Abstract
Advances in our understanding of the pathophysiology of acute myeloid leukemia (AML) have not yet led to major improvements in disease-free and overall survival of adults with this disease. Only about one-third of those between ages 18–60 who are diagnosed with AML can be cured; disease-free survival is rare and current therapy devastating in older adults. In this chapter, challenges in the management of the adult with AML are discussed, including ongoing questions concerning the optimal choice of induction and postremission therapy such as the rationale for and role of allogeneic and autologous stem cell transplantation in a variety of settings, the special considerations pertaining to the older patient, and the development of new, so-called targeted therapies.
In Section I, Dr. Richard Stone reviews state-of the-art therapy in AML in the era of change from a morphological to a genetically based classification system. Questions being addressed in ongoing randomized cooperative group trials include anthracycline dose during induction, the efficacy of drug-resistance modulators, and the utility of pro-apoptotic agents such as the anti-bcl-2 antisense oligonucloetide. Developmental therapeutics in AML include drug resistance modulation, anti-angiogenic strategies, immunotherapy, and signal transduction-active agents, particularly the farnesyl transferase inhibitors as well as those molecules that inhibit the FLT3 tyrosine kinase, activated via mutation in 30% of patients.
In Section II Dr. Margaret O’Donnell discusses the role of stem cell transplantation in AML. Several advances including expanded donor pools, the movement toward peripheral blood stem cell collection, newer immunosuppressive drugs and antifungals, and particularly the advent of nonmyeloablative transplant have made the allogeneic option more viable. The subset-specific role for high-dose chemotherapy with autologous stem cell support and/or for allogeneic transplant in AML patients in first remission is outlined. Although preconceived notions about the role of transplant abound, the clinical data supporting a risk-adapted approach are covered. Finally, guidance concerning the use of nonmyeloablative or reduced-intensity allogeneic transplantation is provided.
In Section III Dr. Mikkael Sekeres reviews the approach to the older patient with AML. Unique biological and therapeutic considerations make AML in this age group a vastly different disease than that in younger adults. The outcome data, including the role of specific anthracylines, hematopoietic growth factors, and drug-resistance modulators, are summarized. Communicating with older adults with AML and their families regarding selection of the optimal treatment strategy, often a stark choice between induction chemotherapy and palliative care, is covered.
I. AML: Current Landscape and Future Directions
Richard M. Stone, MD*
Dana-Farber Cancer Institute, 44 Binney Street, Room D-840, Boston MA 02115-6084
Acute myeloid leukemia (AML) represents a group of clonal hematopoietic stem cell disorders in which both failure to differentiate and overproliferation in the stem cell compartment result in accumulation of non-functional cells termed myeloblasts. While the specific cause for this biological abnormality in any individual patient is usually unknown, the burgeoning understanding of the genetic underpinnings of leukemia is beginning to lead to a wide array of so-called targeted therapies, many of which are in clinical development.
Despite current optimism, most patients with AML will die of their disease. The basic therapeutic approach to patients with AML has changed little over the last 20 years. Nonetheless, before beginning to introduce novel therapies in the clinic, a thorough understanding of the current approach to treatment is required. The evolution of the classification system in AML from morphology to cytogenetic/genetic-based reflects the recognition of the importance of subtype-specific biology.1 The two major prognostic factors in newly diagnosed AML, patient age and chromosome status, form the basis of important treatment decisions. Recently, several molecularly based prognostic factors, such as the adverse impact of an FLT3 tyrosine kinase gene length (or ITD) mutation (repeat of 3–30 amino acids in the juxtamembrane region,2 leading to constitutive activation) and duplication of the MLL gene on the long arm of chromosome 113 have been described; however, the impact of such findings on treatment decisions remains unclear. Patients with treatment-related AML require special consideration because, in the absence of balanced translocations known to carry a favorable prognostic impact, such patients fare poorly with standard approaches. The management of AML in older adults, who have a highly inferior prognosis and an increased rate of treatment morbidity and mortality, is highlighted in Section III.
The approach to adults aged 18–60 years with AML classically involves separate treatment phases. The first consists of induction chemotherapy in which the goal of myelosuppressive chemotherapy is to “empty” the bone marrow of all hematopoietic elements (both benign and malignant) and to allow repopulation of the marrow with normal cells, thereby yielding remission (< 5% marrow blasts). The primacy of the standard regimen of 3 days of an anthracycline and 7 days of cytarabine has not been definitely altered despite clinical trials that have substituted alternative anthracyclines such as idarubicin, added or substituted high-dose cytarabine, or added etoposide. Recent trials by the Cancer and Leukemia Group B (CALGB) have employed high doses of daunorubicin and etoposide.4 While tolerable, whether such higher doses offer disease-free or overall survival benefits remains to be proven by ongoing clinical trials (Table 1 ). Given the high (75%–80%) complete remission rate typically achieved in younger adults with standard chemotherapy, an extremely large trial or a highly effective agent would be required to show superiority over conventional chemotherapy. Most of the trials that have reported an improvement with a new chemotherapeutic agent or with a higher dose of a standard drug during induction have documented a disease-free survival benefit, implying that such remissions yield a lower disease burden. However, the inability to translate such benefits into overall survival improvements suggests a limited ability to affect a major difference in the depth of remission.
It is definitely clear that once remission is achieved, additional therapy is required to reduce the undetectable burden of leukemic cells to a level low enough that long-term disease-free survival (i.e., cure) might be possible. The most effective anti-leukemic approach is allogeneic stem cell transplantation. However, this technique carries a high degree of initial mortality and a significant degree of long-term morbidity in the form of chronic graft-versus-host disease (GVHD), tending to offset the low likelihood of disease relapse. Chemotherapy-based approaches with or without autologous stem cell rescue can be performed relatively safely, but there remains a high chance for disease recurrence. Some of the patients who relapse after chemotherapy or even high-dose chemotherapy with autologous stem cell rescue can be salvaged with an allogeneic transplant performed in early relapse or second remission. The role of autologous and allogeneic (both standard and reduced intensity) strategies in the post-remission management of AML patients with various risks of disease relapse based on karyotype at diagnosis is discussed in Section II.
Specific therapies have proven benefit for small subsets of patients defined by recurring cytogenetic abnormalities. Post-remission chemotherapy with high-dose cytarabine is generally accepted as the best approach for the 15% of patients with favorable prognosis chromosome abnormalities [e.g., t(8:21); or inv(16)].5 While the optimal number of such cycles remains to be defined, at least three are probably required.6 Whether the high-dose cytarabine or repetitive cycles of intensive chemotherapy is the critical factor remains debatable; however, a cure rate of 60%–70% with chemotherapy suggests that the more risky strategy of allogeneic transplant should be reserved for early relapse or second complete remission in this subset of patients. Patients who present with acute promyelocytic leukemia (APL; another 8%–12% of patients) should be treated with all-trans retinoic acid (ATRA) and an anthracycline in induction.7,8 Although many still employ cytarabine in the induction and postremission management of APL patients, its use is probably not necessary.7 ATRA/anthracycline-based postremission therapy should be augmented with a year of maintenance therapy, not generally thought effective in other AML subtypes, with ATRA, probably in combination with oral antimetabolites.9 APL is the one subtype of APL in which molecular monitoring after achievement of complete response (CR) has proven useful. Few patients who achieve polymerase chain reaction (PCR)-negative status after postremission chemotherapy relapse; whereas those with persistently detectable PML-RARα fusion transcripts have a 25% risk of relapse,10 yet whether such relapses can be prevented with additional therapy remains unclear. While the cure rate in APL with “standard” therapy is favorable (60%–70%), the problems of secondary myelodysplasia11 and late central nervous system (CNS) relapses8 have recently been described.
In summary, the young adult who presents with AML should have studies performed on leukemic cells leading to morphologic and cytogenetic classification. Those with non-APL AML should receive induction therapy with 3 days of an anthracyline and 7 days of infusional cytarabine followed by risk-adapted postremission therapy [intensive chemotherapy for those with inv 16 or t(8;21)], allogeneic transplant for those with high-risk cytogenetics, and either intensive chemotherapy, high-dose chemotherapy with autologous stem cell rescue or sibling-matched allogeneic transplant for the remainder. That a “standard approach” to the treatment of AML in the 18–60 year old patient can be described is still compatible with the notion that all should be referred for a clinical trial. Many questions need to be answered, even for those with so-called ‘favorable’ prognoses, that usually involve the addition of a new agent onto a backbone of “standard” therapy. The ideal candidates for investigational therapy, many of whom are described subsequently, with a single agent are those likely to fare poorly with induction chemotherapy, including older adults and those with disease relapse less than a year from diagnosis. While many different chemotherapy regimens exist for AML in relapse, the choice of regimen is less important than the duration of first remission. Those who relapse more than one year after diagnosis have good chance to achieve a second remission after administration of the original induction regimen, or at least one of similar intensity. However a second remission is achieved, the only post-remission therapy with significant utility is allogeneic transplant if possible, or high-dose chemotherapy with autologous stem cell rescue.
