship between the severity of sepsis and the platelet count.10
Pathophysiology
Patients with sepsis may develop de novo ethylenediaminetetraacetic acid (EDTA)-dependent antibodies, which cause platelet clumping in the test tube with a resultant pseudothrombocytopenia.11 Nonimmune destruction of platelets is an important cause of thrombocytopenia in sepsis, a process that is only occasionally associated with underlying disseminated intravascular coagulation (DIC) (see next section). Immune mechanisms may contribute to sepsis-induced thrombocytopenia.12 Nonspecific platelet-associated antibodies can be detected in up to 30% of ICU patients.12 In these cases, nonpathogenic immunoglobulin G (IgG) presumably binds to bacterial products on the surface of platelets, to an altered platelet surface, or as immune complexes. A subset of patients with platelet-associated antibodies has auto-antibodies directed against glycoprotein IIb/IIIa.12 These antibodies have been implicated in the pathogenesis of immune thrombocytopenic purpura and, although not proven, may play a role in mediating sepsis-induced thrombocytopenia. Hematophagocytosis in the bone marrow is a common finding in patients with sepsis and thrombocytopenia.13 The degree to which this pathological process is a cause or simply a marker of sepsis-related thrombocytopenia is not clear. Bone marrow of septic patients with thrombocytopenia infrequently shows hypocellularity with reduced numbers of megakaryocytes. In addition to sepsis-related mechanisms, other causes of thrombocytopenia should be considered in the critically ill patient. For example, thrombocytopenia may occur as a complication of heparin therapy. Other types of drug-induced thrombocytopenia are rare in the ICU setting. Dilutional thrombocytopenia may occur in patients with trauma or complicated surgery. Acute folate deficiency has been described in patients admitted to the ICU.14 Pre-existing underlying disease, including cancer, hypersplenism, and immune thrombocytopenic purpura, may also contribute to a low platelet count.
Clinical manifestations and diagnosis
Thrombocytopenia is a common cause of bleeding in the ICU. Patients with thrombocytopenia may have petechiae, purpura, bruising, or frank bleeding. The diagnosis of thrombocytopenia is made from the complete blood count. A peripheral smear may show evidence of platelet clumping. If that is the case, the platelet count should be remeasured in blood drawn into a tube that contains an anticoagulant other than EDTA. If the thrombocytopenia is associated with consumptive coagulopathy, the INR, PTT, thrombin time, D-dimers, fibrinogen, thrombin-antithrombin complexes, and/or prothrombin fragment 1.2 may be abnormal, and the peripheral smear may show schistocytes. Although patients with sepsis may have increased platelet-associated IgG, this test is nonspecific and does not help in guiding therapy.
Prognosis
Treatment
While guidelines for prophylactic transfusions in patients with chemotherapy-induced thrombocytopenia have been established,17 the threshold for transfusing the thrombocytopenic patient with sepsis is not clear. Patients with sepsis have an underlying shift in the hemostatic balance toward the procoagulant side. Moreover, as we will discuss in the next section, platelets are activated in the setting of sepsis and likely contribute in important ways to the pathogenesis of the syndrome. Therefore, when considering the cost-effectiveness of platelet transfusion, it is important to consider the theoretical risk of accelerating the underlying pathophysiology (i.e., “adding fuel to the fire”). This caveat notwithstanding (and in the absence of evidence-based guidelines), most patients are transfused to achieve a platelet count ≥ 10,000/μL. If the patient has concomitant coagulopathy (e.g., DIC or liver disease), active bleeding, or platelet dysfunction (e.g., uremia), it may be prudent to employ a more liberal transfusion strategy with the goal of maintaining an even higher platelet count.
Scratching the Surface—Applying the Principles of the Host Response to an Understanding of Thrombocytopenia in Sepsis
While the approach to sepsis-associated thrombocytopenia outlined in the previous section provides a practical framework for diagnosing and treating patients, it belies a hidden complexity in the underlying pathophysiology—one that holds an important key to the development and implementation of new therapies. Consider, for a moment, life from the perspective of a platelet. Normally, platelets circulate in a quiescent state, acquiring and delivering information that is essential for maintaining health, occasionally called into action to boost hemostasis, and typically retiring after a 7-day tour-of-duty. In sepsis, the environment is transformed into a virtual battlefield. Circulating platelets are bathed in a sea of inflammatory mediators and activated coagulation factors. Moreover, in traversing the vasculature, platelets interact more frequently with other cell types, including leukocytes and activated endothelial cells. In the process, platelets are sequestered at the level of the endothelium, an effect that appears to vary in intensity from one vascular bed to another.18–,23 These sequestered platelets may be irreversibly activated, destroyed, and/or prevented from returning to the circulating pool. Platelets also participate in and become entrapped by evolving fibrin clots. Previous studies have demonstrated that the coagulation system is activated in the vast majority of patients with severe sepsis.10 For example, D-dimers are elevated in virtually all patients with severe sepsis,24 while protein C levels are decreased in up to 90% of such patients.24,25 Acquired antithrombin III (ATIII) deficiency is also common in the setting of sepsis, with levels below 60% in more than half of patients.26,27 At the extreme, activation of the clotting cascade may lead to excessive depletion of circulating serine proteases and secondary DIC. However, it is important to recognize that overt (decompensated) DIC represents merely the “tip of the iceberg,” occurring in less than one half of patients (based on common criteria used to define the syndrome).28– 33 In summary, platelets in the sepsis syndrome are faced with numerous hurdles and roadblocks that culminate in a shorter circulating half-life and secondary thrombocytopenia.
As much as the platelet may fall victim to the sepsis process, it also contributes in important ways to the underlying pathogenesis. Once activated, platelets aggregate, provide a phospholipid-rich surface for coagulation complexes, and release a host of mediators including proinflammatory molecules such as IL-1β. In addition, platelets may generate procoagulant-rich microparticles, which contribute to a prothrombotic state.34,35 Finally, platelets interact with activated endothelial cells, resulting in amplification of the host response through positive feedback loops.
In summary, thrombocytopenia in sepsis arises from and/or co-exists with intense platelet activation, increased platelet-endothelium interactions, and an underlying hypercoagulable state. As much as a low platelet count may confer increased risk for bleeding, sepsis-associated thrombocytopenia should be recognized for what it is: a surrogate marker for the severity of the host response. Viewed from this perspective, the platelet count is every bit as much an indicator of organ dysfunction as liver enzymes, pulmonary function, or mental status. Indeed, the critical care field has adopted the platelet count as the most common marker for hematological dysfunction in clinical studies of severe sepsis and multiple organ dysfunction syndrome.
