Abstract
Major strides have been made in our understanding of the molecular basis of adult and pediatric leukemias. More than one hundred disease alleles have been identified and characterized in cell culture and murine models of leukemia. In some instances, molecularly targeted therapies have been developed based on these insights that are currently in clinical trials, such as small molecule inhibitors of FLT3. In addition, it has recently been appreciated that, as with normal hematopoiesis, there is a hierarchical organization among leukemic cells that includes a rare population of leukemic stem cells that have properties of self-renewal. Understanding the characteristics of these leukemic stem cells may provide new insights into leukemia therapies that target self-renewal pathways.
In Section I, Dr. Craig Jordan reviews the data that supports the existence of a “leukemia stem cell.” He provides an overview of the functional properties of leukemic stem cells, their relationship to hematopoietic stem cells, and the relevance of leukemic stem cells in other human malignancies including solid tumors. He briefly discusses what is known of the pathways that regulate properties of self-renewal.
Dr. Gary Gilliland provides an overview of the genetics of adult leukemias in Section II and ongoing genome-wide strategies for discovery of new disease alleles. He describes the clinical and therapeutic implications of these findings and provides examples of bench-to-bedside translation of molecularly targeted therapies for AML, including the use of FLT3 inhibitors.
In Section III, Dr. Carolyn Felix reviews recent advances in our understanding of the genetics and therapy of pediatric leukemias. She provides an overview of leukemias that are common in pediatric malignancies but rarely observed in adults, including the TEL-AML1 (ETV6-RUNX1) fusion associated with pediatric B-cell ALL, the OTT-MAL fusion associated with infant megakaryoblastic leukemia, PTPN11 mutations in juvenile myelomonocytic leukemia, and MLL fusion genes in leukemogenesis, among others.
I. The Leukemia Stem Cell
Craig T. Jordan, PhD*
University of Rochester Medical Center, Department of Medicine, 601 Elmwood Ave., Box 703, Rochester NY 14642 Acknowledgments: I am grateful to Drs. Monica Guzman and Fay Young for critical review of this manuscript. CTJ is a scholar of the Leukemia and Lymphoma Society.
While a stem cell origin for myeloid leukemia has been postulated for over three decades, definitive experimental evidence for leukemia stem cells (LSC) has only been generated in recent years. With the development of appropriate functional assays and methods for cell sorting, investigators have isolated and characterized malignant stem cells for both acute and chronic myelogenous leukemia (AML and CML, respectively). These studies have demonstrated the central role of LSC in myeloid leukemia and highlighted the critical need for therapeutic strategies that directly target the LSC population. This section summarizes our current knowledge of LSC, describes the molecular mechanisms that may contribute to stem cell transformation, and discusses emerging new directions for leukemia therapy.
Properties of LSC
Formal proof for malignant stem cells has come through the use of methodologies previously validated for characterization of normal hematopoietic stem cells (HSC). This approach involves fractionation of a cell population, typically by virtue of distinct cell surface markers, followed by functional analysis of individual subsets. Those subpopulations displaying the requisite properties (i.e., self-renewal, multi-potentiality, and proliferation) are then further fractionated and re-analyzed until a relatively pure population of stem cells is achieved. This reductionist approach to stem cell analysis requires two technologies. First, a robust method of functional analysis must be in place to assay the properties of specific subpopulations. For LSC, these assays have come in the form of both long term in vitro culture assays1 and xenogeneic transplantation systems for in vivo analysis. In particular, transplantation of human hematopoietic cells into immune deficient NOD/SCID mice is currently the most commonly used and most physiological method to characterize normal or malignant human stem cells in vivo.2 The second technology required for stem cell analysis is an effective means of cell sorting. This approach requires the identification of specific cell surface markers that define the subpopulation of interest and a sophisticated flow cytometer that can analyze multiple cell surface markers simultaneously. In the case of human AML stem cells, a series of studies have defined their immunophenotype as CD34+, CD38−, CD71−, HLA−DR−, CD90−, CD117−, and CD123+.3 Expression of the last three antigens, CD90, CD117, and CD123, differ from normal HSC and thereby provide the means to separate normal from AML stem cells. Similarly, CML stem cells are known to be CD34+, CD38−.10
In addition to the immunophenotype, recent studies have identified specific developmental, cellular, and molecular features of LSC. Perhaps most central to the criteria by which stem cells are defined is the property of self-renewal. Studies by Bonnet and Dick demonstrated that human AML stem cells derived from multiple French-American-British (FAB) subtypes are able to undergo self-renewal.4 Furthermore, a recent report has further described subclasses of AML stem cells, defined by their relative degree of in vivo developmental potential and self-renewal.5 These studies demonstrate that LSC retain key features of normal stem cells and are biologically distinct from the bulk of leukemia blast cells that do not self-renew. Subsequent studies examined the cell cycle status of LSC and found that both AML and CML stem cells maintain populations that are quiescent.6,7 Although somewhat counterintuitive given that these cells are malignant, these findings indicate another property of normal stem cells that is apparently retained in the LSC population. As discussed below, the relative quiescence of LSC may be a major factor contributing to relapse. There are also unique molecular features of LSC. For example, phenotypically primitive human AML cells upregulate the genes encoding IRF-1 and DAP kinase.8 Similarly, high constitutive levels of NF-κB activity were reported for enriched populations of LSC.9 Likewise, primitive CML cells are capable of autocrine interleukin (IL)-3 growth stimulation and activation of STAT5.10 While the specific role of these genes and pathways remains to be more clearly elucidated, their various activities may provide clues to the underlying mechanisms that control LSC biology. In addition, specific molecular features of the LSC may provide useful targets for therapeutic intervention.
Origin of LSC and Molecular Pathogenesis
As the studies described above have begun to characterize malignant stem cells, investigators have increasingly turned to the question of which types of stem or progenitor cells are capable of transformation to LSC. Of course, the most primitive hematopoietic stem cell (HSC) is one possible target and is a cell type that intrinsically possesses properties that would be advantageous for malignant growth: self-renewal and extensive proliferative capacity. The HSC population is also relatively long-lived. From the standpoint of tumor pathogenesis, if multiple mutations are required for transformation to the malignant state, then presumably only those cells that are maintained for long periods have sufficient opportunity to acquire the requisite mutations. However, recent experimental evidence indicates that other types of early stem or progenitor cells may also serve as targets for transformation. For example, using a mouse model of AML, it was shown that purified populations of common myeloid progenitors (CMP) or granulocyte/macrophage progenitors (GMP) transduced with a retroviral vector encoding the MLL-ENL translocation could generate AML in vivo.11 Interestingly, purified HSC, CMP, and GMP generated indistinguishable disease upon MLL-ENL transformation, although the disease occurred less frequently when derived from the CMP and GMP populations. These findings suggest that for certain types of mutations it may be possible to generate LSC from progenitor cells rather than the more primitive HSC. From a mechanistic viewpoint, the transformation of HSC versus progenitors may occur through disparate processes. For example, HSC possess the ability to self-renew; therefore, initial mutations in the HSC population are not necessarily related to self-renewal. Rather, alterations in survival or differentiation pathways might occur. In contrast, since progenitors such as CMP and GMP do not have significant self-renewal potential, initial mutations would likely serve to increase their self-renewal activity. Were this not the case then short-lived progenitors would probably not persist long enough to undergo subsequent mutations leading to fully transformed LSC. If the theories above are true, then early stages of stem cell pathogenesis may differ substantially depending on the cell of origin. This implies that LSC arising from HSC versus myeloid progenitors may possess distinct biological properties and thus respond differently to various therapeutic approaches.