Developmental Therapeutics in AML
The increased understanding of the pathophysiology of AML has led to the development of a host of new so-called targeted therapies. The success of imatinib in the treatment of chronic myeloid leukemia (CML) and other diseases pathophysiologically based on the constitutive activation of a tyrosine kinase that is inhibited by the drug has spurred the search for similarly effective agents in AML. Table 2 lists some of the newer therapies according to their proposed mechanism of action. Whether any of these will prove to alter the natural history of AML when used either alone or in combination with each other or with standard chemotherapy remains to be determined. That there are so many therapies in development is certainly positive; the challenge posed by this surfeit in a relatively rare disease for which fairly effective therapy already exists remains daunting. Immunological therapies in AML based on enhancement of the host immunity or elegant strategies of making AML cells “visible” to the immune system are mentioned in Section II.
Two agents have recently been approved for use in AML: arsenic trioxide12 and gemtuzumab ozogamicin.13 Their place in the therapeutic armamentarium is now being better defined. Both could be considered models for other new therapies, because they were shown to be effective (in highly varying degrees) in relapsed patients. Post-approval studies are being done to define their role in newly diagnosed patients to increase their overall impact. Arsenic trioxide leads to a four-log reduction in the acute promyelocytic leukemia disease burden in heavily pre-treated relapsed patients12 and is now being evaluated to consolidate first remissions (Table 1 ). Gemtuzumab ozogamicin is an anti-CD33 immunotoxin conjugate that was approved based on a 30% response rate (half of whom were patients who never fully recovered their platelet count) in patients with untreated first relapse who had, in most cases, a first remission of 6 months or longer.13 Disappointment with this drug due to its low single-agent efficacy in treatment of patients with refractory AML as well as its association with hepatic veno-occlusive disease when administered proximal to or following a bone marrow transplant14 has dampened enthusiasm. Nonetheless, based on the apparent safety of combining this agent with chemotherapy in newly diagnosed patients,15 important studies are now underway to determine if gemtuzumab ozogamicin combined with induction or consolidation chemotherapy, or as a single agent in the post-remission setting in older adults might lead to a better outcome (Table 1 ).
Agents Not Expected to Be Effective as Single Drugs
Drug-resistant modulators
The notion that blasts from AML patients, and particularly from older individuals, are likely to express genes capable of mediating drug resistance, most notably the GP170 drug efflux pump, has prompted clinical trials with so-called reversing agents that inhibit this function. Since many of these agents also inhibit the metabolism of chemotherapeutic drugs like anthracyclines, pharmacokinetic considerations are important. Most of the trials that have compared chemotherapy with or without a drug-resistant modulator (DRM) have shown no benefit or have been terminated early due to excess toxicity.16 However, a randomized clinical trial involving cyclosporine A as a reversing agent has been positive,17 prompting the Southwest Oncology Group to treat older adults with chemotherapy (using a novel regimen including continuous infusion daunorubicin) with and without cyclosporine A. Secondly, trials with newer DRMs that do not have pharmacological effects on chemotherapy are also underway (Table 1 ).
A problem with these drugs as well as with many novel approaches is that the mechanism of resistance is likely to be pleiotropic. The inability to initiate cell death in response to chemotherapy is another potential mechanism of resistance. Overexpression of the bcl-2 anti-apoptotic gene has been associated with poor prognosis in AML patients. Preclinical data documenting synergism between chemotherapy and the antisense 18-mer oligonucleotide oblimersen (G3139) have prompted Phase I clinical trials documenting the safety of induction chemotherapy with this agent.18 The CALGB has begun a randomized a trial in older adults that compares chemotherapy alone to chemotherapy administered with oblimersen (Table 1 ). Synergistic antileukemic activity between chemotherapy agents and the proteosome inhibitor bortezomib19 has spurred the clinical development of this agent in conjunction with chemotherapy in AML.
The use of hematopoietic growth factors (HGFs) in AML has received much attention. Once concerns diminished that growth factors would enhance disease resistance via their known ability to stimulate leukemia cells to enter S-phase, a host of clinical trials examined the ability of HGFs to shorten the period of neutropenia and reduce infectious mortality. While the duration of neutropenia was reduced by several days when G-CSF or granulocyte-macrophage colony-stimulating factor (GM-CSF) was used in the postchemotherapy period, the inability of these agents to alter the nadir, allay mucositis, or decrease the death rate diminished their utility. Perhaps more interesting is the use of these agents before or during chemotherapy in an effort to increase the S-phase fraction of cells, thereby using them as chemosensitizing agents. Although most of the trials using HGFs in this fashion have been disappointing, a recent randomized study in which G-CSF was used before and during chemotherapy, yielding a disease free survival benefit,20 has prompted renewed interest in this strategy. At the present time the use of G- or GM-CSF can be considered optional using supportive care guidelines for neutropenic fever while their use as chemotherapy-enhancing agents needs to be explored further before routine use is warranted.
Agents That Promote Differentiation
Almost a quarter century has elapsed since clinical trials suggested that low-dose cytarabine’s effect in AML and myelodysplasia resulted from hematopoietic cell differentiation. In the ensuing years, the enthusiasm for low-dose cytarabine as an effective therapy that induces maturation of AML cells has waned. However, an increased understanding of how the transcription of differentiation-associated genes is regulated has prompted the exploration of new and potentially effective therapies. Among the many biochemical events that must occur as a cell changes from a stem cell to a mature functional blood cell are removal of methyl groups from DNA bases and addition of acetyl groups to the histone DNA protein coat. Such effects change the conformation of the DNA and allow transcription of certain genes. The DNA hypomethylating agent azacitidine21 was recently approved by the US Food and Drug Administration (FDA) for use in all subtypes of myelodysplastic syndrome. Many clinical trials are now underway with so-called histone deacetylase inhibitors, although outcome data are sparse. Given the general hypothesis that AML results from a combination of over proliferation of the stem cell compartment as well as failure to differentiate, it is rational to consider the future use of these differentiating agents in combination with agents that inhibit mitogenic signals.
Signal Transduction Inhibition
The mechanism by which cells receive and transmit mitogenic signals is complex and involves the coordinated action of many different proteins, commonly represented by so-called signal transduction cascades. Insofar as such cascades might be overactive in leukemia cells compared to normal hematopoietic cells, a therapeutic opportunity is provided. Moreover, an activating mutation in one of the proteins responsible for transmitting proliferative signals in AML defines a potential target. Mutations in one of the RAS family proteins, 21-KD guanine-nucleotide binding proteins, have been described in 10%–50% of AML patients. Such activating mutations are thought to allow autonomous growth. RAS proteins require several post-translational modification steps including addition of a farnesyl lipid moiety that allows translocation to the plasma membrane and activation. Farnesyl transferase inhibitors (FTIs) have been shown to inhibit the growth of mutant RAS-transformed cell lines. The FTI in furthest development in AML, tipifarnib (R115777), was associated with responses in advanced-stage patients in a Phase I trial.22 Despite disappointing results in an international Phase II trial in refractory/relapsed patients (although RAS mutations were not required), the drug has been administered to over 100 chemotherapy-naïve AML patients. A preliminary report documented a 20% complete remission rate in this group of poor prognosis, largely older patients who received this oral agent as their initial therapy.23 These exciting results, if confirmed, might provide a new and less toxic therapy compared to chemotherapy for older adults with AML. FTIs are also being evaluated in younger patients in earlier disease states and in combination with chemotherapy. An active area of current research in AML is the development of agents known as FLT3 inhibitors. FLT3 is a transmembrane tyrosine kinase. Approximately 30% of AML patients can be shown to have an activating mutation that generally carries a poor prognosis.2 The activating mutation may be a duplication of between 3 and 60 amino acids in the juxtamembrane region or, less commonly, a point mutation in the activation loop.2 Activating mutations of the FLT3 tyrosine kinase transform leukemic cell lines into growth factor independence and cause a fatal myeloproliferative syndrome in a murine bone marrow transplant model. Small molecules capable of inhibiting FLT3 enzyme activity can selectively kill such transformed cell lines and improve survival in the murine model.2 Thus, the preclinical data supporting the development of these drugs as therapeutic agents in AML is at least as strong as that used to support clinical trials with imatinib in patients with CML. There are two major differences between imatinib’s development and that of the FLT3 inhibitors: 1) CML in chronic phase is probably based solely on the activation of bcr-abl, whereas AML is almost certainly a “multi-hit” disease; 2) Multiple agents and drug companies are developing FLT3 inhibitors. However, just as was the case for imatinib, these drugs have a spectrum of activity beyond FLT3 inhibition alone. For example, PKC-412 (N-benzoylstaurosporine) inhibits FLT3 as well as protein kinase C and the vascular endothelial growth factor receptor. Such a wide spectrum of activity could have positive or negative consequences. SU5416, which inhibits FLT3 reasonably potently, was developed as a c-kit inhibitor. The drug demonstrated modest activity in AML, but no activity was observed in the 7 patients who retrospectively were found to have an activating mutation of FLT3 in their myeloblasts.24 Minor responses have been observed in patients without known FLT3 mutations who have received PKC-412. Available clinical data from early trials with the three drugs specifically developed as FLT3 inhibitors, PKC-412,25 CEP-701,26 and MLN-518,27 are summarized in Table 3 . Biological responses, demonstrated by a major reduction in peripheral blast count, have occurred with each of these drugs. However, they have minimal ability to reduce the bone marrow blast count, and therefore, complete remissions have occurred in 1/42 reported patients. Moreover, the duration of response has been brief. Although much more work with these oral agents needs to be done, initial impressions suggest that (1) the multiplicity of genetic lesions in the typical AML cell may be problematic for expecting these agents to work alone; (2) pharmacokinetic issues require prolonged therapeutic drug levels and the ability to get to the target leukemia progenitor cell; and (3) the contribution of alternative enzymes and pathways are important.28
Nonetheless, further development of these drugs at different doses and schedules and in combination with other signal transduction inhibitors and/or chemotherapy is warranted. Other tyrosine kinase inhibitors, including those that inhibit the vascular endothelial growth factor receptor, are also in clinical trials in AML.29
II. The Role of Autologous and Allogeneic (“Full” and “Mini”) Stem Cell Transplantation in AML
Margaret R. O’Donnell, MD*
City of Hope National Medical Center, 1500 East Duarte Road, Duarte CA 91010
Hematopoietic cell transplantation (HCT) is an effective therapy for AML. Obstacles to broad applicability of HCT therapy for the majority of patients with AML have included inability to control leukemia with primary induction therapy, lack of suitable hematopoietic cell donors, toxicities of HCT conditioning regimens and long-term complications of the transplant procedure. While all antileukemia therapies are complicated by the problems of chemotherapy-related toxicities and disease relapse, allogeneic HCT also exposes patients to the risks of potential failure of engraftment; organ toxicities caused by GVHD and prolonged immunosuppression with its attendant risks of post-HCT infectious complications.