Sepsis Therapy—Implications for Thrombocytopenia
Over the past decade, enormous resources have been expended on sepsis trials, with more than 10,000 patients enrolled in over 20 placebo-controlled, randomized Phase 3 clinical trials. The vast majority of these therapies have failed to improve survival in patients with severe sepsis, including antiendotoxin, anticytokine, antiprostaglandin, antibradykinin, and anti–platelet activating factor (PAF) strategies, ATIII, and tissue factor pathway inhibitor (TFPI).1 At the time of this writing, a total of five Phase 3 clinical trials have demonstrated improved survival in critically ill patients or patients with severe sepsis. These include the use of low tidal volume ventilation,36 activated protein C,24 low-dose glucocorticoids,37 intensive insulin therapy,38 and early goal-directed therapy.39
Many reasons have been postulated to explain the disappointing results in sepsis trials, including poor applicability of animal models to human patients, nonuniformity of supportive care, differences in patient populations, and poor choice of timing or outcome measures. Perhaps more than anything else, these studies have underestimated the redundant, interdependent, and pleiotropic nature of the host response.1 As long as the complexity of the host response remains outside our grasp, the best hope for therapeutic advances will be with broad-based therapy in which multiple components are targeted at the same time (“cluster-bomb” approach), or with more focused therapy directed toward a critical common final pathway of the host response (“smart-bomb” approach). It is noteworthy that the five clinical trials that have shown benefit in patients with sepsis have in common the capacity to attenuate endothelial cell activation. For example, low tidal volume ventilation may reduce stretch- or pressure-induced damage to the pulmonary endothelium; activated protein C may function in part by decreasing endothelial cell activation and apoptosis; low-dose glucocorticoids may dampen the activity of pro-inflammatory transcriptional networks in the endothelium; intensive insulin therapy may have a protective effect on the endothelium either directly through insulin receptors or by normalizing glucose levels; early goal-directed therapy results in more rapid hemodynamic support and hence improved flow over the endothelium. Although a link between therapeutic efficacy and endothelial “health” remains speculative (i.e., largely extrapolated from in vitro studies), the findings point to the endothelium as the primary nerve center of the host response to sepsis.
Is there a connection between systemic therapy for sepsis and treatment of thrombocytopenia? The answer is decidedly—yes. As long as the low platelet count is causally related to the host response, optimal therapy will consist of some combination of low tidal volume ventilation, activated protein C, low-dose steroids, insulin, and early goal-directed therapy. Moreover, in so far as the endothelium contributes to sepsis-associated thrombocytopenia (i.e., through increased platelet–endothelial cell interactions or via endothelial-mediated perpetuation of the pro-inflammatory/pro-coagulant response), the restoration of endothelial health is likely to play an important role in the correction of the platelet count. The extent to which the proven therapies owe their success to protective effects on the endothelium remains to be established.
A few concluding remarks about the use of activated protein C in patients with severe sepsis are in order. As hematologists, most of us think of activated protein C in terms of its capacity to cleave and inactivate factors Va and VIIIa. However, activated protein C also binds to receptors, including the endothelial protein C receptor and the protease activated receptor-1, on the surface of the endothelium (and perhaps white blood cells), resulting in profound anti-inflammatory and pro-survival effects. In fact, existing data suggest that these latter anticoagulant-independent mechanisms (and perhaps other actions yet to be delineated, such as blood pressure control) are primarily responsible for the efficacy of activated protein C in severe sepsis.
A consistent feature of the Phase 3 clinical trial with activated protein C was that the more severe the sepsis (by any number of criteria), the better the response to therapy.24 For example, patients with multiple organ dysfunction or DIC had higher relative and absolute risk reductions in mortality, compared with patients with single organ dysfunction or absence of DIC, respectively. Not surprisingly, the major side effect of activated protein was bleeding. For this reason, patients with a platelet count of < 30,000/μL were excluded from the trial.
And this brings us back full circle to a consideration of the endothelial-platelet axis. If activated protein C is most effective in septic patients at the severe end of the pathophysiological spectrum and if thrombocytopenia is a marker of disease severity, then patients with sepsis-associated thrombocytopenia may theoretically benefit from treatment with activated protein C. In the final analysis, the use of activated protein C in patients with severe sepsis and thrombocytopenia represents a double-edged sword: while treatment is associated with a high risk of bleeding, these patients are the very individuals who may stand to benefit most from the drug. One approach to deal with this problem (favored by this author) is to transfuse patients with platelets to maintain counts > 30,000/μL and treat with activated protein C. This recommendation is not based on evidence and will likely never be tested in a large clinical trial.
Finally, it is interesting to consider how the results of the basic, pre-clinical, and clinical studies may shape the future of research and development in the field of sepsis. If activated protein C does indeed save lives through mechanisms unrelated to its anti-Va and anti-VIIIa activity, then the anticoagulant function (and attendant risk of bleeding) may be viewed as a mere drug toxicity. Based on this hypothesis, one might predict that a re-engineered, activated protein C molecule, in which the anticoagulant activity is selectively ablated, may have an improved therapeutic window.
II. Heparin-Induced Thrombocytopenia and Thrombosis
Theodore E. Warkentin, MD*
Hamilton Regional Laboratory Medicine Program, Hamilton Health Sciences, Hamilton General Hospital, Dept. of Lab. Medicine, 237 Barton Street, East, Level 1, Hamilton Ontario L8L 2X2 Canada
Heparin-induced thrombocytopenia (HIT) is a transient prothrombotic disorder initiated by heparin. Its central feature is thrombocytopenia resulting from immunoglobulin G (IgG)–mediated platelet activation, leading to in vivo thrombin generation and increased risk of venous and arterial thrombosis. HIT antibodies also activate endothelial cells and monocytes. In keeping with the theme of platelet-endothelial interplay, I will discuss coumarin-induced venous limb gangrene, which illustrates the failure of the endothelium-based protein C natural anticoagulant pathway to downregulate excess thrombin in HIT.
Pathogenesis
Exposure to heparin can induce formation of pathogenic antibodies of IgG class that recognize multimolecular complexes of platelet factor 4 (PF4) and heparin on platelet surfaces, leading to platelet activation in vivo from occupancy and cross-linking of platelet FcγIIa receptors (FcγRIIa) by the PF4/heparin/IgG immune complexes.1– 3 PF4 is a 70–amino acid (7,780 Da), platelet-specific member of the C-X-C subfamily of chemokines. Four PF4 molecules self-associate to form compact tetramers (~31,000 Da) of globular structure. PF4 is rich in the basic amino acids lysine and arginine, which form a “ring of positive charge” to which heparin binds. PF4 is stored in platelet α-granules and is bound to heparan sulfate on endothelial cell surfaces. Heparin infusion increases plasma PF4 from trace levels (~3 ng/mL) 15- to 30-fold for several hours by displacing endothelial PF4.