The issue of whether LSC originate from HSC or progenitors may also be relevant to current models of leukemogenesis. The specific mutations leading to myeloid leukemia have been under intensive investigation for many years. As discussed in detail in Section II, studies in both primary human and mouse model systems have identified various classes of mutations that form the basis for theories on how certain mutations cooperate with others to mediate leukemogenesis.12 In testing these theories, it has been possible to recapitulate both CML and AML in mouse models; however, the relative effects of various mutations at the stem or progenitor cell level are not well understood. Certainly, mutations leading to aberrant self-renewal are implicated in pathogenesis; however, other changes in processes such as survival, apoptosis, and differentiation have not been clearly defined for the LSC. As studies attempt to translate research findings into clinically meaningful strategies, it will be important to define those processes that are truly unique to LSC and that represent the most attractive targets for therapeutic intervention.
Current Therapies and Future Directions
Our increasing understanding of LSC has allowed a reassessment of our clinical approach to AML and CML. With current therapy, the long-term prognosis for AML is poor. With imatinib therapy survival is projected to vastly increase for CML, yet relapses and resistance remain a significant problem and blastic transformation remains refractory to therapy. For many years, the primary chemotherapy for AML has consisted of induction with cytarabine (Ara-C) + anthracycline, followed by consolidation with Ara-C. While this approach achieves remission for a majority of patients, relapse is common and long-term survival rates remain low. The lack of durable response suggests that these drugs can usually ablate leukemic blast cells but may not effectively target the LSC population. As noted above, LSC are often quiescent, which may make the malignant population refractory to standard chemotherapy, or at least no more susceptible than normal HSC. Indeed, recent experimental evidence indicates that both Ara-C and anthracyclines are less effective for primitive AML cells than leukemic blasts.9,13 Interestingly, both of these drugs, like the vast majority of chemotherapy agents, are known to upregulate NF-κB activity. If NF-κB mediates a survival function, as is commonly seen in many cancers, then the combination of high NF-κB activity and a quiescent cell cycle status may make LSC resistant to standard chemotherapy agents. To develop more effective approaches for leukemia, it may be necessary to inhibit NF-κB (or at least not induce its activity) and approach therapy in a cell cycle–independent fashion. With these criteria in mind, there appear to be at least three general strategies that may more effectively target the LSC population.
Targeting LSC surface molecules
The use of monoclonal antibodies for cancer therapy has become an accepted methodology. A number of cell surface epitopes have been targeted and clearly provide benefit for several different types of cancer. For AML, studies using a CD33 targeted approach have shown promise and may lead the way to other similar strategies.14 While the expression of CD33 on AML stem cells has not been characterized in detail, the partial efficacy of CD33-based approaches suggests the antigen may be found on LSC in some cases. Aside from monoclonal antibodies, studies have also employed cytokine molecules fused to toxins as a means to exploit expression of receptors found on AML cells. Specifically, an IL-3 fusion to diphtheria toxin has been generated and shown to target primary leukemic blasts as well as the LSC population.15 Thus, the concept of identifying and targeting antigens found on the surface of LSC appears to be a very useful direction for future research.
Inducing LSC-specific immunity
While initial treatment of leukemia uses chemotherapy to induce remission, the most effective current means of achieving long-term disease-free survival is allogeneic stem cell transplantation. The ensuing graft-versus-leukemia effect that is mediated by allogeneic donor cells can presumably, in some cases, target leukemia stem cells. Direct evidence for this concept was recently reported in a study using T cell clones specific to minor histocompatibility antigens on LSC.16 These findings indicate that with appropriate graft engineering, it may be possible to more effectively elicit anti-LSC immunity. While this approach does not appear to distinguish LSC from HSC, in the context of stem cell transplant the donor stem cells can generally supply the necessary hematopoietic functions to reconstitute hematopoiesis in the patient.
Targeting LSC-specific molecular pathways
As understanding of the unique molecular properties of the LSC has grown, investigators have also started to consider targeting such pathways as a means to specifically induce LSC death. Interestingly, a variety of rational drug designs may impinge upon the LSC population and provide useful agents for future therapies. For example, several recent studies have tested small molecule inhibitors of the Flt3 transmembrane tyrosine kinase. Activating mutations in Flt3 occur frequently in AML, and signaling via this pathway is a strong growth stimulus.17 While a specific role for Flt3 in stem cell pathogenesis has not yet been defined, downstream targets of Flt3 such as Akt and NF-κB are implicated in LSC survival. Thus, inhibition of Flt3 may sensitize LSC to cell death.
Another approach to leukemia therapy might involve the use of proteasome inhibitor drugs. Agents of this nature are known to inhibit NF-κB and to be highly toxic to LSC in vitro. Importantly, normal HSC are relatively resistant to proteasome inhibition, thus suggesting a substantial therapeutic index can be achieved between LSC and HSC. Furthermore, proteasome inhibition can serve to sensitize LSC to anthracyclines, suggesting that such drugs may be useful as a means to augment current standard chemotherapy.18 While proteasome inhibitors undoubtedly function through multiple pathways, it appears a central activity is to block the survival signals regulated by NF-κB. This observation is consistent with studies in many cancer types, indicating a role for NF-κB in promoting tumor growth and survival. A related strategy for blocking survival signals in LSC would be to inhibit signaling in the PI3 kinase (PI3K) pathway. Recent studies by Xu et al indicate that constitutive activation of this pathway is common in AML and that inhibition of PI3K signaling may target the LSC population.19 Since the PI3K/Akt pathway is known to activate NF-κB, combinations of proteosome inhibitors and PI3 kinase inhibitors would be predicted to strongly inhibit NF-κB function and block LSC growth.
Specific methods for targeting the CML stem cell may also relate to PI3K/Akt and NF-κB signaling pathways, both of which are known to be active in CML cells. However, not all pathways relevant to the survival of leukemic cells are necessary for survival of LSC. This was recently demonstrated in studies of imatinib in which the CML stem cell population was compared to bulk CML cells. While imatinib was highly toxic to more differentiated CML cells, it was only cytostatic to primitive CML cells in vitro.20 These findings indicate that molecular mechanisms known to mediate the growth or survival of the more differentiated leukemic cells of CML must be re-validated at the stem cell level to establish their relative role in LSC. To date, this would apply to agents such as farnesyl transferase inhibitors, Jak/stat inhibitors, HSP90 inhibitors, etc., none of which have yet been characterized with regard to their effects on AML or CML LSC.
Summary
A better understanding of human LSC is likely to provide exciting opportunities to develop improved leukemia therapies. Such approaches can be tailored to the unique biological properties of the LSC, while protecting the critical functions of normal HSC. As outlined above, several different approaches are emerging that show promise as more specific means to target the LSC population. In addition, powerful animal model systems have recently been described that will permit detailed analysis of LSC and the molecular mechanisms that control their growth and survival.21 Such models also offer excellent systems in which novel experimental therapies can be evaluated.
For the future, critical questions require further investigation: What types of stem or progenitor cells are capable of becoming LSC and what mutations can mediate their transformation? What mechanisms control the growth and survival of LSC? How do LSC respond to various therapeutic challenges? Does the origin of LSC affect their response to different therapies? And what type of intervention will induce LSC-specific cell death in vivo while sparing normal HSC? With the experimental tools currently in hand, each of these questions can be addressed and should lead to a variety of new therapeutic options for the treatment of myeloid leukemia.