In the last decade, several developments have made allogeneic HCT a more “user-friendly” treatment modality. Expansion of the pool of donors in national and international registries as well as the establishment of cord blood banks has vastly increased the likelihood of success in identifying a suitable HLA match for patients who lack matched family donors, although cord blood HCT is still utilized primarily for pediatric recipients. The refinement of tissue typing using molecular probes permits better matching of unrelated donor-recipient pairs, thereby decreasing the risks of both graft rejection and GVHD.
There has also been a recent change in the type of hematopoietic progenitor cell (HPC) product used for graft reconstitution. Autologous transplant procedures had transitioned from marrow to cytokine-primed (G-CSF and/or GM-CSF) peripheral blood stem cells (PBSC) for ease of collection and rapid engraftment by the early 1990s. However, expectation of higher rates of GVHD due to the 10-fold increase in T cells in PBSC products delayed their use in allogeneic settings until the latter-half of the 1990s. In 2003, National Marrow Donor Program (NMDP) statistics showed 60% of unrelated donor products collected for transplants for AML were PBSC. Collection via apheresis is physically easier for many donors. The 4- to 5-fold higher number of CD34+ cells in a PBSC product facilitates more rapid engraftment in comparison with a marrow product (median of 17 days versus 24 days for granulocytes and median of 28 days versus 47 days for platelets, respectively), which decreases the risk for bacterial infections in the early post-transplant period.1 Despite initial concerns that a 10-fold increase in the number of CD3+ cells in the PBSC graft would lead to higher incidence of severe GVHD, this has not been demonstrated in clinical trials. Several studies have shown no significant difference in the incidence of acute GVHD; however, higher rates of late onset chronic GVHD (beyond 6 months) have been reported.2 In both a large randomized trial and a retrospective review of International Bone Marrow Transplant Registry (IBMTR) data, PBSC grafts were associated with improved relapse-free survival in patients with leukemia beyond first remission (CR1).1,3,4 In addition to producing a HPC product that results in more rapid engraftment, G-CSF exposure also shifts donor T cells to Type 2 cytokine secretion (interleukin [IL]-4 and IL-10) and downregulates Type 1 (IL-2 and interferon γ) cytokine production, phenomena which may be associated with less severe acute GVHD.
Improvements in post-transplant supportive care and the development of newer immunosuppressive agents have also had an impact on transplant-related toxicities. More refined PCR-based screening for cytomegalovirus reactivation allows pre-emptive therapy tailored to viral load. Newer less toxic antifungal agents such as voriconazole and caspofungin have decreased early mycotic infections and make it feasible to provide long-term fungal prophylaxis in patients at high risk due to chronic GVHD. Newer immunosuppressive agents, such as sirolimus and mycophenolate mofetil (MMF) are being incorporated into GVHD prophylactic regimens to decrease the incidence of acute and chronic GVHD in high-risk populations including unrelated donor recipients and older (> 50 years) patients.5,6
However, the change that may have the greatest impact on AML treatment is the advent of reduced intensity or nonmyeloablative HCT. Reduced-intensity HCT relies upon the graft-versus-leukemia effect of the allograft rather than the direct tumoricidal activity of the conditioning regimen. Because of the paucity of any direct antileukemic effect of the conditioning regimens, the truly nonmyeloablative (NMABT) regimens can only be used in patients with low volumes of disease, whereas the reduced-intensity conditioning regimens, which usually contain fludarabine and some alkylating agent, do have direct antileukemia activity and can be used with more extensive disease. The shortened duration of cytopenias and the minimal mucosal toxicity of the newer reduced-intensity conditioning regimens provide a reasonably safe transplant option for patients two decades older than the population that was treated with traditional fully ablative high-dose chemoradiotherapy regimens.
Choosing the Type and Timing of HCT (To Transplant or Not to Transplant)
The possible need for HCT as a component of future therapy should be acknowledged at diagnosis in AML. For all patients under age 56 who do not have significant co-morbid conditions, HLA typing should be part of the patient’s initial evaluation, and family typing should be initiated when the cytogenetic information is available. Only a minority of patients will have a favorable karotype that would obviate consideration of allogeneic HCT either as part of consolidation or for salvage of resistant disease. For patients with poor-risk cytogenetics, antecedent myelodysplasia or therapy-related AML, an unrelated donor search should be promptly instituted in patients lacking a family donor. Early donor identification allows optimal timing for HCT as consolidation or salvage therapy. In older patients (56–70 years), however, it is reasonable to delay HLA typing of family members until remission is achieved and the patient has recovered a good performance status since there is substantial attrition in the rank of candidates for the investigational use of reduced-intensity or nonmyeloablative HCT as consolidation due to failure to achieve a remission or decline of functional status as a consequence of induction therapy.
The decision to proceed to either autologous or allogeneic HCT is strongly influenced by the following factors: expectations of outcome with the prevailing non-HCT conventional therapy, the effectiveness of salvage options for disease relapse, the toxicities of transplant including long-term complication of infertility and second malignancy, and the individual patient’s co-morbid conditions. The patient’s cytogenetic risk group and time to achieve remission are currently the major disease-specific factors to be considered in opting for HCT as consolidation therapy, whereas donor availability and the impact of recipient performance status on the ability to tolerate the rigors of even a reduced-intensity HCT are important factors to consider at relapse. While there are several studies with long-term outcome data comparing myeloablative conditioning regimens followed by either autologous or allogeneic HCT, the follow-up data on reduced intensity HCT is much shorter and the patient populations are not comparable.