The HIT immune response is polyspecific, i.e., antibodies are directed against multiple neoepitope sites.4–,6 Only a minority of PF4/heparin-reactive HIT sera activate platelets in vitro,7 based on antibody affinity for PF4/heparin (or PF4 alone)8 and titer.9 The affinity of HIT-IgG for PF4/heparin is intermediate between that of relatively low- and relatively high-affinity antigens (β2-glycoprotein I and tetanus toxoid, respectively), although binding of both Fab arms of HIT-IgG to multimolecular PF4/heparin complexes could significantly increase affinity.10 Heparin is surprisingly nonspecific in creating neoepitope(s) on PF4: indeed, a nonheparin polyanion (polyvinyl sulfonate) with PF4 is used to detect HIT antibodies in a commercial assay.11
HIT can be viewed as a transient, drug-induced autoimmune disorder, as HIT-IgG recognize neoepitopes on PF4 (not heparin) (Figure 4; see Appendix, 603). Indeed, some HIT-IgG recognize PF4 bound to solid phase, even in the absence of heparin,2,8,10 and activate platelets in vitro without added heparin.8,12 Perhaps, heparin-independent platelet activation by HIT-IgG explains the occasional patient with onset of thrombocytopenia and thrombosis beginning several days after stopping heparin (delayed-onset HIT).12,13 HIT is transient, as PF4-reactive antibodies generally decline to undetectable levels within a few weeks or months of an episode of HIT.14 Moreover, HIT antibodies are not regenerated more quickly (if at all) in former HIT patients reexposed to heparin following disappearance of antibodies (i.e., there is no anamnestic response).14,15
Thrombin generation in HIT patients (marked increase in thrombin-antithrombin complexes16,17) results from procoagulant platelet changes (microparticles18) and possibly by tissue factor produced by endothelium3 and monocytes.19 It remains unproven whether activation of endothelium and monocytes occurs in vivo. Kwaan and Sakurai20 found hyperplastic endothelial cells, and immunoglobulin deposition within platelet thrombi and proliferative endothelial cells, in ischemic tissues from patients with HIT. Differential activation of endothelium (microvascular > macrovascular) has been reported.21 Only IgG antibodies activate platelets, and I believe it remains unclear whether HIT can be caused by IgA and IgM antibodies.
Various animal models for HIT have been reported. However, only one model22 recapitulates several key clinical and laboratory features of HIT by using double-transgenic FcγRIIa/hPF4 mice, i.e., mice with platelets bearing human FcγRIIa and human PF4 (mice lack platelet Fcγ receptors, and murine PF4 is not recognized by HIT antibodies). When these mice are treated with an HIT-mimicking murine monoclonal antibody that recognizes hPF4/heparin and are then given heparin, severe thrombocytopenia and fibrin-rich thrombi in multiple organs result.
Thrombosis in HIT
HIT is remarkably prothrombotic (Table 3).23–,27 Thus, heparin use produces a cohort of HIT patients with high thrombotic risk, a scenario that ironically occurs most often in postoperative patients receiving low-dose heparin for antithrombotic prophylaxis.28
A wide variety of thrombotic complications can occur in HIT (Table 4). One characteristic (albeit rare) “bleeding” event—adrenal hemorrhage—is associated with adrenal vein thrombosis, producing hemorrhagic infarction; when necrosis is bilateral, acute adrenal failure results.
Frequency of HIT
The frequency of HIT varies widely, depending on the type of heparin (bovine lung unfractionated heparin [UFH] > porcine mucosal UFH > low–molecular weight heparin [LMWH]) and the patient population (surgical > medical > obstetrical).7,28,29 In addition, the risk of HIT increases each day that heparin continues beyond day 4 (although immunization risk declines after day 10).25 The highest frequency of HIT (~5%) has been reported in postorthopedic surgery patients receiving up to 2 weeks of UFH.24,25,28,29 Differing risk in various clinical settings influences the intensity of platelet count monitoring for HIT30,31: for example, in patients at highest risk of HIT (e.g., postsurgical patients receiving prophylactic-dose UFH), at least alternate-day platelet count monitoring is appropriate. In contrast, routine monitoring may not be useful in pregnant women receiving LMWH (negligible risk). HIT occurred in about 0.5% of postorthopedic surgery patients receiving LMWH for up to 2 weeks.7
Laboratory Testing
HIT antibodies are detected using either PF4-dependent “antigen” assays or platelet “activation” (functional) assays.30 The latter can be further divided into those utilizing washed platelets and those employing citrate-anticoagulated platelet-rich plasma (PRP). Washed platelet activation assays are more sensitive and specific for HIT antibodies than PRP-based assays. The major drawback of washed platelet assays is their technically demanding nature. Several commercial PF4-dependent antigen assays are now available. Two that utilize conventional solid-phase enzyme immunoassay (EIA) technology are very sensitive for HIT; however, clinically insignificant HIT antibodies are also often detected among patients who have received heparin 5 to 100 days earlier. Further, antibodies against “minor” non-PF4/heparin antigens (e.g., interleukin-8) are not detected by PF4-dependent EIA. A particle gel assay that utilizes PF4 coated onto spheres has been developed.32 This rapid assay (< 30 minutes) may be somewhat less sensitive than the solid-phase EIA but has fewer positive reactions among healthy controls.
HIT: A Clinicopathologic Syndrome
In my view, HIT should be seen as a “clinicopathologic syndrome,” i.e., the diagnosis should be based upon clinically evident abnormalities (usually, thrombocytopenia with or without new thrombosis) and a (generally “strong”) positive test for HIT antibodies.30 Estimation of the pretest probability of HIT, e.g., by using a scoring system (Figure 5 ), and interpreted together with HIT antibody test results (including magnitude of a positive result), may improve diagnostic accuracy (Bayesian approach).33 Some might argue that a high pretest probability of HIT obviates the need for laboratory confirmation of HIT antibodies. While the fact that tests are least useful when the pretest probability is either very high or very low is something of a truism, sometimes thrombocytopenia is caused by disorders that strongly mimic HIT (pseudo-HIT34), and negative results using sensitive tests (e.g., the combination of a washed platelet activation assay and a PF4-dependent EIA) are needed to rule out HIT. For example, cancer-associated disseminated intravascular coagulation (DIC) can present with thrombocytopenia and venous limb ischemia during the transition from heparin to warfarin and thus resemble HIT.34,35
Natural History of Isolated HIT
Patients with “isolated HIT” are those suspected of having HIT because of thrombocytopenia alone and not because a new (HIT-associated) thrombosis draws attention to the platelet fall.28 Several retrospective cohort studies36–,39 indicate that 25%–50% of these patients develop clinically evident thrombosis after stopping heparin (with or without substitution by warfarin), usually within the first week (for review, see Warkentin23,40). The risk of fatal thrombosis is 4% to 5%.40 Further evidence includes a cohort study41 that found subclinical thrombosis in 8 of 16 (50%) patients who underwent routine lower-limb duplex ultrasonography for isolated HIT. Interestingly, early heparin cessation does not reduce risk of thrombosis in patients with isolated HIT37; this implies that routine platelet count monitoring for HIT might not prevent thrombosis if the response is merely to stop the heparin rather than to substitute an alternative anticoagulant.
Treatment
Management primarily involves stopping all heparin and (usually) initiating an alternative anticoagulant in those patients strongly suspected as having HIT, even when thrombosis is not clinically apparent (Figure 5 ). This recommendation31 is based upon the unfavorable natural history of isolated HIT and risk of thrombosis during delay in obtaining HIT antibody test results.
Several nonheparin anticoagulants are rational therapies for HIT, including direct thrombin inhibitors (DTIs), such as argatroban, lepirudin, or bivalirudin, and a nonheparin glycosaminoglycan with predominant anti-factor Xa activity, danaparoid (no longer available in the US). A novel anticoagulant with anti-Xa (and anti-IXa) activity, fondaparinux, theoretically should be effective for HIT, but experience is limited, and optimal dosing is not established. Only 1 (retrospective) study compared 2 of these agents (lepirudin and danaparoid),42 and so the relative efficacy of these agents is uncertain. Thus, drug selection should be based primarily upon drug availability, individual patient characteristics, and prior physician experience and preference.