II. Genetics and Targeted Therapy of Acute Myeloid Leukemias
D. Gary Gilliland, PhD, MD*
Brigham and Women’s Hospital, Howard Hughes Medical Institute, Harvard Medical School, 1 Blackfan Circle, Room 5120, Boston MA 02115
Acute myeloid leukemia is a genetically and phenotypically heterogeneous disease. However, we have made significant progress in identifying mutant genes that are causally implicated in disease pathogenesis. These insights in turn have generated strategies for improving treatment outcome and minimizing toxicity of therapies. The genotypic diversity of AML augments the challenge of developing molecular targeted therapy for each genotypic variant. However, there are common themes emerging in the signal transduction pathways and transcriptional programs associated with malignant transformation. As discussed in Section I, the capacity of leukemic progenitors to self-renew appears to be a shared theme among all leukemias and may provide new insights into therapies that target self-renewal. In addition, a number of chromosomal translocations and point mutations in myeloid leukemias target pathways that confer proliferative and survival advantage to hematopoietic progenitors. Other mutations target hematopoietic transcription factors, and phenotypically result in impaired hematopoietic differentiation. These observations suggest novel approaches to therapy that focus on interrupting the proliferative and/or survival pathways, and/or developing agents that can override the block to hematopoietic differentiation. In this section we will discuss several of these novel approaches that are based on our understanding of the genetics of AML.
Mutational Analysis of AML
Multistep pathogenesis
AML, like other human cancers, is the consequence of more than one mutation. Epidemiologic and genotypic data have shown that many AML cells have more than one recurring mutation, either as point mutations, gene rearrangements and/or chromosomal translocations. Data from animal models of leukemia also strongly support a multistep pathogenesis of disease. For example, expression of PML-RARα [associated with t(15;17) in acute promyelocytic leukemia] in transgenic murine models results in leukemia with a long latency (6 months or longer) and incomplete penetrance (~15%–30%), indicating a requirement for a second mutation. Rare inherited leukemia syndromes provide additional evidence that leukemogenesis requires more than one mutation, such as the familial platelet disorder with propensity to AML syndrome (FPD-AML syndrome). FPD-AML syndrome is an autosomal dominant preleukemic disorder that is caused by loss of function mutations in the hematopoietic transcription factor RUNX1 (AML1). Although these mutations are present in the germline, affected individuals do not develop leukemia until later in life, often with acquisition of karyotypic abnormalities in bone marrow cells. These observations indicate that second mutations are required for development of leukemia in inherited leukemias. Furthermore, as discussed in more detail in Section III, there is evidence from analysis of pediatric leukemias that multiple steps are required. The work of Greaves and colleagues demonstrates that certain leukemia-associated translocations, including t(4;11) associated with the MLL-AF4 fusion or t(12;21) associated with the ETV6-RUNX1 (also known as TEL-AML1), are present in utero during development, but leukemias do not develop until later in life, again indicating a need for a second mutation to develop AML.
Analysis of the spectrum of mutations that have been identified in human acute leukemias has suggested that disease alleles can be divided into two broad complementation groups: those that confer a proliferative and/or survival advantage to hematopoietic progenitors and those that impair hematopoietic differentiation and confer properties of self-renewal to the hematopoietic cell at a particular stage of differentiation.
Proliferation and/or survival mutations in AML
The first “complementation group” of mutant genes in AML confers proliferative and/or survival advantage to hematopoietic progenitors, usually as a consequence of aberrant activation of signal transduction pathways. Examples in myeloid leukemias include activating mutations in RAS family members, in the receptor tyrosine kinases KIT and FLT3 (discussed in more detail below), loss of function of NF-1, and more recently putative gain-of-function mutations in the hematopoietic phosphatase SHP-2. Of note, although these mutations collectively account for as many as 50% of cases of AML, with rare exception, only one of these is mutant in any given patient. This epidemiologic observation suggests that these mutations can be viewed as a complementation group and that any one of these is sufficient to contribute proliferative and survival advantage to a leukemic cell.
FLT3 is the most commonly mutated gene in AML, and is constitutively activated by acquired mutation in approximately 30%–35% of AML. In 20%–25% of cases of AML, there are internal tandem duplications (ITD) in the juxtamembrane domain of FLT3 ranging in size from several to > 50 amino acids. In each case, the consequence of the ITD is constitutive activation of FLT3 tyrosine kinase activity. These mutations are always in frame, and the diversity of mutations among patients has suggested that they may be loss-of-function mutations in an autoinhibitory domain. Recent structural data supports this hypothesis. The crystal structure of the FLT3 juxtamembrane and catalytic domains shows that a 7–amino acid extension of the juxtamembrane domain is intercalated into the activation loop and would be predicted to inhibit kinase activation. ITD mutations in AML occur exclusively within this 27–amino acid stretch, and would be predicted to result in loss of structure and subsequent activation of the tyrosine kinase. In addition, mutations also occur in the activation loop of FLT3 in about 5%–10% of AML and result in constitutive kinase activation. These mutations in the context of other tyrosine kinases result in folding out of the activation loop, providing access of the catalytic site to ATP and substrate. There are rare examples of both ITD and activation loop mutations in the same allele of FLT3, suggesting that the combination of mutations may hyperactivate the kinase and provide added proliferative advantage to cells that harbor both mutations.
Most studies indicate that pediatric and adult AML patients with FLT3 mutations have a poor prognosis. FLT3 has not been reported as a poor prognostic indicator in adults with AML over the age of 65, which may reflect the overall worse prognosis of this group compared to younger individuals (reviewed in 1).
FLT3-ITD confers IL-3 independent growth to the murine hematopoietic cell line Ba/F3, which is normally dependent on IL-3 for growth and survival. Conversion of these cells to factor-independent growth is a surrogate for transformation of hematopoietic cells. FLT3-ITDs activate several signal transduction pathways in Ba/F3 cells that are known to confer proliferative and/or survival advantage, including the RAS/MAPK, STAT, and PI3K/AKT pathways.1,2 Furthermore, FLT3-ITD induces a myeloproliferative disease (MPD) in primary hematopoietic progenitors in a murine bone marrow transplantation (BMT) assay with a median latency of about 45 days. These animals do not develop AML, but rather a leukocytosis with normal maturation and differentiation of myeloid lineage cells, and splenomegaly due to extramedullary hematopoiesis.3 These data indicate that FLT3-ITDs alone are not sufficient to induce an AML phenotype in primary murine hematopoietic progenitors. In addition, the FLT3-ITD–induced MPD is not transplantable into secondary recipient mice. These data suggest either that FLT3-ITD mutations do not confer certain properties of self-renewal (such as serial transplantability) or that in the BMT assay, retroviral transduction of FLT3-ITD does not target a population of cells that have inherent self-renewal capacity. However, retroviral transduction with other leukemia-associated oncogenes such as MLL-AF4 (see below) can confer properties of self-renewal to committed progenitors in the BMT assay.
The FLT3-ITD phenotype is similar to that reported in the murine BMT assay for other constitutively activated tyrosine kinases associated with myeloproliferative phenotypes in humans, including BCR-ABL, TEL-PDGFβR, TEL-ABL or TEL-JAK2.4– 7 These diseases are not transplantable into secondary recipient mice using the stringent criteria of disease induced by transplantation of 10,000 cells into sublethally irradiated recipient mice. Taken together these data indicated that constitutive activation of tyrosine kinases is sufficient to induce a myeloproliferative phenotype, but not AML. Hence, it seems likely that in order for activated tyrosine kinases to contribute to the development of frank leukemia they must work in concert with other mutations that confer self-renewal to the blood cell.
Mutations associated with AML that affect hematopoietic differentiation
A second broad complementation group in leukemias primarily comprises mutations in transcription factors or transcriptional co-activators that are important for normal hematopoietic development. We will review several of the more common groups of mutations.
Core Binding Factor in Acute Leukemias
Chromosomal translocations associated with AML frequently target transcription factors or transcriptional co-activators that are important for normal hematopoietic development. Multiple translocations target the core binding factor (CBF) in acute leukemias (reviewed in 7). Of these, the most extensively studied are the RUNX1-ETO, CBFβ-SMMHC and TEL-RUNX1 fusions. CBF is a heterodimeric transcription factor comprised of the RUNX1 (also known as AML1) and CBFβ subunits. Homozygous loss of function of either RUNX1 or CBFβ in genetically engineered mice results in a complete lack of definitive hematopoiesis, indicating that both components of CBF are essential for hematopoietic development.