Comparisons of Autologous Transplantation, Allogeneic and Reduced-Intensity HCT
Autologous transplantation has been used primarily as a component of consolidation following initial remission; less commonly, it can be used as a component of salvage therapy for patients in second remission. To minimize both the total body leukemic burden and the contamination of the PBSC product, current practice is to administer one to two cycles of a high-dose cytarabine-based consolidation to achieve “in vivo purging” prior to collection of G-CSF–mobilized PBSC. To assure that an adequate hematopoietic progenitor cell product of at least 5 × 106 CD34+ cells/kg recipient body weight can be collected, it is recommended that no more than 2 cycles of consolidation occur before PBSC collection. While the nonhematologic toxicities of the conditioning regimens used for autologous HCT are significantly greater than those seen with high-dose cytarabine consolidation, the hematologic recovery is quite rapid (8–11 days) following infusion of autologous G-CSF–mobilized PBSC. The combined treatment-related mortality (TRM) for one cycle of high-dose cytarabine-based consolidation followed by 1200 cGy fractionated total body irradiation (FTBI), etoposide and cyclophosphamide (Cy) and autologous HCT at our institution ranged between 3% and 6% in three sequential trials inclusive of patients up to age 60. Attrition between consolidation and autologous transplant ranged from 18% to 24% due to relapse, inadequate HPC collection or treatment-related toxicities such as invasive fungal infection and cytarabine-induced neurotoxicity, making this a reasonable consolidation option for most patients up to age 60.7
Over the last 15 years, the upper age limit considered to be acceptable for a fully ablative allogeneic HCT has advanced from 40 to “robust” 60 year olds (perhaps in response to the maturing age of the transplanters themselves as much as to improvements in supportive care and GVHD prophylaxis).8 If an HLA-matched donor has been identified while the patient was receiving induction therapy, there is no advantage to administering postremission chemotherapy prior to allogeneic HCT.9 GVHD and transplant-related toxicity remain the major obstacles to successful outcome with allogeneic HCT. The introduction of two new immunomodulatory drugs for GVHD prophylaxis may have an important impact on both these problems. Investigators from the Dana-Farber recently reported a Phase II trial in which sirolimus was substituted for methotrexate (MTX) in a tacrolimus-based GVHD prophylaxis regimen for PBSC HCT utilizing a fully myeloablative conditioning regimen.5 Engraftment was prompt and only 3/30 (10%) patients developed acute GVHD (all Grade II) compared to the expected incidence of 35%–40% acute GVHD in sibling-donor HCT with tacrolimus/MTX. TRM was only 6% at 1 year compared to 25%–30% in most traditional allogeneic studies. Chronic GVHD developed in 40% of the study patients. Toxicities included significant hyperlipidemia, thrombotic microangiopathy and a 10% incidence of veno-occlusive disease primarily in patients with recent exposure to gemtuzumab ozogamicin. Other studies of the substitution of mycophenolate mofetil (MMF) for MTX in GVHD prophylaxis regimens have demonstrated decreased regimen-related toxicities of severe mucositis and interstitial pneumonias, as well as more rapid engraftment compared to a MTX-containing regimen (14–16 days versus 19–22 days for neutrophil engraftment).10,11
Improvements in molecular matching for unrelated donor-recipient pairs have significantly improved outcomes for this group, although there continues to be an approximately 10% higher incidence of TRM when sibling allogeneic HCT is compared to allogeneic HCT from molecularly matched unrelated donors for patients in CR1. Because of the perception of higher TRM, the profile of patients who undergo unrelated donor HCT in CR1 is skewed toward those with poorer risk cytogenetics, prolonged time to achieve a remission, or a history of antecedent myelodysplasia or therapy-related AML. The majority of unrelated donor transplants are still being performed for AML patients beyond CR1.
The foremost advantage of the nonmyeloablative or reduced-intensity HCT is the reduction of TRM. Mucositis in the immediate post-HCT period is much reduced due to the low antiproliferative activity of the conditioning regimen and the substitution of MMF for MTX in the GVHD prophylaxis regimen. The reduction in mucosal disruption led to a decreased incidence of acute (12%) and chronic (19%) GVHD for reduced-intensity fludarabine/melphalan versus 36% acute and 40% chronic GVHD for patients receiving ablative sibling HCT in an MD Anderson Cancer Center study.11 Infectious complications are also reduced, with a 9% incidence of bacteremia at day 100 for nonmyeloablative conditioning versus 27% for standard HCT and a difference in day 100 survivals of 93% versus 81%, respectively, in a case-matched control series from Seattle.12 However, other investigators have reported higher rates of CMV reactivation before day 100 and persistent risk of bacteremia and fungal infections late post-HCT with nonmyeloablative regimens because of the prolonged immunosuppressive effects of fludarabine even in the absence of GVHD.
Table 4 provides a schematic of current thinking on the role of the various types of HCT in the therapy of AML. A caveat for interpretation of all consolidation trials in which HCT is a component is the existence of selection bias. There have been several large national or co-operative group trials conducted between 1986 and 1995 that tried to answer the question of the role of transplantation in consolidation in younger patients with AML.13–,15 Patients with sibling donors were assigned to the allogeneic marrow transplantation and the remainder were randomized, between a variety of consolidation (2–4 cycles) chemotherapy versus autologous marrow transplant after 1–4 cycles of consolidation. The conclusions ranged from no survival advantage to either form of HCT compared with consolidation chemotherapy in the initial analysis of the US National Trial involving the Eastern Cooperative Oncology Group (ECOG), Southwest Oncology Group (SWOG) and CALGB to a significant difference in disease-free survival for both allogeneic (55%) and autologous HCT (48%) compared to chemotherapy (30%) in the European Organisation for Research and Treatment of Cancer (EORTC) trial (albeit with no overall survival advantage as a high percentage [58%] of chemotherapy relapses were salvaged with transplant). When the US National Trial was reanalyzed using cytogentic risk groupings there were clear differences in favor of HCT (allo and auto) for favorable risk and for allogeneic in patients with poor risk cytogenetics.15 In all these trials as well as the MRC10 trial, there was considerable patient attrition from achieving remission to initiating any form of consolidation.16 Fully one third of younger (< 55 yr) patients received no documented form of consolidation; 50%–80% of patients actually received the designated HCT treatment. While it is appropriate to compare treatment strategies on an intent-to-treat basis from the time CR is achieved, the interposition of multiple nontransplant therapies before HCT may cause high attrition rates that provide a negative bias, while analyses that start only at time of transplant may give an overly optimistic prediction of outcome.
Consolidation
Good-risk cytogenetics
Given the high curability of acute promyelocytic leukemia (APL) with conventional chemotherapy, there is no role for autologous or allogeneic HCT for patients in CR1 who achieve a molecular remission by the completion of consolidation. Several series have demonstrated that autologous HCT can produce durable second remissions in 75%–100% of the patients in whom a molecularly negative product can be collected.17 However, for patients with persistence of the PML/RAR gene product, allogeneic transplantation should be considered since the relapse rate following infusion of a molecularly positive product exceeds 85%.
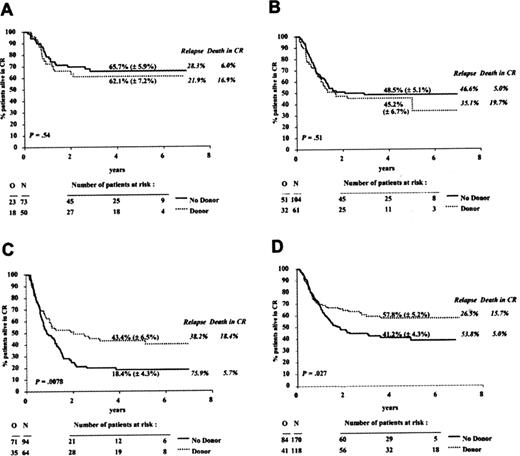
Relapse free-survivals of 60%–83% have been reported for patients with t(8;21) or inv(16) using intent-to-treat analysis from the start of high-dose cytarabine consolidation through autologous HCT with relapse rates of 15%–20% post-HCT and TRM of 4%–8% overall.18,19 While these disease-free survival (DFS) rates are higher than the DFS seen with multiple cycles of high-dose cytarabine-based consolidation and the time to completion of treatment may be shorter, long-term toxicities associated with autologous HCT need to be factored into the decision to pursue autologous HCT in individual patients. Many with expertise in AML would reserve autologous HCT for relapse in patients with good risk cytogenetics. In patients who opt for conventional chemotherapy, one should consider cryopreserving autologous PBSC as a “back-up” for salvage therapy in the event of relapse in patients who lack a histocompatible sibling. Autologous HCT can provide long-term salvage for 40% of patients who achieve a second remission overall; factors that correlate with better outcome include cytogenetics and duration of first remission.20 Sibling allogeneic HCT in CR1 in the group with favorable cytogenetics had only 62% DFS with a 17% TRM in the EORTC/GIMEMA (Gruppo Italiano Malattie Ematologiche dell’ Adulto) trial based on intent-to-treat from time of remission that compared autologous to allogeneic transplant during the time period of 1993–1999 (Figure 1A ). The high TRM associated with allogeneic HCT indicates that allogeneic HCT has no advantage over conventional chemotherapy or autologous HCT until the toxicity profile improves.19
Intermediate cytogenetics
The European Bone Marrow Transplant (EBMT) group reported an overall 2-year leukemia-free survival of 49% for 1040 patients who received autologous unpurged marrow transplant during the period of 1986–1994.21 The TRM was 11%, with a median time to granulocyte and platelet recovery of 29 days and 42 days, respectively. Factors that influenced outcomes were time to achieve remission (1 vs 2 inductions) for leukemia-free survival and older age (> 35 years) for TRM. Karyotypic analysis was not included in that study. In the EORTC/GIMEMA study, patients with normal karyotype who underwent autologous HCT had a 48% DFS with a 5% TRM compared to 45% DFS and 20% TRM in patients transplanted from a sibling donor.14 In a consortium study that included 128 patients up to age 65 receiving autologous HCT in CR1, Linker et al reported a 51% DFS for patients with intermediate risk cytogenetics, with a combined TRM of 3% for both consolidation and transplant.18 Since TRM in allogeneic recipients increases significantly with age, autologous transplant may offer equivalent DFS with less morbidity for older patients (≥ 50 years) than standard myeloablative allogeneic transplant. Younger (≤ 40 years) patients with a sibling donor can expect a better relapse-free survival of 62% with a 15% relapse rate and TRM of 15%–20%. Since TRM is 10%–18% higher for unrelated-donor HCT using myeloablative conditioning, a donor search is rarely initiated during CR1 for intermediate-risk patients in the absence of other risk factors such as antecedent MDS.