Lepirudin, argatroban, and danaparoid have been most studied for HIT, but only danaparoid was assessed in a randomized clinical trial (against dextran-70).43 The low-dose danaparoid regimen (750 U, 2 or 3 times a day, subcutaneously) approved in some jurisdictions for prophylaxis or treatment of HIT is less effective than a therapeutic-dose protocol.42 Thus, danaparoid (like DTIs) should generally be given in therapeutic doses when treating HIT, preferably with initial intravenous bolus.
DTIs in HIT: Which Agent to Use?
Table 5 provides an indirect comparison of lepirudin and argatroban for treating HIT (with or without thrombosis).23 Both agents showed improved outcomes compared with historical controls.17,38,39,44–,46 However, important differences in the conduct of the trials preclude definitive conclusions regarding relative efficacy.23 For example, treatment duration with argatroban was substantially shorter than in the lepirudin trials, and fewer argatroban-treated patients (eventually) received coumarin: both factors increase the likelihood that lepirudin would show improved outcomes, even if both agents had identical efficacy. Also, whereas the pivotal lepirudin trials required serological confirmation of HIT, patients entered the argatroban trials based upon a clinical diagnosis of HIT. Since some patients did not have HIT, this had the potential effect of underestimating efficacy based upon the primary end point (composite of all-cause mortality, limb amputation, and new thrombosis): this is because mortality rates tend to be relatively high in patients who are clinically suspected to have HIT but who test negative for HIT antibodies, likely because of the high mortality of many non-HIT disorders, e.g., septicemia (Warkentin and Kelton, unpublished data).
Pharmacokinetic factors influence choice of therapy. For example, lepirudin is renally excreted, whereas argatroban undergoes hepatobiliary excretion. Therefore, argatroban is preferred in patients with renal insufficiency, whereas lepirudin may have advantages in patients with hepatic disease. Another consideration is that the polypeptide nature of lepirudin results in many patients forming antihirudin antibodies.47 Although these are usually clinically insignificant, a recent report48 indicates that a lepirudin bolus can cause anaphylaxis, particularly in patients who received lepirudin within the previous 3 months (risk, ~1/600). Thus, physicians might omit the lepirudin bolus, or prescribe argatroban, in patients recently treated with lepirudin.
Warfarin-associated venous limb gangrene
Coumarins (e.g., warfarin, phenprocoumon) have the potential to cause microvascular thrombosis in patients with uncontrolled thrombin generation related to HIT or other hypercoagulability disorders.16,35,49–52 The typical clinical presentation is a HIT patient with acute deep vein thrombosis treated with coumarin in whom distal extremity ischemia coincides with the international normalized ratio (INR) rising above the therapeutic range. Ischemia can progress to venous limb gangrene, resulting in acral limb loss despite palpable arteries. Less often, coumarin-induced microvascular thrombosis produces “classic” skin necrosis in central (nonacral) tissue sites, such as breast, abdomen, or thigh.
In theory, coumarin-induced necrosis can be avoided by deferring oral anticoagulants until HIT has substantially resolved (or administering vitamin K if HIT is diagnosed only after coumarin has been initiated). After platelet count recovery to at least 100 (preferably, 150) × 109/L on DTI treatment, overlapping coumarin should be started cautiously, beginning with a maintenance (rather than loading) dose, ensuring at least 4–5 days of overlap, and stopping the DTI only when the INR has been within the target range for 2 consecutive days and the platelet count has recovered to a stable plateau.31 A practical problem is that the INR is prolonged by DTIs,53–,56 particularly argatroban, and that the INR rises further during combined DTI/coumarin. Indeed, the appropriate target INR can be as high as 4.0–7.0 in patients receiving combined therapy with argatroban and warfarin, if a thromboplastin with high international sensitivity index is used.53 The lack of effect on the INR, and long half-lives, are advantages of danaparoid and fondaparinux in transitioning to oral anticoagulants.
Special Topics
HIT during pregnancy
Lepirudin, bivalirudin, argatroban, danaparoid, and fondaparinux are drugs in category B, indicating absence of fetal damage in certain high-dose animal studies but limited (if any) human data. Danaparoid and fondaparinux57 do not appear to cross the placenta, whereas DTIs can cross the placenta in low doses58 and have caused embryopathy in rabbits given high doses of hirudin.59 Furthermore, a zebrafish model reveals thrombin to play a role in embryogenesis.60 Thus, danaparoid and fondaparinux may be preferable for treatment of HIT during (early) pregnancy.
HIT during renal failure
HIT and cardiac surgery
There are 3 major approaches (for review, see Warkentin and Greinacher63): (1) await disappearance of HIT antibodies, and then give UFH during cardiac surgery; (2) combine intraoperative UFH with potent antiplatelet therapy (e.g., epoprostenol or tirobifan); and (3) use alternative anticoagulant for cardiopulmonary bypass (CPB), such as bivalirudin, lepirudin, or danaparoid. The last approach requires special intraoperative monitoring (e.g., ecarin clotting time for bivalirudin or lepirudin, anti-factor Xa assays for danaparoid) and has other drawbacks, such as lack of antidotes and drug accumulation if postoperative renal (lepirudin) or hepatic (argatroban) failure occurs. Moreover, special steps are required by the anesthesiologist to avoid pump clotting, or to permit reinfusion of pump contents into the patient following CPB.63
III. The Antiphospholipid Syndrome: Pathogenic Mechanisms, Diagnosis, and Treatment
Jacob H. Rand, MD*
Montefiore Medical Center, 111 E 210th Street, Core Lab Office, Silver Zone, Bronx NY 10467
The antiphospholipid (aPL) antibody syndrome is an autoimmune condition in which vascular thrombosis and/or recurrent pregnancy losses occur in patients with laboratory evidence for autoantibodies against phospholipids or phospholipid-binding protein cofactors. The thrombi generally occur in large veins and arteries, but any portion of the vasculature may be affected. Rarely, patients present with a catastrophic form of the condition that is marked by disseminated small and large vessel occlusions with multiorgan damage.1
Confident diagnosis of this autoimmune prothrombotic condition is often challenging since antibodies against anionic phospholipids such as cardiolipin are prevalent in the general population and can arise in other conditions such as infectious diseases. A classification of the various patients having aPL antibodies is shown in Table 6 . Research criteria have been developed to identify patients with the “definite” autoimmune aPL syndrome2 (Table 7 ).
Antigenic Targets of aPL Antibodies
The initial immunoassays for detecting this disorder were designed to quantify the “biologic false-positive” syphilis test for which the major target antigen is the anionic phospholipid cardiolipin (diphosphatidylglycerol). The actual targets of “aPL” antibodies from patients with this autoimmune syndrome (in contrast to most patients who develop these antibodies in response to infection) are generally believed to be phospholipid-binding protein cofactors, the major one being the serum protein, β2-glycoprotein I (β2GPI), also known as apolipoprotein H; however, antibodies may also be directed to complexes of phospholipid-binding proteins and phospholipids.