Based on the critical role of CBF in hematopoietic development, it might be anticipated that an acquired gene rearrangement or mutation that results in CBF loss of function would impair hematopoietic differentiation. Indeed, several lines of evidence indicate that the leukemia-associated fusion proteins are dominant negative versions of CBF that inhibit rather that activate CBF target genes by aberrant recruitment of co-repressor complexes including histone deacetylases. Genetic experiments bear out this notion. For example, use of homologous recombination strategies to express RUNX1-ETO or CBFβ-SMMHC from their endogenous promoters in mice results in a phenotype nearly identical to that of the RUNX1 or CBFβ knock-outs, namely a loss of definitive hematopoiesis (reviewed in 7). Although these data clearly indicate a requirement for RUNX1 for hematopoietic development during embryogenesis, recent data indicate a less stringent requirement for RUNX1 in adult hematopoiesis. Using a conditional Runx1allele, Hirai and colleagues recently demonstrated that deletion of Runx1 in the adult hematopoietic compartment yields relatively modest defects when compared with the complete lack of definitive hematopoiesis when Runx1 is absent during development. In the conditional experiments defects in B and T cell development, as well as thrombocytopenia, were noted but had surprisingly few effects on myeloid maturation. These observations add further complexity to the role of RUNX1 loss of function in leukemogenesis in adults.
Loss of CBF function also plays an important role in the pathogenesis of leukemias in pedigrees with inherited predisposition to develop leukemia. For example, the familial platelet disorder associated with propensity to develop AML (FPD/AML syndrome) is an autosomal dominant disorder that is caused by haploinsufficiency of the RUNX1 gene.8,9 Although patients carry germline mutations in RUNX1, they do not develop leukemia until later in life, often with acquired cytogenetic abnormalities in bone marrow cells indicative of acquisition of second mutations. Furthermore, in sporadic AML,9,10 there are loss of function point mutations of RUNX1 in about 3%–5% of cases, most of which impair RUNX1 DNA binding activity. In many of these AML patients there is loss of function of both alleles of RUNX1, suggesting that homozygous loss may contribute to disease progression or severity of disease.
Mutations and gene rearrangements affecting CBF function are important in pathogenesis of AML, but they are not sufficient to cause AML. For example, conditional alleles of RUNX1-ETO expressed in adult hematopoietic progenitors fail to induce AML unless the animals are treated with chemical mutagens such as ethyl-nitrosourea (ENU) to induce AML11 or are infected with replication-competent retroviruses that insert into various sites of the genome, generating second mutations. However, RUNX1-ETO expression does confer an “immortalization” phenotype in that RUNX1-ETO expressing progenitors can be propagated in serial transfer assays in vitro.11 It is not clear whether RUNX1-ETO expression actively induces a transcriptional program that confers the immortalization phenotype, or whether the phenotype simply reflects a block in differentiation at the level of a hematopoietic stem cell that has self-renewal capacity.
Mutations involving retinoic acid receptor-alpha:
Acute promyelocytic leukemia (APL) is always associated with chromosomal translocations involving the retinoic acid receptor alpha (RARα) locus on chromosome 17 and one of five different partner genes. Each of these is associated with APL characterized by a block in differentiation at the promyelocyte stage of hematopoietic development. The most extensively studied, and the most common fusion gene in APL, is PML-RARα associated with t(15;17). PML-RARα is a dominant negative form of RAR that inhibits rather than stimulates expression of retinoic acid target genes due to recruitment of the co-repressor complex, in a manner similar to the RUNX1-ETO, CBFβ-MYH11 and ETV6-RUNX1 fusions. Expression of PML-RARα is associated with inhibition of differentiation and increased cell self-renewal. All-trans-retinoic acid (ATRA), a ligand for RARα, is effective therapy for APL, especially when given in combination with conventional induction chemotherapy. The efficacy of ATRA in treatment of APL is related to the ability of ATRA to bind to the fusion protein, with resultant dissociation of the co-repressor complexes, engagement of co-activation complexes by the chimerical receptor and subsequent degradation of the fusion protein.12 Promyelocytes are then able to undergo a normal hematopoietic differentiation program that ultimately results in apoptotic cell death. The ability of ATRA to reverse transcriptional repression by PML-RARα by release of co-repressors suggested that inhibitors of histone deacetylase, a key component of the co-repressor complexes, might have therapeutic efficacy not only in APL but in other leukemias characterized by aberrant recruitment of the nuclear co-repressor complex, such as RUNX1-ETO and CBFβ-MYH11.13
Like RUNX1-ETO, expression of PML-RARα and/or its reciprocal RARα-PML is not sufficient to induce AML. Transgenic murine models of PML-RARα–induced AML were developed in which expression of PML-RARα has been directed to the promyelocyte compartment using promyelocyte specific promoters, including the Cathepsin G promoter14,15 and the MRP8 promoter.16 However, although the fusion gene is present in the germline and expressed during embryonic and adult development, these animals do not develop AML until 3–6 months after birth, with a modest penetrance of only 15%–30%, and often with acquisition of secondary cytogenetic abnormalities.14,15 Although co-expression of the reciprocal RARα-PML and PML-RARα under the control of the Cathepsin G promoter in double transgenic mice increases penetrance to about 60%, double transgenic mice do not have shortened latency of disease. These data indicate that second mutations are necessary in pathogenesis of APL in this murine model system.
Additional data that support a need for more than one mutation in pathogenesis of APL are derived from genotypic analysis of humans with APL. At least 30% of APL patients harbor activating mutations in FLT3-ITD mutations in addition to the t(15;17) that gives rise to the PML-RARα fusion (reviewed in 17). These mutations are not observed in normal individuals; thus, their concordance in this context indicates that in at least a subset of patients both mutations are required for pathogenesis of APL.
Other transcription factors and transcriptional co-activators (not discussed in more detail here due to space constraints) that have been identified in AML associated with chromosomal translocations include the HOX family of transcription factors that is involved in more than a dozen different chromosomal translocations, the MLL gene as described in Section III, that is involved in more than 40 different translocations, and the CBP, p300, MOX and TIF2 genes.
In addition, emerging evidence indicates that, as for RUNX1, loss of function point mutations in hematopoietic transcription factors such as GATA-1, C/EBPα, and PU.1 may play an important role in leukemogenesis.
Cooperativity in AML
Genotypic analysis of known leukemia oncogenes thus indicates that there are at least two broad complementation groups of mutations. One class of mutations, exemplified by FLT3-ITD or oncogenic RAS mutations, confers a proliferative and/or survival advantage to hematopoietic progenitors but has minimal effects on differentiation programs in hematopoietic progenitors. Mutations involving a component of the signal transduction pathway are each relatively frequent in AML, but only very rarely is more than one such mutation observed together in the same patient. In contrast, mutations resulting in loss of function of hematopoietic transcription factors result in a block in differentiation at a specific stage in hematopoietic development as exemplified by the PML-RARα fusion that is associated with a block in differentiation at the promyelocyte stage. Again, although CBF mutations and PML-RARα mutations occur in a significant proportion of AML patients, they are never observed together in the same patient, suggesting that they also make up a complementation group. These mutations may also confer an immortalization phenotype but are not sufficient to cause AML.18– 21 Based on these observations, and genotypic data, a model emerges for pathogenesis of AML in which there are at least two broad classes or complementation groups of mutations. When mutations that confer proliferative and/or survival advantage are expressed alone, they result in an MPD with leukocytosis and normal differentiation. When mutations such as RUNX1-ETO are expressed alone, they impair differentiation and confer an immortalization phenotype, reminiscent of the behavior of hematopoietic progenitors in myelodysplastic syndrome. In this model, co-expression of a mutant that confers a proliferative and/or survival advantage, such as a FLT3-ITD, and a mutation that impairs hematopoietic differentiation, such as PML-RARα, would result in AML.