Poor-risk cytogenetics
A donor search for a related or unrelated donor should be initiated as soon as the patient is identified as having poor-risk cytogenetics as this group may not achieve remission and may require allogeneic HCT for early salvage. Patients with poor-risk cytogenetics are proportionally underrepresented in autologous transplant series, accounting for less than 10% of patients because remissions are more difficult to achieve and the recovering marrow may have underlying myelodysplasia, which impairs the ability to collect adequate numbers of stem cells with normal proliferative capacity. In most series, the results of autologous HCT for patients in this group are poor, with DFS of 20% or less.21 In contrast, sibling allogeneic transplant for this group results in a 40%–45% DFS for patients ≤ 45 years of age19 (Figure 1C ). Most unrelated donor transplants in CR1 are performed in patients with poor-risk cytogenetics. Data from the NMDP demonstrated an overall 40% 5-year relapse-free survival for all AML patients in CR1 transplanted between 1987 and 2001, which is comparable to the 44% reported by the EORTC for sibling HCT of patients with poor-risk karyotypes.
Reported outcomes for reduced intensity transplants in patients with AML in CR1 are based on small samples. The group from the University of Michigan treated 21 patients over age 55 (median 61 years) with MDS or AML who had HLA-matched sibling donors with the reduced intensity regimen of fludarabine (Flu) and busulfan (Bu) with tacrolimus and MMF for GVHD prophylaxis.22 Seven of 12 patients who were in CR at time of transplant remain in remission with a median follow-up of 1 year (range 210–733 days). There were 3 relapses and 2 treatment-related deaths. All the patients with active disease died (5 relapse and 4 TRM) with a median survival of 108 days. Overall, the incidence of acute GVHD was 38% with a maximum severity of grade 2. TRM at day 100 for the whole group was 19% and was only 8% in the patients who did not have active disease at transplant.
The German Cooperative Transplant Study Group reported on 113 AML patients treated with Bu/Flu (93 patients) or Flu/TBI (20 patients) with a radiation dose of either 400 or 800 cGy. The reduced intensity regimens were chosen for these patients because of age > 50, co-morbid condition or prior HCT; the median age was 51 and more than half the patients received unrelated donor allografts.23 The minority of patients (22%) were in CR1, and half the patients were in relapse at HCT. Five patients with relapse did not clear their disease and 1 did not engraft. Acute GVHD (Grade 2–4) occurred in 42% of patients and chronic GVHD occurred in 35% of patients at risk after day 100 with the majority of these cases being of limited extent. The 2-year DFS was 50% for those in CR1, 40% for those in CR2 or 3, and 15% for those with relapsed disease. Causes of death were evenly divided between relapse and treatment-related toxicity (29 patients each). In addition to remission status, performance status (≤ 70%) and donor type (sibling versus unrelated donor) were predictive factors for event-free survival.
Salvage
The majority of allogeneic transplants are performed as salvage for patients who have relapsed after conventional chemotherapy or autologous HCT. The decision regarding reinduction prior to salvage transplant is influenced by (1) the availability of an identified donor and (2) the likeliness of achieving a remission, which in turn is based on cytogenetic risk group, duration of CR1 and comorbid conditions. Data from EBMT, IBMTR and the NMDP show DFS of 44% with sibling allografts and 30% with matched unrelated donor allografts at 5 years for patients transplanted in second remission and DFS of 35%–40% in sibling transplants and 10% in unrelated donor transplants for patients with induction failures or HCT in relapse.24 It should be noted that the majority of these patients received marrow rather than PBSC. In the Seattle study comparing sibling marrow versus PBSC in patients with more advanced disease, there was a significant difference in DFS (57% vs 33%) using PBSC compared to marrow.1 Additional data from the IBMTR also showed an advantage for PBSC compared to marrow for patients transplanted in second remission.3 The reduced intensity regimens have had a tremendous impact on TRM in the setting of second transplants or secondary MDS/AML following prior autologous HCT, increasing the DFS from 16% with standard Bu plus Cy conditioning to 40% with Flu/Bu or Flu/Melphalan conditioning.25 The recent formation of the Blood and Marrow Transplant Clinical Trials Network (CTN) to facilitate the conduct of large scale comparative transplant trials should enable the transplant community to expeditiously define the role of newer reduced-intensity conditioning regimens and GVHD prophylaxis regimens in exploiting the antileukemic effect of donor alloreactivity, minimizing transplant-related toxicities and broadening the applicability of HCT as a curative treatment strategy for patients with AML.
III. AML in Older Adults: Are We Listening?
Mikkael A. Sekeres, MD, MS*
The Cleveland Clinic Foundation, Hematology and Medical Oncology, Desk R35, 9500 Euclid Avenue, Cleveland OH 44195
AML is a disease of older adults. In the US, the median age is 68 years and the age-adjusted population incidence is 17.6 per 100,000 for people 65 years of age or older, compared with an incidence of 1.8 per 100,000 for people under the age of 65 years.1 Therefore, of the estimated 11,900 new AML diagnoses in the US in 2004, over half will affect patients 60 years of age or older, a population considered “elderly” in the leukemia literature.2– 5 This number will only increase as the population of US citizens 65 years or older, estimated to be approximately 35 million in the year 2000, is expected to double by the year 2030.
Older adults with AML, when compared to younger patients with the same disease, have a poor prognosis and represent a discrete population in terms of disease biology, treatment-related complications, and overall outcome. As a result, older patients require distinctive management approaches to determine whether standard treatment, investigational treatment, or low-dose therapy or palliative care is most appropriate. Particularly in this population, the tradeoff between potential for cure or survival prolongation and quality of life must be weighed carefully.
Disease Biology
Cytogenetics
Older adults with AML have a lower incidence of favorable chromosomal abnormalities [including the core binding factor abnormalities, such as t(8;21) or abnormalities of chromosome 16, or the t(15;17) associated with APL] and a higher incidence of unfavorable abnormalities (including complex cytogenetics or abnormalities of chromosomes 5, 7, or 8) compared to younger adults with AML (Table 5 ).6–,8 The rare older patient fortunate enough to have good-risk cytogenetic abnormalities may have a survival advantage over other older patients,8,9 though it is not clear that this finding holds for patients older than 65 years.
Bone marrow biology
In older adults, AML is more likely to arise from a proximal bone marrow stem cell disorder, such as myelodysplastic syndrome (MDS), and with leukemia-specific abnormalities in more than one hematopoietic cell lineage. This may explain the different disease behavior in this group, as well as prolonged neutropenia following chemotherapy.3 Older adults with AML also are more likely to have reduced proliferative capacities in normal hematopoietic stem cells, which may affect blood count recovery following intensive chemotherapy.
Drug-resistance genes
The expression of genes that mediate drug resistance occurs with increased frequency in this age cohort. MDR1, the so-called P-glycoprotein (gp170) chemotherapy efflux pump, was found in 71% of leukemic blasts in subjects in a SWOG study of AML patients over the age of 55 years, compared to a prevalence of 35% in younger AML patients.10 MDR1/P-glycoprotein expression is associated with lower complete remission (CR) rates and more chemo-resistant disease.
Prior stem cell insult
Older adults with AML are more likely to have a secondary leukemia arising from an antecedent MDS or from prior treatment with chemotherapy or radiation therapy for another cancer. Patients with this type of AML are predisposed to having abnormalities in chromosome 5 and/or 7.7 Secondary AML (AML that arose after MDS, myeloproliferative disorders, and therapies of malignancy) comprises 24%–56% of AML diagnosis in older patients,10,11 compared to a prevalence of approximately 8% in younger AML patients in the Medical Research Council (MRC) AML 10 trial.7 AML arising from prior bone marrow stem cell disorders or antecedent hematologic disorders, particularly when the process is greater than 10 months in duration prior to the development of AML, is less responsive to chemotherapy, resulting in shorter event-free survival, a lower CR rate, and conferring a worse prognosis.12
Response to Therapy
Older adults are not as tolerant of or responsive to remission induction and consolidation chemotherapy compared to their younger counterparts. High mortality rates likely result from inherent disease biology, an increased prevalence of comorbid disease, and a differential metabolism of induction regimen drugs, particularly cytarabine, resulting in supratherapeutic drug levels.4 Concern over potential treatment-related toxicities may result in undertreatment of disease. Paradoxically, administration of full-dose daunorubicin, for example, may result in a reduction in early deaths by effecting a more rapid CR.