β2GPI is a member of the complement control protein, or short consensus repeat (SCR), superfamily. The protein, which contains 5 repeating SCR stretches of ~60 amino acid residues,3 is thought to insert into phospholipid bilayer through a cationic segment near the carboxyterminus of its fifth SCR domain (Figure 6; see Appendix, page 604). It appears likely that dimerization of the protein, via aPL IgG recognition of epitopes that are present in aminoterminal portion of the molecule, increases the affinity of the antibody-protein complex for membrane phospholipids.3,4 A similar process has been shown to occur with the second major cofactor, prothrombin. This high-affinity phospholipid-binding complex is the likely basis for the lupus anticoagulant (LA) phenomenon, in which these antibodies inhibit phospholipid-dependent coagulation reactions. Divalent immune complex formation also appears to play a role in the development of arterial thrombosis in an animal model of photochemically induced vessel damage;5 a monoclonal anti-β2GPI and its Fab2 fragments, but not the Fab1 fragments, were shown to promote thrombosis in this model.
The biologic function of β2GPI has not been established. β2GPI deficiency occurs without any apparent consequences in otherwise normal, healthy humans. Homozygous β2GPI-null mice appear to be without disease, anatomically and histologically.6 Since fewer than expected numbers of homozygous β2GPI-null offspring are born from heterozygous parents, it was suggested that the protein may play a role early in the reproductive process.6 β2GPI has been shown to bind to endothelial cells via annexin II, a protein that also serves as a receptor for plasminogen and tissue plasminogen activator.7 As further discussed below, it has been proposed that antibody binding to β2GPI on the endothelial surface can increase the expression of adhesion molecules on the membrane.
Other phospholipid-binding proteins have also been shown to be recognized by aPL antibodies or to be cofactors for antibody recognition of phospholipids. These include prothrombin (coagulation factor II), coagulation factor V, protein C, protein S, annexin A5, high- and low-molecular-weight kininogens, and recently factor VII/VIIa.8 Antibodies of some aPL patients recognize heparin and inhibit the formation of thrombin-antithrombin complexes. The antigenic targets in the aPL syndrome and in heparin-induced thrombocytopenia (HIT) share a similar pattern; both consist of protein epitopes that are bound to polyanions—β2GPI linked to anionic phospholipids in the aPL syndrome and PF4 linked to heparin in HIT.
Proposed Mechanisms for Thrombosis
Multiple mechanisms have been offered to explain how aPL antibodies may promote thrombosis (see Rand9 for review). The bulk of evidence accumulated from animal models of the aPL syndrome indicates that aPL antibodies play a causal role in the development of thrombosis and pregnancy loss. A direct causal relationship between aPL antibodies and thrombotic manifestations or pregnancy losses in humans has not yet been proven.
Reversal of endogenous anticoagulant mechanisms
aPL antibodies can affect several endogenous anticoagulant mechanisms in vitro. Annexin A5 is a potent anticoagulant protein that forms 2-dimensional crystalline lattices over phospholipid surfaces (Figure 7A )10 and shields the phospholipid from availability for coagulation enzyme reactions. Annexin A5 is reduced on syncytiotrophoblasts of aPL and preeclamptic placentas and on cultured human trophoblasts and endothelial cells exposed to aPL IgG antibodies.11 aPL IgGs from patients with the aPL syndrome reduce the binding of annexin A5 to phospholipid-coated microtiter plates, an effect that is dependent upon anti-β2GPI antibodies and correlates with clinical thrombosis.11 Recently, atomic force microscopic imaging has provided direct morphologic evidence that aPL monoclonal antibodies can disrupt the ordered structure of the 2-dimensional annexin A5 crystal shield over phospholipid bilayers (Figure 7B ),12 allowing more phospholipid to be available for coagulation reactions.
aPL antibodies have been shown to interfere with every aspect of the thrombomodulin-protein C-protein S system, inhibiting the formation of thrombin (through the inhibition of prothrombinase activity), decreasing protein C activation by the thrombomodulin-thrombin complex, inhibiting the assembly of the protein C complex, inhibiting activated protein C activity, and binding to factors Va and VIIIa in manners that protect them from proteolysis by activated protein C.13 Protein C can be a target of anticardiolipin antibodies (aCL) in the presence of cardiolipin and β2GPI, leading to protein C dysfunction. In addition to these effects, patients with the aPL syndrome frequently have protein S deficiency.14
aPL antibodies may increase tissue factor activity by inhibiting tissue factor pathway inhibitor (TFPI) activity.15 The recent finding that low TFPI levels are a risk factor for deep vein thrombosis16 supports the plausibility of this concept. aPL antibodies may reduce antithrombin activity in 2 ways. Some aPL have been shown to cross-react with heparin and heparinoid molecules (which are highly polyanionic) and thereby inhibit antithrombin activity. In addition, some aPL antibodies recognize an epitope on thrombin that reduces its inactivation by antithrombin.17
Effects on endothelium
Several effects of aPL antibodies upon vascular endothelium have been described. aPL antibodies can injure and/or activate cultured human vascular endothelial cells. This activation is mediated by anti-β2GPI and results in the increased expression of cell adhesion molecules18 and of tissue factor. The activation of endothelial cells by anti-β2GPI is followed by redistribution of nuclear factor kappa B (NFκB) from the cytoplasm to the nucleus, a process that is accompanied by increased expression of tissue factor and of the leukocyte adhesion molecules ICAM-1, VCAM-1, and E-selectin. Inhibition of the nuclear translocation of NFκB abolished the response to these antibodies. In an immortalized human microvascular endothelial cells model, the signaling cascade has been shown to involve TRAF6 and MyD88 but not TRAF2 and to show the same time kinetics as interleukin (IL)-1 receptor-activated kinase phosphorylation, suggesting an involvement of the toll-like receptor family.19 The pattern of effects of the anti-β2GPI antibodies is comparable to activation by lipopolysaccharide or IL-1 but different from activation by tumor necrosis factor. The aPL antibody-induced adhesion of leukocytes to endothelium with concurrent thrombosis was confirmed in vivo in a murine model of vascular injury, where it was shown to be mediated by ICAM-1, VCAM-1, and P-selectin.20 Complement activation has also been proposed to play a role in the mechanisms of aPL-associated thrombosis and pregnancy loss.21 aPL antibodies that recognize annexin A5 induce apoptosis in endothelial cells.22 Endothelial-derived microparticles are detectable in normal human blood and are increased in patients with lupus anticoagulants (LA).23 Significantly increased plasma levels of endothelin-1, which is thought to play a role in arterial tone, vasospasm, and thrombotic arterial occlusion, were found in aPL syndrome patients with arterial thrombosis. Human monoclonal aCL induced an increase of prepro-endothelin-1 messenger RNA levels.
Effects on platelets
aPL antibodies also activate platelets and stimulate platelet aggregation. Prothrombotic properties of aPL antibodies may be explained in part by their ability to enhance the activation of platelets pretreated with low doses of adenosine diphosphate or thrombin receptor agonist peptide, an effect that is reduced by the antimalarial drug hydroxychloroquine in a dose-dependent manner.24 There is increased platelet activation in the primary aPL syndrome and systemic lupus erythematosus, as evidenced by increased platelet CD63 expression in primary aPL syndrome and in SLE patients with and without secondary aPL syndrome, and increased PAC-1 binding in primary aPL syndrome and SLE patients without aPL syndrome. Monoclonal aCL with anti-β2GPI specificity also promoted platelet interaction with the subendothelium under flow conditions when the cofactor was present.25
Other mechanisms
aPL antibodies have also been reported to promote tissue factor synthesis by leukocytes. Stimulation of monocytes from aPL syndrome patients with β2GPI induced substantial monocyte tissue factor, whereas no induction was observed with cells from patients having aPL antibodies without clinical problems; this effect required CD4+ T-lymphocytes and class II major histocompatibility complex (MHC) molecules.26 In one study, the ability of IgG to stimulate monocyte tissue factor expression was associated with the presence of decreased free protein S and increased prethrombotic markers.27
It has also been suggested that fibrinolysis may be impaired in the aPL syndrome, since women with the disorder have been described to have elevated plasminogen activator inhibitor-1 levels. Fibrinolysis may also be impaired via anti-β2GPI-mediated inhibition of the autoactivation of factor XII and the ensuing reductions of kallikrein and urokinase.