To test this hypothesis, bone marrow from transgenic C3H/C57BL6 mice expressing the PML-RARα fusion under the control of the Cathepsin G promoter was harvested and transduced with retrovirus containing a FLT3-ITD mutant.14,15,22 In control experiments, PML-RARα transgenic bone marrow transduced with an empty vector control resulted in an APL-like disease in secondary recipient mice, with a latency of approximately 6 months and a penetrance of about 15%–30%, in consonance with previous reports.22 In an additional control experiment, FLT3-ITD retrovirus transduced into the C3H/C57BL6 wildtype background resulted in T cell lymphomas with a latency of 3–6 months.
PML-RARα bone marrow transduced with FLT3-ITD resulted in a shortened latency with 100% penetrance of disease. This murine model not only provides experimental evidence for cooperation between these two types of mutations but also provides a system in which FLT3 inhibitors can be tested alone and in combination with ATRA in treatment of APL. Indeed, initial data in a similar system using MRP8–PML-RARα transgenic mice and FLT3-ITD indicates that small molecule inhibitors of FLT3 have at least additive effects with ATRA in treating APL in this model system.23 Additional experimentation will be required to assess the transforming properties of oncogenic RAS expressed from its endogenous reporter and co-expression of oncogenic RAS with potential cooperating mutations.
Inhibition of FLT3 as a Strategy for Improving Outcome in AML
FLT3 mutations are an independent poor prognostic indicator in AML patients under the age of 65 in most studies (reviewed in 17) and are thus an attractive target for therapeutic intervention. This approach of targeting constitutively activated kinases with selective small molecule inhibitors has been validated by Druker, Sawyers, Kantarjian and colleagues in demonstrating efficacy of the ABL kinase inhibitor imatinib (Gleevec) in BCR-ABL–positive CML.24,25
Similar strategies were used to identify selective inhibitors of FLT3. Our group has developed cell-based screens for specific inhibitors of FLT3. Using this approach, we identified several FLT3 selective inhibitors with properties suitable for use in human clinical trials. These include PKC412 (in collaboration with Novartis Pharma AG)26 and MLN518 (CT53518; in collaboration with Millennium).27 Other agents with similar activity include SU11248, SU5614 and SU541628–,30 from SuGen,31,32 and CEP-701 developed by Small and colleagues in collaboration with Cephalon.29,30,33 Each of these inhibitors is selective rather than specific. For example, MLN518 is also a potent inhibitor of KIT and PDGFR; CEP-701 also inhibits TRKA; PKC412 inhibits KIT, PDGFR and protein kinase C; SU11248 also inhibits KIT and PDGFR.
Preclinical activity of FLT3 inhibitors
Each of the inhibitors listed above induces apoptosis in cell lines harboring activating mutations in FLT3 Ba/F3 cells transformed with FLT3-ITD, and this effect can be rescued by addition of IL-3. Several of these inhibitors also induce apoptotic cell death in human AML cell lines containing the FLT3-ITD mutation, and in some cases even in AML cell lines overexpressing wild type FLT3.26,29,33 It is not clear why in some studies inhibitors appear to be generally more effective for cells expressing mutant receptors. These observations indicate that it may be appropriate to test FLT3 inhibitors in AML patients with overexpression of wild type FLT3 as well as mutant FLT3.
Murine models were also developed to test FLT3 inhibitors in preclinical analysis. These include injection of FLT3-ITD–transformed Ba/F3 cells into syngeneic recipient mice27,30 and murine bone marrow transplant models of FLT3-ITD–induced disease.26,27 In each of the model systems animals treated with FLT3 inhibitors demonstrate statistically significantly prolonged survival, indicating that these agents are effective in vivo and have appropriate pharmacokinetic properties for inhibition of FLT3 in vivo. Based on these data, several Phase I/II trials of FLT3 inhibitors in the treatment of AML were begun. Most trials have focused on treatment of relapsed AML patients who have a mutant FLT3. Some FLT3 inhibitors such as PKC412 and CEP-701 that were already tested in Phase I trials for other disease indications are currently in Phase II trials for AML. Other agents, such as MLN518, are currently in Phase I trials. Although it is still early in the evaluation process, recent reports suggest that several of these agents have reasonable safety profiles and have activity in this clinical context. Extensive additional testing will be necessary to determine whether these agents will have a place in the armamentarium used to treat AML; whether they can be used in combination or sequentially with available therapies for AML; and whether they will be equally efficacious in cases harboring mutant FLT3 or overexpressing wild-type FLT3.
Future Directions
Promising initial data indicate that mutant tyrosine kinases can be targeted for therapeutic intervention by small molecule inhibitors. It may therefore be useful to screen for additional mutations in tyrosine kinases in AML. Genome-wide approaches that are being employed include genome-wide high-throughput DNA sequence analysis of the tyrosine “kinome” (i.e., the complete coding sequence of all ~90 known tyrosine kinases) as well as screens for activation of tyrosine kinases by formation of fusion kinase genes due to small genomic deletions as in the case of the hypereosinophilic syndrome. Small molecules that inhibit farnesyl transferase (FTIs) and thereby inhibit Ras and other farnesylated proteins are being actively investigated, and appear to have activity in AML. Clinical responses are not directly correlated with mutational status of Ras and appear not to be well correlated with inhibition of farnesyltransferase itself. Identification of the targets of FTI may be a fruitful line of inquiry for developing other therapies. In addition, small molecules with potential to override the block in hematopoietic differentiation, such as histone deacetylase inhibitors, have shown promising activity in clinical trials. Some of these also appear to have activity in enhancing degradation of FLT3 and BCR-ABL, and thus may offer overlapping and non-crossresistant activities as therapeutic agents in AML.
Summary
Novel therapeutic approaches to AML can be envisioned based on a comprehensive effort to identify disease alleles that are causally implicated in disease pathogenesis. These include strategies that target the block in differentiation, with ATRA treatment in APL as the paradigm for this approach. In addition, inhibitors of proliferative and/survival mutations such as FLT3 may also prove therapeutically useful. Future directions include identification of additional mutant forms of proliferative and survival promoting proteins that may be targets for small molecule inhibitors using genome-wide mutational analysis in the tyrosine kinome, and screens for compounds that override the block in differentiation. Eventually, it may be possible to use combinations of molecularly targeted therapies such as FLT3 inhibitors plus ATRA in selected clinical contexts to improve outcome and reduce toxicity.
It should also be noted that, to the extent that these therapies are successful, we can anticipate the development of resistance to single agents such as FLT3 inhibitors and ATRA. ATRA resistance develops in the majority of APL patients, which is one reason that current therapy always includes the combination of ATRA with conventional induction chemotherapy. Imatinib resistance is well described, particularly in CML blast crisis patients, and we should anticipate this problem and begin to develop strategies to circumvent or prevent resistance to FLT3 inhibitors. It may be possible to address the problem of resistance to small molecule kinase inhibitors using alternative inhibitors with different chemical structures.
III. Recent Advances in Genetics and Therapy of Infant Leukemias
Carolyn A. Felix, MD*
Associate Professor of Pediatrics, University of Pennsylvania School of Medicine; Attending Physician, The Children’s Hospital of Philadelphia; Division of Oncology, Abramson Research Center, Room 902B, 3615 Civic Center Boulevard, Philadelphia PA 19104-4318 C.A.F. is supported by NIH Grants CA77683, CA85469, CA80175, Leukemia and Lymphoma Society Translational Research Award, Leukemia and Lymphoma Society SCOR Grant, Joshua Kahan Foundation and Friends of Joseph Claffey Fund.