The outcome of older adults with AML is also worse. Adults under the age of 60 years treated with an induction regimen consisting of an anthracycline combined with cytarabine have a 65%–73% chance of attaining a complete remission (CR), while those over 60 years of age have a 38%–62% chance of a CR.2–,4,13–,15 Patients who fall into the “very elderly” category (80 years or older) can attain a CR with intensive therapy, but their chance of doing so is 30%, and only 7% of treated patients are alive at one year. Similar numbers hold for patients 70 years of age or older.9 Moreover, long-term survival occurs in approximately 30% of younger adults (or 45% of those entering a CR), compared to only 5%–15% long-term DFS in adults over the age of 60 years.2–,4,15
Indications for Treatment
For 85%–95% of older AML patients, any therapy ultimately will be purely palliative. Treatment options range from supportive care (blood and platelet transfusions when needed, antibiotics to treat infections, and growth factor support) to low-dose chemotherapy (e.g., hydroxyurea or low-dose cytarabine) or investigational agents as part of clinical trials, and high-dose chemotherapy (anthracycline- or anthracenedione-based remission induction therapy). Two trials have randomized older patients to receive either immediate remission induction chemotherapy with an anthracycline-based regimen versus a less aggressive or palliative approach.16,17 Only one of these studies16 demonstrated a survival advantage for patients receiving induction chemotherapy (21 vs 11 weeks, P = 0.015), for a median survival time only 16 days longer than the median amount of time they spent hospitalized to receive therapy. Thus, the decision of whether or not to offer remission induction therapy (on the part of physicians) or to receive it (on the part of patients) is not straightforward.
In making this decision, older patients overestimate the potential benefit they may derive from intensive chemotherapy and may not recall all treatment options. One study18 found that 74% of older patients estimated their chance to be cured by remission induction therapy to be ≥ 50%, and almost 90% estimated their chance of being alive in one year to be ≥ 50%. In contradistinction, physicians caring for these patients estimated the chance of cure to be ≤ 10% almost 89% of the time. Nearly two-thirds of patients did not recall being offered treatments other than the one they chose, despite physician documentation of alternatives in all cases. Patients choosing to receive remission induction therapy spent 79% of their days during the first 6 weeks of the study period either hospitalized or being seen in clinic, compared to 14% of days for patients choosing less aggressive therapy or best supportive care. Thus, treatment decisions should be based on individual patient preferences after an informed discussion has taken place that incorporates risk estimates modified to a patient’s performance status, comorbidities, and leukemia-specific risk factors, without using absolute cutoffs for prognostic factors or chronological age. For example, an active, “younger” older adult with de novo AML and favorable cytogenetics should be presented with different prognostic information than a bed-bound septuagenarian with secondary disease and complex cytogenetics. Ideally, this discussion should include specifics about prognosis and treatment-related complications, and the potential impact therapy will have on a patient’s quality of life. When available and clinically appropriate, older patients should always be offered the opportunity to participate in clinical trials for up-front therapy.
Treatment Options
Remission induction therapy
As with younger AML patients, the backbone of remission induction in older adults consists of an anthracycline (daunorubicin or idarubicin) or anthracenedione (mitoxantrone) and cytosine arabinoside (Ara-C), a regimen that has not changed in over two decades. Typically, daunorubicin is given at a dose of 45 mg/m2/d × 3 days, or mitoxantrone or idarubicin are given at doses of 12 mg/m2/d × 3 days, in combination with Ara-C, which is administered as a continuous infusion at 100 or 200 mg/m2/d × 7 days (frequently referred to as 7+3 chemotherapy). While studies have compared different anthracyclines and anthracenediones, varied doses and schedules, and added additional agents with some improvement in CR rates, they have not demonstrated an improvement in overall survival (OS) rates (Table 6 ).2,5,13,15,19–,21 For example, a recent study from the ECOG randomized older AML patients to remission induction therapy with either daunorubicin, idarubicin, or mitoxantrone along with a standard dose of Ara-C.9 The outcome was not significantly different, with CR rates of 40%, 43%, and 46% and median survivals of 7.7, 7.5, and 7.2 months, respectively. Once a decision has been made to initiate intensive chemotherapy, it should not be delayed, as this may impact outcome.9,22
Hematopoietic growth factors
In the majority of AML patients, death results from bleeding or infectious complications. This is particularly true in older adults with AML. The utility of hematopoietic growth factors (HGF) for ameliorating the myelosuppressive complications of AML therapy in older adults has been studied extensively.3,14 These trials were also designed to determine whether or not HGF had detrimental effects due to inappropriate stimulation of leukemic cell proliferation and thus resistance, or whether they had beneficial effects in “priming” leukemic cells to proliferate prior to the administration of S-phase specific chemotherapy agents such as Ara-C.9,23 With the exception of one ECOG study that demonstrated a CR rate and overall survival benefit in patients randomized to the GM-CSF arm (compared to patients receiving no growth factor support), these trials found that while HGF are safe, reduce the duration of neutropenia (by a range of 2–6 days), and do not support leukemia cell proliferation, they also do not reliably improve the CR rate, the length of hospitalization, or the induction death rate or prolong survival.
Post-remission chemotherapy
No randomized trial has ever demonstrated that any amount of post-remission therapy in older AML patients provides better outcomes than no post-remission therapy. That being said, the only studies demonstrating that long-term DFS is possible in older AML patients have included remission induction and post-remission therapy. It is reasonable, then, to administer post-remission therapy consisting of a repeat of remission induction therapy, single-agent Ara-C, or 2 days of an anthracycline or anthracenedione (the same type of drug given at the same doses as with remission induction therapy) combined with 5 days of Ara-C, again given at the same dose as with remission induction therapy (frequently referred to as post-remission therapy). There does not appear to be any additional survival benefit attained from administering more than 1–2 cycles of post-remission therapy or in treating older AML patients with maintenance therapy. In the Medical Research Council (MRC) AML 11 trial, 371 patients who entered a complete remission following anthracycline- or anthracenedione-based remission induction therapy were randomized to receive either 1 cycle of daunorubicin, Ara-C, and thioguanine (DAT) consolidation therapy, or DAT along with 3 additional cycles of Ara-C–based consolidation therapy (for a total of 4 cycles of post-remission therapy).15 Of those randomized to the long consolidation course, 61% were able to complete all 4 cycles. Survival was similar at 5 years for patients randomized to the short and long consolidation arms.
Postremission bone marrow transplantation
An even more aggressive approach than induction therapy followed by consolidation consists of bone marrow transplantation. Nonmyeloablative allogeneic bone marrow transplants take advantage of a graft-versus-leukemia effect using a less-intensive preparative regimen with lower up-front mortality. The reduced TRM and ability to perform these transplants in the outpatient setting make them an appealing option for the older AML patient with few comorbidities and an adequate performance status. Preliminary studies that include older AML patients have demonstrated that durable complete remissions are attainable with this treatment, though with limited follow-up. One study of 19 patients with myeloid malignancies (17 of whom had advanced MDS or AML) and a median age of 64 years (range, 60–70 years) demonstrated a 68% survival at a median follow-up of 825 days following nonmyeloablative transplantation.24 These early data have prompted cooperative groups to explore the role of bone marrow transplantation in older AML patients in first CR.
Newer approaches
Remission induction therapy for older adults with AML is no panacea, with median and 5-year survival rates resembling those of patients with advanced lung cancer.1 It is thus reasonable to consider investigational agents for older AML patients as initial therapy, particularly those that may be associated with less TRM. Potential targets for antileukemia therapy include specific signaling molecules required for the maintenance of the leukemic state, such as tyrosine kinases; overexpression of bcl-2, an anti-apoptosis signal; DNA methylation, associated with suppression of regulatory genes and with disease progression; indirect pathways that maintain leukemogenesis, including angiogenesis and drug resistance; and investigational agents with mechanisms of action that differ from anthracyclines, anthracenediones, and Ara-C, such as nucleoside analogs, farnesyl transferase inhibitors (FTIs), and MDR modulators, alone or in combination with standard therapies.25–,29 These approaches are discussed in more detail in Section I. The efficacy of the FTI Zarnestra will be examined in newly diagnosed AML patients over the age of 70 years in the US cooperative group setting (SWOG 0432). One recent trial from the Dutch-Belgian Hemato-Onocology Cooperative Group (HOVON) randomized predominantly older patients, most of whom had a diagnosis of advanced MDS or AML, to Ara-C and G-CSF or the same regimen plus fludarabine for 2 cycles followed by Ara-C and daunorubicin for 1 cycle.30 This study is typical of many novel combination trials in older AML patients in that, while the CR rate was improved in AML patients receiving fludarabine (95% vs 71%, P = 0.046), survival was not impacted.