Diagnosis
The diagnosis of the aPL syndrome in clinical practice can often be problematic, especially since many patients referred to hematologists have isolated or equivocal laboratory abnormalities. Adding to the difficulty is the high prevalence of positive tests—between 3% and 10%—in the asymptomatic general population. The two general approaches to detecting aPL antibodies are through coagulation tests that report the LA effect and through enzyme-linked immunosorbent assays (ELISAs) using solid-phase phospholipids (most commonly cardiolipin or phosphatidylserine) and protein cofactors (most commonly β2GPI) as antigenic targets. At present, no single test is sufficient to diagnose the disorder. The panel of tests performed should at least include a coagulation screening test for LA, syphilis testing, and assays for antibodies against aCL, phosphatidylserine (aPS), and β2GPI.
Lupus anticoagulant tests
It is paradoxical that one of the key methods for diagnosing this thrombophilic syndrome detects autoantibodies that inhibit phospholipid-dependent coagulation reactions in vitro. LAs are not associated with bleeding problems unless there are other hemostatic defects, e.g., hypoprothrombinemia, thrombocytopenia, platelet function abnormalities, or specific inhibitors of blood coagulation factors or severe acquired factor X deficiency).28
The LA phenomenon can be understood to be a surrogate marker for identifying high-affinity aPL antibodies. As described above, this is most likely due to the divalent interactions between the Fab2 portions of the antibodies and the cofactors β2GPI and prothrombin.4 The prothrombotic effects of these antibodies may be a consequence of this high-affinity antibody-antigen-membrane interaction (Figure 8; see Appendix, page 605). High-affinity antibodies may play a similar role with respect to activation events initiated on endothelial cell membranes.
This utility for identifying high-affinity aPL antibodies would explain why LA, as a surrogate “functional” test, better predicts the risk of thrombosis than the antiphospholipid immunoassays.29,30 A meta-analysis of the risk for aPL-associated venous thromboembolism in individuals with aPL antibodies without underlying autoimmune disease or previous thrombosis followed for a 15-year period showed the mean odds ratios to be 1.6 for aCL antibodies, 3.2 for high titers of aCL, and 11.0 for LA.30 Similarly, data from the Hopkins Lupus Cohort, a large prospective study, showed that in these patients the presence of LA is a better predictor of the risk of venous thrombosis than aCL.31
A number of different methods have been devised to detect the LA phenomenon. The consensus criteria for defining the LA phenomenon provide diagnostic principles but do not specify the methods to be utilized.32 The criteria stipulate that the tests should report (1) the prolongation of a phospholipid-dependent coagulation test, (2) evidence of inhibitor activity in the test plasma determined by mixing tests with pooled normal plasma, and (3) confirmation that the inhibitory effect is due to blocking phospholipid-dependent coagulation (i.e., neutralization of the inhibitory effect by adding excess phospholipids or by changing the source of phospholipid). LA tests, and their interpretations, are notoriously fickle; even specialized laboratories will frequently disagree about the interpretation of an LA effect in given plasmas.
The dilute Russell viper venom time (dRVVT) is considered to be one of the most sensitive LA tests. The test is performed by adding Russell viper venom (RVV) to a sample containing diluted rabbit brain phospholipid and patient plasma. RVV directly activates coagulation factor X, leading to the formation of fibrin clot. LA prolongs the dRVVT by interfering with assembly of the prothrombinase complex. To ensure that prolongation of the clotting time is not due to coagulation factor deficiency (e.g., liver disease, warfarin treatment), a mixture of patient and control plasma is also tested.
The LA phenomenon is one of the frequent causes of a prolonged activated partial thromboplastin time (aPTT). The currently available reagents for performing aPTTs vary widely in their sensitivity to LAs. These differences may be exploited for identifying LAs based upon the differential results with LA-sensitive and -insensitive reagents. When the aPTT is prolonged and not “correctable” by mixture with normal plasma, the presence of an “anticoagulant” or “inhibitor” should be suspected. The LA is differentiated from inhibitors of specific coagulation factors (most commonly, factor VIII) and from anticoagulants such as heparin by using specific assays to exclude these possibilities. If the aPTT is normalized when an “LA-insensitive” aPTT reagent is used or when frozen washed platelets are added to the aPTT assay—the platelet neutralization procedure—then an LA effect is likely present. Confirmation of a prolonged aPTT with the LA-insensitive reagent points toward a true coagulation factor deficiency or an acquired coagulation factor inhibitor. In rare patients, both types of anticoagulants—LA and specific coagulation factor inhibitors—coexist. Several other LAs may also be used. The kaolin clotting time is similar to the aPTT but uses a different activator (kaolin) and limited phospholipid concentrations to better detect interference from aPL antibodies. The tissue thromboplastin inhibition test is a diluted prothrombin time assay. The textarin/ecarin test depends on the different coagulation mechanisms initiated by 2 snake venoms—textarin activates prothrombin via a phospholipid-dependent pathway, and ecarin activates prothrombin in the absence of phospholipid.
Immunoassays
The most frequently used immunoassay for the aPL syndrome is the ELISA for aCL antibodies. High levels of aCL antibodies predict an increased risk of thrombosis. During a 10-year follow-up of asymptomatic patients with raised levels of aCL antibodies, about 50% of patients subsequently developed clinical manifestations of the syndrome.33 Also, the presence of elevated titers of aCL 6 months after an episode of venous thromboembolism predicts an increased risk of recurrence and of death.33 aPL syndrome has been described primarily with elevated aCL IgG antibodies but also occurs with elevated IgM antibodies, and infrequently with IgA antibodies. With respect to stroke, elevated anticardiolipin antibodies of IgG or IgM isotype are a significant risk factor.34 Antiphospholipid antibodies are also an independent risk factor for stroke in young women.35
Despite the important role of β2GPI protein as an antigenic target in the aPL syndrome, the usefulness of clinical tests for anti-β2GPI antibody activity is limited by the low sensitivity (40%–50%) of these tests. Nevertheless, their specificity for the aPL syndrome has been estimated to be approximately 98%. Theoretically, tests for antibodies against phosphatidylserine (located on the plasma membrane of cells) are more pathophysiologically relevant than aCL antibodies (located on intracellular membranes not exposed to plasma). aPS antibodies correlate more specifically with aPL syndrome than aCL antibodies do.36 The risk of stroke with elevated aPS antibodies is comparable to the risk with aCL antibodies.34
Prothrombin is the second major cofactor for aPL antibodies. Although antiprothrombin antibodies occur in 30% of patients with SLE and were previously reported to be significantly associated with thrombosis, their usefulness has been questioned.37 The presence of these antibodies correlates with hypoprothrombinemia and thrombocytopenia.