Unique Leukemias in the Pediatric Population
Among the de novo forms of leukemia generally restricted to the pediatric age group, leukemias with translocations of the MLL gene at chromosome band 11q23 occur primarily in infants and young children and comprise the majority of leukemias in the infant population. The distinct biology, age at presentation and poor outcome of MLL-rearranged leukemias place these diseases at one extreme of the spectrum of pediatric leukemias. This section will review the pathobiology, treatment and epidemiology of this unique subset of leukemia, for which new therapies and preventive approaches clearly are needed.
The most common childhood leukemia and the most common childhood cancer in developed countries is CD10+ B lineage or common acute lymphocytic leukemia (ALL), which is characterized by the t(12;21) translocation in 25% of cases, making this the most common chromosomal abnormality in pediatric cancer (reviewed in 1). The t(12;21) results in fusion of the TEL gene to CBFA2 (AML1) and is believed to cause leukemia by recruitment of complexes containing histone deacetylases to AML1 target genes, which is associated with transcriptional repression (reviewed in 1). The TEL-AML1 translocation has a prenatal, non-constitutional origin. Although the peak incidence of TEL-AML1 leukemia occurs at 2 to 5 years of age, new data indicate that the postnatal latency is variable and can be protracted.2 Secondary alterations, most often loss of the normal TEL allele, are needed for leukemia to emerge (reviewed in 1). The TEL-AML1 translocation is generally associated with a favorable prognosis (reviewed in 1).
Recent advances have occurred in characterizing leukemias associated with constitutional disorders. For example, the acute megakaryoblastic leukemia (AMKL) affecting patients with Down syndrome is associated with mutations in the GATA1 gene that result in the formation of a truncated GATA1 protein with altered transcriptional function. The discovery of GATA1 mutations in the transient MPD of infants with Down syndrome, a condition associated with subsequent AMKL in 30% of cases, indicated that the GATA1 mutation is an early event in leukemogenesis in these patients.3 It was also recently observed that expression of the cystathione-β-synthase (CBS) gene at chromosome band 21q22.3 at levels higher than predicted by gene dosage from the extra chromosme 21 is associated with increased Ara-C sensitivity in Down syndrome myeloblasts.4 The increased CBS expression appears to be related to transcriptional regulation of the CBS promoter.4 The Children’s Cancer Group (CCG) reported on the largest uniformly treated cohort of pediatric patients with Down syndrome and AML or myelodysplastic syndrome (MDS) (n = 161), in which AMKL comprised 70% of cases.5 Due to excessive toxicities in patients with Down syndrome who had been randomized to receive intensive timed induction or HSC transplantation, which became apparent at an interim analysis, 161 subsequent uniformly treated patients all were non-randomly assigned to standard timed induction and excluded from transplantation.5 Age at diagnosis was the most important prognostic factor in the uniformly treated cohort, with an event-free survival (EFS) rate of 86% in patients < 2 years old at diagnosis.5
Noonan syndrome, a constitutional disorder with facial abnormalities, short stature, heart defects, and juvenile myelomonocytic leukemia (JMML), is associated with germline mutations in the PTPN11 (SHP2) gene.6 This led to the new finding that somatic PTPN11 mutations can result in JMML, MDS and AML in pediatric patients without Noonan syndrome, implying a role for PTPN11 mutations in disease pathogenesis.6PTPN11 mutations lead to increased activity of the SHP2 phosphatase, which in turn can activate the RAS/MAPK cascade.6 Germline NF1 mutations and somatic RAS mutations are known to activate the same pathway in other cases of JMML.
Progress also has been made in identifying the OTT and MAL genes involved in the t(1;22)(p13;q13) translocation, which is specifically associated with another subtype of AMKL peculiar to the infant population.7
Clinical Spectrum of Acute Leukemia in Infants
Leukemia is the second most common malignancy in the first year of life. The annual incidence in the US is 37 per million infants, and there are ~126 new cases of infant ALL and ~67 new cases of infant AML per year (reviewed in 8). Infant ALL often presents with features associated with poor outcome including young age, high WBC count, bulky extramedullary and central nervous system (CNS) disease, a CD10-negative, early pre-B immunophenotype, and poor early response to treatment. Infant ALL has the worst prognosis of all of the pediatric leukemias, with EFS rates of ~20%–40%. An ultra high risk subgroup within infant ALL with age < 3 months at diagnosis, WBC count > 100,000/μL or the t(4;11) translocation, has an EFS of only ~5%. Most relapses (~70%) occur within 1 year from diagnosis. There also are significant regimen-related toxic deaths. AML in young children up to 4 years old is associated with a high incidence of MLL translocations and FAB M4 and FAB M5 morphologies. Because of the poor outcome for most pediatric patients with AML, the infant age itself is not an independent prognostic factor.
Molecular Pathobiology of Infant Leukemias with MLL Translocations
MLL translocations disrupting an 8.3 kb breakpoint cluster region (bcr) between exons 5 and 11 are present in ~80% of cases of infant ALL and ~80% of FAB M4/M5 leukemias in infants and young children and comprise the hallmark aberrations of most infant leukemias (reviewed in 8). The ~100 kb MLL gene at chromosome band 11q23 contains 36 exons and encodes a 430 kDa, 3969 amino acid protein with regional amino acid similarity to the Drosophila trithorax (trx) protein in the N-terminal DNA binding AT-hook motifs, the central zinc fingers comprising a plant homeodomain (PHD) and the C-terminal SET domain.9Drosophila trx group (trxG) and Polycomb-group (PcG) proteins maintain expression or repression, respectively, of homeotic gene complexes during embryogenesis; trx maintains but does not initiate expression of HOX genes (reviewed in 10). The mammalian homologues of these proteins, MLL and BMI-1, are antagonistic regulators of HOX gene expression. MLL maintains HOX gene expression early during mammalian skeletal, craniofacial, neural and hematopoietic development (reviewed in 10). MLL functions in a large supercomplex of at least 29 proteins that is involved in the remodeling, acetylation, deacetylation, and methylation of nucleosomes and histones (reviewed in 10). Its SET domain has specific histone H3 lysine-4-specific methyltransferase activity that regulates HOX promoters (reviewed in 10). MLL is cleaved by Taspase1 into an N terminal fragment with transcriptional repression properties and a C terminal fragment with transcriptional activation properties that associate in an intramolecular complex. The association of the MLL N and C terminal fragments is important for proper nuclear sublocalization of MLL and proteolytic cleavage is essential for proper expression of HOX target genes (reviewed in 10).
MLL translocations involve many partner genes that encode diverse partner proteins.9 More than 40 partner genes have been identified through the molecular cloning of MLL genomic breakpoint junction sequences or chimeric transcripts (reviewed in 10). Among these fusion partners is MLL itself; MLL self-fusions result in partial duplications of several exons. Many MLL partner proteins have structural motifs of nuclear transcription factors, proteins involved in transcriptional regulation or other nuclear proteins. Other MLL partner proteins are found in the cytoplasm, at the cell membrane, endoplasmic reticulum and Golgi apparatus, or the ribosome. Only a subset of the partner genes appear to be involved in ALL. AF-4 at chromosome band 4q21 is the most common partner gene of MLL in infant ALL. ENL at chromosome band 19p13 is another common partner gene in ALL. Both encode transcription factors (reviewed in 8). MLL translocations with AF-4 and ENL account for 70%11 and 13%,11 respectively, of MLL translocations in infant ALL. However, in AML the partner genes are more diverse.
MLL translocations are believed to cause leukemia by creating fusion proteins from the der(11) transcripts consisting of the amino terminus of MLL and the carboxy terminus of the partner protein (reviewed in 9). Various murine models, most of which are retroviral transplantation models, have established that the der(11) protein products are leukemogenic (reviewed in 10), but the role of the partner proteins in leukemogenesis is uncertain. Intriguingly, some of the partner genes are members of the same gene families. For example, the partner genes LAF-4, AF4, AF5q31 are members of the LAF-4 family.12 The partner genes hCDCrel, MSF (AF-17q25) and SEPTIN6 are SEPTIN family members (reviewed in 10). Whether the partner proteins alter the function of MLL in a similar fashion is not yet known, but several MLL fusion partners have transcriptional activation function (reviewed in 10). In experiments to understand aberrant gene activation in leukemias with MLL translocations, it recently was observed that the Hoxa9 and Meis1 genes are key targets in MLL-ENL–mediated immortalization (reviewed in 10).