Supportive/palliative care
Intensive chemotherapy provides only marginal, if any, survival benefit to older AML patients, so non-intensive (or non-chemotherapy-based) approaches are reasonable. We use the phrase aggressive supportive care to emphasize that symptoms will be treated vigorously and to distinguish this modality from hospice. Blood and platelet transfusions should be administered to alleviate symptoms stemming from anemia and thrombocytopenia, and antibiotics started when appropriate. Low-dose chemotherapy should only be used in the setting of leukocytosis and/or associated symptoms. Any recommendations for the institution of neutropenic precautions (i.e., avoiding crowds, refraining from ingestions of raw foods) must be balanced with the lack of evidence supporting the benefit of these maneuvers and the impact such restrictions will have on a patient’s quality of life. Hospice services should be instituted within 6 months of anticipated demise. While some hospice organizations prohibit blood product transfusions, we consider these to be palliative in this population as they may result in improved quality of life in terminal cancer patient populations.
Selected clinical trials in acute myeloid leukemia (AML).
| Abbreviations: APL, acute promyelocytic leukemia; ATRA, all-trans retinoic acid; ara-C, cytosine arabinoside; 6MP, 6-mercaptopurine; AML, acute myeloid leukemia; PBSCT, peripheral blood stem cell transplantation; IL, interleukin; MDR, multidrug resistance; BMT, bone marrow transplantation; G-CSF, granulocyte colony-stimulating factor | |
| US Intergroup | APL: ATRA/daunorubicin/ara-C induction, followed by As203 + daunorubicin/ATRA × 2 vs daunorubicin/ ATRA × 2 consolidation, followed by ATRA/6MP/methotrexate vs ATRA × 1 yr for maintenance. |
| AML (age > 70 years and not a candidate for chemotherapy): Oral tipifarnib (R115777) at 2 doses and 2 schedules | |
| CALGB | AML (age > 60): dauno/ara-C vs dauno/ara-C/oblimersen AML (age < 60): dauno/ara-C/etoposide, followed by post-chemotherapy/PBSCT, followed by IL-2 vs observation |
| ECOG | AML (age > 60): dauno/ara-C ± MDR modulator (LY335979) |
| AML (age < 60): daunorubicin (45 mg/m2 × 3 d)/ara-C vs daunorubicin (90 mg/m2 × 3 d)/ara-C, followed by ± gemtuzumab ozogamicin (GO) prior to autoPBSCT (if no sib donor) | |
| SWOG | AML (age > 55): continuous infusion daunorubicin/ara-C ± cyclosporine A followed by assignment to mini-allo BMT (if HLA-matched sibling donor) |
| AML (age ≤ 55): daunorubicin/ara-C vs daunorubicin/ara-C + GO | |
| EORTC | AML (age > 60): ida/ara-C vs ida/ara-C/GO |
| AML (age < 60): ida/ara-C vs ida/high dose ara-C, followed by intensive consolidation/allogeneic BMT, followed by IL-2 | |
| HOVON | AML (age > 60): dauno (45 mg/m2)/ara-C vs dauno (90 mg/m2)/ara-C f/b intermediate dose ara-C, followed by GO × 4 vs observation |
| AML (age < 60): ida/ara-C ± G-CSF vs ida/high-dose ara-C ± G-CSF, followed by mitoxantrone/etoposide (good risk) or Mito/etop vs autoBMT or alloBMT (if sibling donor) | |
| MRC | APL: ATRA/dauno/high-dose ara-C/6-thioguanine (MRC) vs ATRA/ida (Spanish), followed by MRC or Spanish chemo ± GO |
| AML (age > 60): intensive chemo (dauno/ara-c at various doses) vs non-intensive chemo (hydroxyurea/low dose ara-C ± ATRA) | |
| AML (age < 60): dauno/high dose ara-C/6-thioguanine vs fludarabine/ara-C/G-CSF/ida +/- GO, followed by allo BMT (if sibling; and will be nonmyeloablative if > 50, ‘full’ if under 35 years old) | |
| Abbreviations: APL, acute promyelocytic leukemia; ATRA, all-trans retinoic acid; ara-C, cytosine arabinoside; 6MP, 6-mercaptopurine; AML, acute myeloid leukemia; PBSCT, peripheral blood stem cell transplantation; IL, interleukin; MDR, multidrug resistance; BMT, bone marrow transplantation; G-CSF, granulocyte colony-stimulating factor | |
| US Intergroup | APL: ATRA/daunorubicin/ara-C induction, followed by As203 + daunorubicin/ATRA × 2 vs daunorubicin/ ATRA × 2 consolidation, followed by ATRA/6MP/methotrexate vs ATRA × 1 yr for maintenance. |
| AML (age > 70 years and not a candidate for chemotherapy): Oral tipifarnib (R115777) at 2 doses and 2 schedules | |
| CALGB | AML (age > 60): dauno/ara-C vs dauno/ara-C/oblimersen AML (age < 60): dauno/ara-C/etoposide, followed by post-chemotherapy/PBSCT, followed by IL-2 vs observation |
| ECOG | AML (age > 60): dauno/ara-C ± MDR modulator (LY335979) |
| AML (age < 60): daunorubicin (45 mg/m2 × 3 d)/ara-C vs daunorubicin (90 mg/m2 × 3 d)/ara-C, followed by ± gemtuzumab ozogamicin (GO) prior to autoPBSCT (if no sib donor) | |
| SWOG | AML (age > 55): continuous infusion daunorubicin/ara-C ± cyclosporine A followed by assignment to mini-allo BMT (if HLA-matched sibling donor) |
| AML (age ≤ 55): daunorubicin/ara-C vs daunorubicin/ara-C + GO | |
| EORTC | AML (age > 60): ida/ara-C vs ida/ara-C/GO |
| AML (age < 60): ida/ara-C vs ida/high dose ara-C, followed by intensive consolidation/allogeneic BMT, followed by IL-2 | |
| HOVON | AML (age > 60): dauno (45 mg/m2)/ara-C vs dauno (90 mg/m2)/ara-C f/b intermediate dose ara-C, followed by GO × 4 vs observation |
| AML (age < 60): ida/ara-C ± G-CSF vs ida/high-dose ara-C ± G-CSF, followed by mitoxantrone/etoposide (good risk) or Mito/etop vs autoBMT or alloBMT (if sibling donor) | |
| MRC | APL: ATRA/dauno/high-dose ara-C/6-thioguanine (MRC) vs ATRA/ida (Spanish), followed by MRC or Spanish chemo ± GO |
| AML (age > 60): intensive chemo (dauno/ara-c at various doses) vs non-intensive chemo (hydroxyurea/low dose ara-C ± ATRA) | |
| AML (age < 60): dauno/high dose ara-C/6-thioguanine vs fludarabine/ara-C/G-CSF/ida +/- GO, followed by allo BMT (if sibling; and will be nonmyeloablative if > 50, ‘full’ if under 35 years old) | |
Categories of novel therapies for acute myeloid leukemia (AML).
| Abbreviations: IL, interleukin; GM-CSF, granulocyte-macrophage colony-stimulating factor |
| Drug-resistance modifiers |
| Proteosome inhibitors |
| Pro-apoptotic approaches |
| Signal transduction inhibitors |
| “RAS”-targeted (e.g., farnesyl transferase inhibitors) |
| Tyrosine kinase targeted (e.g., FLT3, c-kit) |
| Downstream signal inhibitors |
| Immunotherapeutic approaches |
| Antigens known |
| anti-CD33 |
| anti-GM-CSF receptor |
| Antigens unknown |
| stimulate immune system (IL-2, GM-CSF) |
| present tumor antigens effectively |
| dendritic cell fusion |
| transfer hematopoietic growth factor genes |
| Abbreviations: IL, interleukin; GM-CSF, granulocyte-macrophage colony-stimulating factor |
| Drug-resistance modifiers |
| Proteosome inhibitors |
| Pro-apoptotic approaches |
| Signal transduction inhibitors |
| “RAS”-targeted (e.g., farnesyl transferase inhibitors) |
| Tyrosine kinase targeted (e.g., FLT3, c-kit) |
| Downstream signal inhibitors |
| Immunotherapeutic approaches |
| Antigens known |
| anti-CD33 |
| anti-GM-CSF receptor |
| Antigens unknown |
| stimulate immune system (IL-2, GM-CSF) |
| present tumor antigens effectively |
| dendritic cell fusion |
| transfer hematopoietic growth factor genes |
FLT3 inhibitors in clinical development (modified from Wadleigh et al29 ).