Treatment
Patients with spontaneous thromboembolism and the aPL syndrome should be treated with long-term oral anticoagulant therapy. Results of studies vary as to the recommended intensity of anticoagulant therapy. A retrospective study concluded that an international normalization ratio (INR) of ≥ 3.0 was necessary to protect patients from recurrence of venous or arterial thrombosis.38 However, prospective studies on the treatment of venous thromboembolism concluded that an INR in the range of 2.0–3.029,39 or 2.0–2.8540 is effective.
A high titer of aCL alone is not sufficient to justify prophylactic anticoagulation therapy in asymptomatic patients. The same conclusion should be applied to most patients with LAs who have not experienced thrombotic or embolic events. Anticoagulant therapy may be considered for occasional asymptomatic patients: patients with convincing family histories for thromboembolic complications of the aPL syndrome who themselves manifest significant laboratory abnormalities, patients with SLE who have significant aPL laboratory abnormalities, and patients who also have other reasons for being at increased risk for thrombosis (e.g., severe valvular heart disease).41 The antimalarial drug hydroxychloroquine may be considered for treating patients with SLE who have aPL antibodies but not thrombosis since it appears to have an antithrombotic effect in these patients.42
Women with a history of recurrent spontaneous pregnancy losses and evidence of aPL antibodies should be treated with a combination of low-dose aspirin (75–81 mg daily) and unfractionated heparin (5000 units subcutaneously every 12 hours).43 Treatment with low-molecular-weight heparins has been studied44,45 and appears to be efficacious, but these drugs are not approved by the Food and Drug Administration for pregnancy. Anticoagulant treatment should be continued for the period of the puerperium—i.e., an additional 6 weeks—to reduce the risk of thromboembolism.
Corticosteroid treatment should only be considered for patients who are refractory to anticoagulant therapy, who have a severe immune thrombocytopenia, or who have a contraindication to heparin therapy. Treatment with the combination of prednisone and heparin should generally be avoided, since this combination will markedly increase the risk of osteopenia and vertebral fractures. Patients with the catastrophic aPL syndrome require aggressive immunosuppressive therapy in addition to anticoagulation.
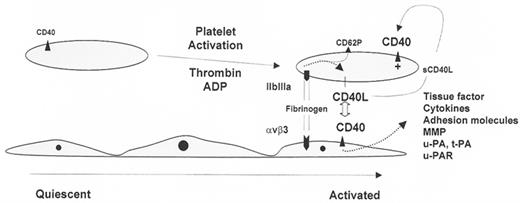
CD40-CD40L system as an example of platelet-endothelial interaction.
Shown are three endothelial cells and two platelets (not drawn to scale). Platelet activation results in increased expression of CD40 and CD40 ligand (CD40L). GPIIbIIIa-dependent adhesion of platelets to the endothelium results in CD40L-induced activation of endothelial cells with secondary induction of tissue factor, cytokines, adhesion molecules, metalloproteinases (MMP), urokinase-type plasminogen activator (u-PA), tissue-type plasminogen activator (t-PA), and urokinase receptor (u-PAR). Thus, the platelet indirectly orchestrates (via the endothelium) changes in coagulation, leukocyte trafficking, and extracellular matrix modeling/turnover. At the same time, the inter-action between platelet and endothelial cells results in GPIIbIIIa-mediated outside-in signaling with secondary induction of CD40L and CD62P (P-selectin) expression on the platelet surface. In addition, soluble trimeric CD40L (sCD40L), released from activated platelets, may engage platelet CD40 in an autocrine or paracrine manner (indicated by +), resulting in shape change and α-granule release.
Based in part on
CD40-CD40L system as an example of platelet-endothelial interaction.
Shown are three endothelial cells and two platelets (not drawn to scale). Platelet activation results in increased expression of CD40 and CD40 ligand (CD40L). GPIIbIIIa-dependent adhesion of platelets to the endothelium results in CD40L-induced activation of endothelial cells with secondary induction of tissue factor, cytokines, adhesion molecules, metalloproteinases (MMP), urokinase-type plasminogen activator (u-PA), tissue-type plasminogen activator (t-PA), and urokinase receptor (u-PAR). Thus, the platelet indirectly orchestrates (via the endothelium) changes in coagulation, leukocyte trafficking, and extracellular matrix modeling/turnover. At the same time, the inter-action between platelet and endothelial cells results in GPIIbIIIa-mediated outside-in signaling with secondary induction of CD40L and CD62P (P-selectin) expression on the platelet surface. In addition, soluble trimeric CD40L (sCD40L), released from activated platelets, may engage platelet CD40 in an autocrine or paracrine manner (indicated by +), resulting in shape change and α-granule release.
Based in part on
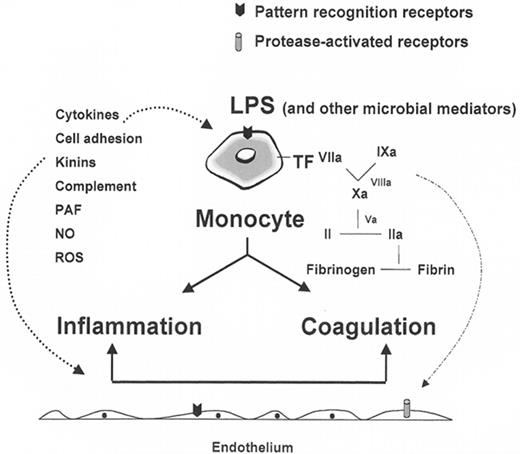
Schematic of the innate immune response.
Shown are the monocyte, the endothelium, inflammatory and coagulation pathways, and receptors for lipopolysaccharide (LPS) and serine proteases.
Abbreviations: TF, tissue factor; PAF, platelet activating factor; NO, nitric oxide; ROS, reactive oxygen species.
Schematic of the innate immune response.
Shown are the monocyte, the endothelium, inflammatory and coagulation pathways, and receptors for lipopolysaccharide (LPS) and serine proteases.
Abbreviations: TF, tissue factor; PAF, platelet activating factor; NO, nitric oxide; ROS, reactive oxygen species.
[on page 507] . A diagnostic and treatment approach to suspected heparin-induced thrombocytopenia (HIT).
Physicians usually must make initial treatment decisions based upon the probability—judged clinically—that a patient has HIT (an 8-point scoring system, the 4 T’s, for judging pretest probability of HIT is shown). Treatment decisions range from stopping heparin and substituting an alternative anticoagulant (high probability of HIT) to continuing heparin (low probability of HIT). Results of diagnostic testing for HIT antibodies can influence whether an alternative anticoagulant is continued until platelet count recovery, or (low molecular weight) heparin is continued or resumed. Assessment for thrombosis should include routine imaging for lower-limb DVT, as subclinical lower-limb DVT occurs frequently in HIT and influences treatment duration. The transition from alternative anticoagulation to coumarin (eg, warfarin, phenprocoumon) should be managed cautiously, as HIT is a risk factor for coumarin-induced necrosis, including venous limb gangrene associated with DVT.