Murine models of MLL-associated leukemias have shown a latency period before overt disease develops, suggesting that additional genetic changes are required to generate leukemia.12 Gene expression profiling showed that FLT3 overexpression was common in MLL(+) leukemias with a lymphoid phenotype, leading to characterization of FLT3 mutations as important secondary alterations.13
Outcome and Treatment Options for Leukemia in Infants
MLL-rearranged infant leukemias are generally resistant to cytotoxic chemotherapeutic agents.11 Survival rates are poor following intensive chemotherapy with or without HSC transplantation. The t(4;11) is generally associated with poor outcome in infant ALL and is a special prognostic factor. In CCG trials there were only rare survivors of infant ALL with t(4;11); however, an EFS of ~50% was found with other MLL translocations or normal karyotypes (reviewed in 8). In BFM ALL trials the outcome was also worse for the t(4;11) subgroup (reviewed in 8). In studies conducted by St. Jude’s and POG and in a review of 212 cases of infant ALL, any MLL translocation conferred poorer EFS.11 The adverse effect of MLL translocations is age-dependent with poorer outcome in infants than in children (reviewed in 8,11). The poor clinical response to treatment in infant ALL is mirrored by in vitro drug resistance to glucocorticoids and L-asparaginase used routinely in its treatment.14 In contrast, infant ALL cells exhibit high in vitro sensitivity to Ara-C.14 These patterns of sensitivity and resistance have implications for the design of clinical trials for infant ALL.
In a POG study of 478 children on the impact of chromosomal abnormalities in AML on prognosis, all MLL translocations were associated with an inferior outcome compared to other abnormalities or a normal karyotype. The 4-year EFS was only ~24% for cases with MLL translocations compared to 34% for the entire group, and there was no difference in outcome among cases with the various types of MLL translocations.15 In contrast, earlier studies suggested a worse prognosis for infant AML when certain MLL translocations occurred in congenital cases, and a more favorable outcome for cases with t(9;11) (reviewed in 8). It is also noteworthy that cases of AML harboring t(9;11) are especially sensitive in vitro to agents such as Ara-C, etoposide, and anthracyclines used in AML treatment regimens compared to AML cases with other chromosomal abnormalities.16 Additional studies are warranted to investigate the impact on outcome of sensitivity to specific agents in subsets of AML with MLL translocations and incorporate this information into future clinical trials.
Recent molecular profiling studies support possibilities for optimized and expanded treatment options for the infant population with MLL-rearranged leukemias. For example, gene expression profiling revealed that the molecular basis for the sensitivity of infant ALL to Ara-C involves differential expression of mRNAs encoding Ara-C metabolizing enzymes.17 Specifically, cases of MLL-rearranged infant ALL express significantly less dCK mRNA and significantly more hENT1 mRNA compared to cases of ALL without MLL translocations in infants and children.17 High-dose Ara-C has been incorporated into various current clinical trials for infant ALL.11 Preclinical data have shown sensitivity of MLL-rearranged leukemias with FLT3 mutations or FLT3 overexpression to tyrosine kinase inhibitors, suggesting that FLT3 may also prove an important therapeutic target suggested by gene expression profiling.13
In addition, leukemia-specific fusion proteins from MLL translocations offer “vistas” to design leukemia-specific agents (reviewed in 10). Chromosomal translocations creating leukemia-specific fusion proteins via breakage within introns and the generation of unique in-frame fusion transcripts may afford new opportunities for targeting the mRNA with antisense, ribozymes or RNA interference, or other custom therapeutic agents (reviewed in 10). Several investigators showed that experimental downregulation of translation of the MLL fusion proteins MLL-AF-9, MLL-ENL, MLL-ELL and MLL-CBP with antisense oligodeoxynucleotides is feasible (reviewed in 10).
Natural History and Epidemiology of Infant Leukemias with MLL Translocations
The gravity of this problem, for which current treatments offer little hope, mandates the development of not only novel treatments, but also strategies for prevention. Therefore, the etiologic agent(s) and the timing and the nature of the DNA damage leading to MLL translocations in leukemia in infants have been topics of investigation. The finding of identical MLL rearrangements in the leukemias from pairs of monozygous twins where both twins were affected, but not in their constitutional DNA, established that MLL translocations in infant leukemias are non-hereditary, non-constitutional, in utero events and, further, suggested that there was metastasis of cells with the translocation from one twin to the other via the placenta (reviewed in 8). The retrospective finding of leukemia-associated MLL genomic breakpoint junction sequences by PCR analysis of genomic DNAs contained in bloodspots on neonatal Guthrie cards of infants who were diagnosed later with leukemia showed that MLL translocations also occur in utero in the non-twin cases (reviewed in 8). Molecular cloning and analysis of MLL genomic breakpoint junctions sequences in infant leukemias suggested staggered and/or multiple sites of breakage as elements of damage and DNA repair by nonhomologous end-joining.18 This led to the conclusion that DNA damage and repair underlie the formation of the translocations.
Evidence of damage in the genomic breakpoint junction sequences and the non-constitutional, in utero origin of the translocations indicate that the damage to MLL and partner genes occurs in utero. Because MLL translocations are much less frequent in de novo leukemias of older patients but frequent in leukemias following chemotherapeutic DNA topoisomerase II poisons, e.g., etoposide, it has been proposed that leukemia in infants may have an etiology resembling treatment-related cases (reviewed in 8). The chemotherapy-leukemia association in the treatment-related cases suggests that chromosomal breakage resulting from DNA topoisomerase II cleavage and its attempted repair may play a role in the formation of these translocations.
Agents interact with DNA topoisomerase II via either or both of two broad mechanisms:
(1) Many agents convert DNA topoisomerase II into a cellular “poison” by stabilization of the cleavage complex (i.e., the covalent complex created when each of the two enzyme subunits forms a covalent bond with the 5′-phosphate terminus of the DNA strand created in the scission). This can occur by increasing the forward rate of cleavage or decreasing the reverse rate of religation, each of which has the overall effect of increasing cleavage.19 This is the mechanism of action of epipodophyllotoxins and other chemotherapeutic drugs that have been associated with leukemia as a treatment complication; all function as “poisons” of the enzyme. “Poison” effects on the DNA topoisomerase II cleavage-religation equilibrium have been demonstrated for several naturally occurring compounds. Some (e.g., quercitin, genistein, genistin) but not all bioflavonoids, as well as catechins and quinolones, have been shown to increase DNA topoisomerase II cleavage in in vitro assays.20 Various bioflavonoids, catechins and quinolones specifically increase the forward rate of cleavage.20,21 Several of these agents induce the formation of DNA topoisomerase II covalent complexes in hematopoietic cells.22 The formation of DNA adducts or abasic sites from oxidative damage within the 4-base 5′ overhang also can have the effect of a position-specific DNA topoisomerase II poison and can be associated with cleavage stimulation.23
(2) The second broad mechanism of action of compounds targeting DNA topoisomerase II involves true catalytic inhibition of enzymatic function and activity.24 Some chemotherapeutic and naturally occurring compounds interact with DNA topoisomerase II by this second mechanism or have mixed effects (Table 1 ). This is the mechanism of action of several other agents contained in dietary items that interact with DNA topoisomerase II (e.g., caffeine, ellagic acid, daidzein).25– 27
Ross et al conducted an epidemiologic case-control study in the CCG by administration of a food frequency questionnaire via telephone in order to determine whether maternal exposure to dietary items containing compounds that interact with DNA topoisomerase II was associated with an increased risk of leukemia in infants.28 Ten of the 26 foods (beans, fresh vegetables, canned vegetables, fruit, soy, regular coffee, black tea, green tea, cocoa, wine) contained compounds that interact with DNA topoisomerase II (Table 1 ). When a combined exposure variable for these ten foods was created to define low, medium and high exposure, a significant positive association within the AML stratum was observed.28 However, foods containing compounds acting as DNA topoisomerase II poisons, compounds with effects of catalytic inhibition and compounds with mixed effects were not examined separately.28 An association between maternal alcohol consumption during pregnancy and infant AML was also suggested; red wine is a source of the DNA topoisomerase II poison quercetin.29 When items such as onions and apples were repeatedly consumed, bioavailability analyses revealed a long elimination half-life and quercetin accumulation in the circulation.30 Maternal plasma, cord plasma and amniotic fluid measurements in a Japanese population during birth demonstrated maternal-fetal transfer of soy isoflavonoids, which interact with DNA topoisomerase II.31 In Sprague Dawley rats, placental transfer of the soy isoflavone genistein was shown following dietary and gavage administration of doses relevant to human consumption of soy products.32 These observations indicate that maternal-fetal exposure to naturally occurring compounds that interact with DNA topoisomerase II occurs and support the epidemiologic association of maternal diet with infant AML.