Abbreviations: aPTT, activated partial thromboplastin time; ASR, acute systemic reaction; DVT, deep vein thrombosis; INR, international normalized ratio; LMW, low molecular weight; PF4, platelet factor 4.
[on page 507] . A diagnostic and treatment approach to suspected heparin-induced thrombocytopenia (HIT).
Physicians usually must make initial treatment decisions based upon the probability—judged clinically—that a patient has HIT (an 8-point scoring system, the 4 T’s, for judging pretest probability of HIT is shown). Treatment decisions range from stopping heparin and substituting an alternative anticoagulant (high probability of HIT) to continuing heparin (low probability of HIT). Results of diagnostic testing for HIT antibodies can influence whether an alternative anticoagulant is continued until platelet count recovery, or (low molecular weight) heparin is continued or resumed. Assessment for thrombosis should include routine imaging for lower-limb DVT, as subclinical lower-limb DVT occurs frequently in HIT and influences treatment duration. The transition from alternative anticoagulation to coumarin (eg, warfarin, phenprocoumon) should be managed cautiously, as HIT is a risk factor for coumarin-induced necrosis, including venous limb gangrene associated with DVT.
Abbreviations: aPTT, activated partial thromboplastin time; ASR, acute systemic reaction; DVT, deep vein thrombosis; INR, international normalized ratio; LMW, low molecular weight; PF4, platelet factor 4.
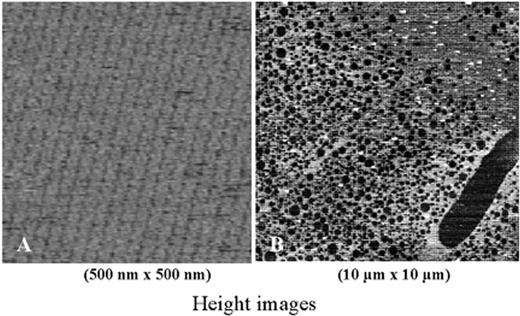
Annexin A5—effect of aPL antibody.
(A) An atomic force image of the 2-dimensional crystallization of annexin A5 over a phospholipid bilayer. Each of the circular structures that compose each row is a trimer of annexin A5. The crystal covers nearly 100% of the phospholipid bilayer.
(B) The effects of a monoclonal aPL antibody together with β2GPI on the crystal structure of annexin A5. The aPL-β2GPI results in marked disruptions of the annexin A5 crystal.
Abbreviations: aPL, antiphospholipid; β2GPI, β2-glycoprotein I.
Annexin A5—effect of aPL antibody.
(A) An atomic force image of the 2-dimensional crystallization of annexin A5 over a phospholipid bilayer. Each of the circular structures that compose each row is a trimer of annexin A5. The crystal covers nearly 100% of the phospholipid bilayer.
(B) The effects of a monoclonal aPL antibody together with β2GPI on the crystal structure of annexin A5. The aPL-β2GPI results in marked disruptions of the annexin A5 crystal.
Abbreviations: aPL, antiphospholipid; β2GPI, β2-glycoprotein I.

![Figure 5. [on page 507] . A diagnostic and treatment approach to suspected heparin-induced thrombocytopenia (HIT). / Physicians usually must make initial treatment decisions based upon the probability—judged clinically—that a patient has HIT (an 8-point scoring system, the 4 T’s, for judging pretest probability of HIT is shown). Treatment decisions range from stopping heparin and substituting an alternative anticoagulant (high probability of HIT) to continuing heparin (low probability of HIT). Results of diagnostic testing for HIT antibodies can influence whether an alternative anticoagulant is continued until platelet count recovery, or (low molecular weight) heparin is continued or resumed. Assessment for thrombosis should include routine imaging for lower-limb DVT, as subclinical lower-limb DVT occurs frequently in HIT and influences treatment duration. The transition from alternative anticoagulation to coumarin (eg, warfarin, phenprocoumon) should be managed cautiously, as HIT is a risk factor for coumarin-induced necrosis, including venous limb gangrene associated with DVT. / Abbreviations: aPTT, activated partial thromboplastin time; ASR, acute systemic reaction; DVT, deep vein thrombosis; INR, international normalized ratio; LMW, low molecular weight; PF4, platelet factor 4.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2003/1/10.1182_asheducation-2003.1.497/5/m_warkentin_fig5.jpeg?Expires=1765989592&Signature=VX5R5A5oSj2emv4y9eysT-eIR4yt1vWCRQq8GJGPQGfy-h1SdII1-~C7VF7PhErhIp0RbVM7R6Zt6C4I2Q3BG5Q0K3OWvzb152ZKJm4HYf16z~6yVVILm8fAKcKkhQojvgvgkzLJNOP7E0H0iyS~KKdhtRBGg0-n43N9iBDH5C77-f-ibCCm2ubVZ4kit4~-N1kjaHCB-Q438j4cve32dQ5bL36h8gGU8x6xr4rrJpBCgz8dFdsfte82846q~UoV5Y~1kY5xtdp1oC8AsgZwYmyMihoydvPxAukyAEM8nqqAjK4-KeGyUack8-SsqlZ-Eue-7xOQwpX02pFgIvTtpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 5. [on page 507] . A diagnostic and treatment approach to suspected heparin-induced thrombocytopenia (HIT). / Physicians usually must make initial treatment decisions based upon the probability—judged clinically—that a patient has HIT (an 8-point scoring system, the 4 T’s, for judging pretest probability of HIT is shown). Treatment decisions range from stopping heparin and substituting an alternative anticoagulant (high probability of HIT) to continuing heparin (low probability of HIT). Results of diagnostic testing for HIT antibodies can influence whether an alternative anticoagulant is continued until platelet count recovery, or (low molecular weight) heparin is continued or resumed. Assessment for thrombosis should include routine imaging for lower-limb DVT, as subclinical lower-limb DVT occurs frequently in HIT and influences treatment duration. The transition from alternative anticoagulation to coumarin (eg, warfarin, phenprocoumon) should be managed cautiously, as HIT is a risk factor for coumarin-induced necrosis, including venous limb gangrene associated with DVT. / Abbreviations: aPTT, activated partial thromboplastin time; ASR, acute systemic reaction; DVT, deep vein thrombosis; INR, international normalized ratio; LMW, low molecular weight; PF4, platelet factor 4.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2003/1/10.1182_asheducation-2003.1.497/5/m_warkentin_fig5.jpeg?Expires=1766166483&Signature=X4OB9YF5fjtZEBb-jRI45~d~BCLWmeNvquzq2nnCLaaRmkQIvXdDVQXA9rObv7TYi8tAbiaNWBnFWtnUrblbdFRxvzsmGmeBSO8pABypWliy4BmK1Oy333dqApimBQ~qg5h9b~2HhcICjsjbngPZVx8iX2rQJOyYChVX9wUATINRXFjg0kr7PH7wXwewFtvBZ3WOcF5xQqdV~7WdJS04ojAiCdvI5lD~n0ZXAH7BDYAaMaWJ8Ju9YRcniUiq-9sTvdP~GVl6ezzxZ4JgwIfqoErdNl4fAuTjTwRfZ0DsgWk5k7FQT0S-3FfiVKZlPAtIkZ-EY0csGtri3ddJOLZPPg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)