Molecular epidemiology also supports the role of DNA topoisomerase II in infant leukemia. It has been demonstrated in a British population and replicated in an independent US population that an inactivating C609T polymorphism in the enzyme NAD(P)H: quinone oxidoreductase 1 (NQO1), which detoxifies the benzene metabolite 1,4-benzoquinone and other simple quinones, confers susceptibility to infant ALL and AML with MLL translocations, particularly cases with t(4;11) (reviewed in 33). Of relevance to this finding, it was recently established that benzoquinone interacts with DNA topoisomerase II in the manner of a “poison” and induces the formation of DNA topoisomerase II covalent complexes in hematopoietic cells.34 There are numerous potential dietary and environmental sources of benzoquinone and related compounds (reviewed in 33) that were not included in the original food-frequency questionnaire. This could explain the predominant association of the food items containing DNA topoisomerse II-interacting compounds in the classical epidemiologic study conducted by Ross et al with infant AML.28
Marijuana use, pesticide exposure during pregnancy and exposure to the anti-inflammatory drug dipyrone (reviewed in 29) have been associated with the development of leukemia in the young and, in the case of pesticides and dipyrone, MLL-rearranged leukemia in particular.35 Whether these agents affect DNA topoisomerase II cleavage is unknown. High birthweight and a history of maternal fetal loss are associated with an increased risk of leukemia in infants (reviewed in 29). These data further indicate that prenatal events and exposures are important for leukemogenesis in infants. The C677T polymorphism in the gene encoding methylenetetrahydrofolate reductase (MTHFR) has been suggested to have a protective effect against leukemias with MLL translocations.36
DNA Damage-Repair Model of MLL Translocations in Leukemia in Infants
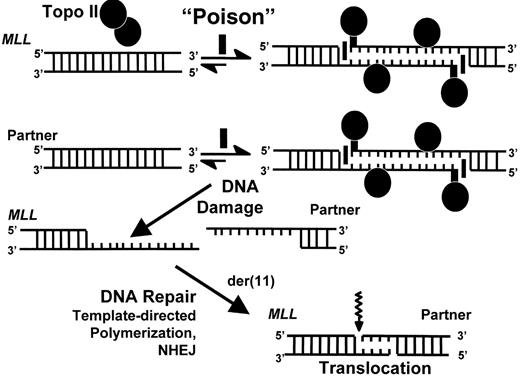
In leukemia in infants, the analysis of MLL genomic breakpoint junction sequences has shown duplicated regions up to several hundred bases long from MLL and/or from its partner gene on both derivative chromosomes or deletions of several hundred bases (reviewed in 18). In contrast, in chemotherapy-related leukemias the interchromosomal DNA recombinations occur with gains or losses of no or, more often, only a few bases (summarized in 37). DNA topoisomerase II creates 4-base staggered double-stranded breaks in DNA, but also introduces single-stranded nicks as kinetic intermediates of double-stranded breaks (reviewed in 18). Some agents cause a higher proportion of double-stranded breaks and the kinetics of the cleavage varies.38 The precision of the breakpoint junction sequences and the results of DNA topoisomerase II in vitro cleavage assays in treatment-related leukemias are consistent with the processing of 4-base, staggered double-stranded breaks.37 In the infant leukemias, the breakpoint junction sequences and in vitro cleavage assays suggest a mechanism in which two type II DNA topoisomerases introduce separate single-stranded nicks in duplex DNA that are staggered by up to several hundred bases. Subsequent template-directed polymerization of the single-stranded overhangs between the staggered nicks then would generate the sequence duplications.39 This leads to a DNA damage-repair model in which various naturally occurring DNA topoisomerase II poisons induce DNA topoisomerase II-mediated damage in leukemia in infants (Figure 1 ). The large deleted regions observed in other infant cases are consistent with multiple sites of breakage or, alternatively, more extensive processing (reviewed in 18).
An alternative mechanism was suggested in which cleavage by an apoptotic nuclease within the MLL bcr is responsible for the DNA damage in these translocations,40 and the exact DNA damage mechanism remains controversial. Nonetheless, the potential to identify the relevant damaging exposure(s) in the uniquely finite time in utero during which the DNA damage must occur holds promise for development of approaches for the prevention of the major form of leukemia in infants.
Single-strand nick model of DNA topoisomerase II-mediated damage in genesis of MLL translocations in infant leukemia.
DNA damage results when natural compound with properties of a DNA topoisomerase II poison induces DNA topoisomerase II-mediated single strand nicks on opposite DNA strands in MLL as well as in its partner gene and disrupts the cleavage-religation equilibrium. This creates long overhangs that serve as templates for polymerization, which results in sequence duplication. MLL and its partner gene contain a few bases of homology immediately at their points of fusion that enable DNA repair by non-homologous end joining. The schematic shows formation of the der(11) genomic breakpoint junction by attempted repair of DNA topoisomerase II-mediated damage. Similar events ensue in the creation of the genomic breakpoint junction on the other derivative chromosome.
Abbreviations: NHEJ, non-homologous end joining
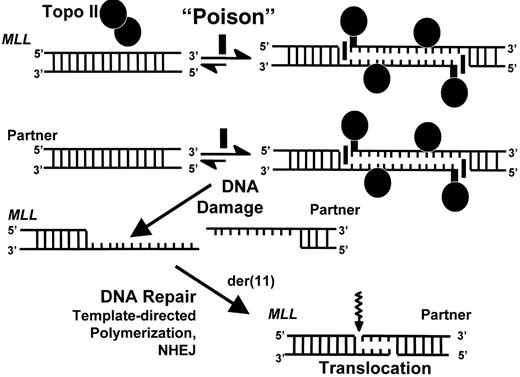
Single-strand nick model of DNA topoisomerase II-mediated damage in genesis of MLL translocations in infant leukemia.
DNA damage results when natural compound with properties of a DNA topoisomerase II poison induces DNA topoisomerase II-mediated single strand nicks on opposite DNA strands in MLL as well as in its partner gene and disrupts the cleavage-religation equilibrium. This creates long overhangs that serve as templates for polymerization, which results in sequence duplication. MLL and its partner gene contain a few bases of homology immediately at their points of fusion that enable DNA repair by non-homologous end joining. The schematic shows formation of the der(11) genomic breakpoint junction by attempted repair of DNA topoisomerase II-mediated damage. Similar events ensue in the creation of the genomic breakpoint junction on the other derivative chromosome.
Abbreviations: NHEJ, non-homologous end joining