| Tyrosine Kinase Inhibitor . | Class . | Receptor Inhibitor Activity† . | FLT3 IC50‡ . | Clinical Trials/ Comments . | Toxicity . |
|---|---|---|---|---|---|
| † Receptor inhibitor activity in descending order of potency | |||||
| ‡ FLT3 autophosphorylation in vitro 1 | |||||
| Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; MPD, myeloproliferative disease; MM, multiple myeloma | |||||
| PKC-412 | Benzoylstaurosporine | PKC PDGFR KDR KIT FLT3 ABL | 528 nM | Phase II: AML with/without FLT3-ITD In FLT 3 mut pts (n = 20), 35% significant reduction in blast count25 | Nausea, emesis, fatigue |
| CEP-701 | Indolocarbazole | FLT3 TRKA KDR PKC PDGFR EGFR | 2–3 nM | Phase II: AML with FLT3-ITD Several pts had reduced blast counts, autophosphorylation inhibited26 | Nausea, emesis, fatigue |
| MLN-518 | Piperazinyl quinazoline | KIT PDGFR FLT3 FMS | 170–220 nM | Phase I: AML/MDS with/without FLT3-ITD Phase II: AML with FLT3-ITD A few pts with FLT3 ITD treated at higher doses had biological response27 | Generalized weakness, fatigue, nausea and vomiting |
| SU5416 | Indolinone | FLT3 KDR KIT | 250 nM | Phase II: Refractory AML/MDS/MPD/MM Phase II: Refractory AML (c-KIT positive). No responses in FLT3 ITD pts.24 | Fatigue, nausea, sepsis and bone pain |
| Tyrosine Kinase Inhibitor . | Class . | Receptor Inhibitor Activity† . | FLT3 IC50‡ . | Clinical Trials/ Comments . | Toxicity . |
|---|---|---|---|---|---|
| † Receptor inhibitor activity in descending order of potency | |||||
| ‡ FLT3 autophosphorylation in vitro 1 | |||||
| Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; MPD, myeloproliferative disease; MM, multiple myeloma | |||||
| PKC-412 | Benzoylstaurosporine | PKC PDGFR KDR KIT FLT3 ABL | 528 nM | Phase II: AML with/without FLT3-ITD In FLT 3 mut pts (n = 20), 35% significant reduction in blast count25 | Nausea, emesis, fatigue |
| CEP-701 | Indolocarbazole | FLT3 TRKA KDR PKC PDGFR EGFR | 2–3 nM | Phase II: AML with FLT3-ITD Several pts had reduced blast counts, autophosphorylation inhibited26 | Nausea, emesis, fatigue |
| MLN-518 | Piperazinyl quinazoline | KIT PDGFR FLT3 FMS | 170–220 nM | Phase I: AML/MDS with/without FLT3-ITD Phase II: AML with FLT3-ITD A few pts with FLT3 ITD treated at higher doses had biological response27 | Generalized weakness, fatigue, nausea and vomiting |
| SU5416 | Indolinone | FLT3 KDR KIT | 250 nM | Phase II: Refractory AML/MDS/MPD/MM Phase II: Refractory AML (c-KIT positive). No responses in FLT3 ITD pts.24 | Fatigue, nausea, sepsis and bone pain |
Treatment outcomes and toxicity for autologous and allogeneic hematopoietic cell transplantation (HCT) for consolidation or salvage therapy.
| . | Autologous HCT . | Allo HCT (Sib) . | AlloHCT/MUD . | Reduced-Intensity AlloHCT + MUD . |
|---|---|---|---|---|
| * Because of high salvage rate and long-term sequelae, many would reserve autologous HCT for relapse for patients with favorable cytogenetics. | ||||
| ** Most of these patients represent patients with high grade myelodysplastic syndrome (MDS) in which an unrelated donor search was initiated prior to leukemic evolution. | ||||
| *** DFS interval will be 5 years unless otherwise noted | ||||
| Abbreviations: AML, acute myeloid leukemia; DFS, disease-free survival; TRM, treatment-related mortality; HCT, hematopoietic cell transplantation; MUD, matched unrelated donor; allo, allogeneic; CR, complete response | ||||
| Consolidation(CR1) | ||||
| Cytogenetic risk | ||||
| t(15;17) | No role | No role | No role | No role |
| t(8;21)inv(16) | DFS 60%–80%* TRM 4%–8% | DFS 65% TRM 18% = No Role | No role | No role |
| Intermediate | DFS 42%–55% TRM 4%–6% | DFS 48%–62% TRM 16%–20% | Insufficient data for nonmyeloablative | |
| Poor | DFS 18%–25% TRM 4%–8% | DFS 35%–45% TRM 18%–20% | 5-yr DFS 30%–40% TRM 30% | Reduced intensity DFS 50% for older AML CR1 at 2 yr |
| Salvage | ||||
| CR2 | DFS 30% overall DFS 60%–80% for t(15;17) | DFS 40% | Pediatric 40% Adult 5-yr DFS 30% TRM 30% | 2-yr DFS 40%–50%reduced intensity |
| Relapse | Not an option unless “back-up” product from CR1 available | DFS 20%–30% | Pediatric DFS 20% 10%–15% | 2-yr DFS 10%–30% Adult 5-yr DFS depending on the volume of residual disease |
| Induction Failure | No Role | DFS 30%–40% (3 yrs) (20% for untreated secondary AML) | DFS 20%–30%** | 1-yr DFS 15%–30% (sibling) |
| . | Autologous HCT . | Allo HCT (Sib) . | AlloHCT/MUD . | Reduced-Intensity AlloHCT + MUD . |
|---|---|---|---|---|
| * Because of high salvage rate and long-term sequelae, many would reserve autologous HCT for relapse for patients with favorable cytogenetics. | ||||
| ** Most of these patients represent patients with high grade myelodysplastic syndrome (MDS) in which an unrelated donor search was initiated prior to leukemic evolution. | ||||
| *** DFS interval will be 5 years unless otherwise noted | ||||
| Abbreviations: AML, acute myeloid leukemia; DFS, disease-free survival; TRM, treatment-related mortality; HCT, hematopoietic cell transplantation; MUD, matched unrelated donor; allo, allogeneic; CR, complete response | ||||
| Consolidation(CR1) | ||||
| Cytogenetic risk | ||||
| t(15;17) | No role | No role | No role | No role |
| t(8;21)inv(16) | DFS 60%–80%* TRM 4%–8% | DFS 65% TRM 18% = No Role | No role | No role |
| Intermediate | DFS 42%–55% TRM 4%–6% | DFS 48%–62% TRM 16%–20% | Insufficient data for nonmyeloablative | |
| Poor | DFS 18%–25% TRM 4%–8% | DFS 35%–45% TRM 18%–20% | 5-yr DFS 30%–40% TRM 30% | Reduced intensity DFS 50% for older AML CR1 at 2 yr |
| Salvage | ||||
| CR2 | DFS 30% overall DFS 60%–80% for t(15;17) | DFS 40% | Pediatric 40% Adult 5-yr DFS 30% TRM 30% | 2-yr DFS 40%–50%reduced intensity |
| Relapse | Not an option unless “back-up” product from CR1 available | DFS 20%–30% | Pediatric DFS 20% 10%–15% | 2-yr DFS 10%–30% Adult 5-yr DFS depending on the volume of residual disease |
| Induction Failure | No Role | DFS 30%–40% (3 yrs) (20% for untreated secondary AML) | DFS 20%–30%** | 1-yr DFS 15%–30% (sibling) |
Characteristics of older and younger adults with acute myeloid leukemia (AML).
| Characteristic . | Older AML PatientsA . | Younger AML PatientsA . |
|---|---|---|
| A In general, Older AML Patients are defined as ≥ 60 years of age; Younger AML Patients as < 60 years of age. | ||
| B New diagnoses, per 100,000 US citizens per year. Older/younger division occurs at 65 years. | ||
| C Percentages rounded to nearest whole number | ||
| D Rates following remission-induction therapy with an anthracycline- or anthracenedione-based regimen. | ||
| Population incidenceB | 17.6 | 1.8 |
| Favorable cytogeneticsC | ||
| t(8;21) | 2% | 9% |
| inv 16 or t(16;16) | 1–3% | 10% |
| t(15;17) | 4% | 6–12% |
| Unfavorable cytogeneticsC | ||
| −7 | 8–9% | 3% |
| +8 | 6–10% | 4% |
| Complex | 18% | 7% |
| MDR1 expression | 71% | 35% |
| Secondary AML | 24–56% | 8% |
| Treatment-related mortalityD | 25–30% | 5–10% |
| Complete remissionD | 38–62% | 65–73% |
| Long-term survivalC | 5–15% | 30% |
| Characteristic . | Older AML PatientsA . | Younger AML PatientsA . |
|---|---|---|
| A In general, Older AML Patients are defined as ≥ 60 years of age; Younger AML Patients as < 60 years of age. | ||
| B New diagnoses, per 100,000 US citizens per year. Older/younger division occurs at 65 years. | ||
| C Percentages rounded to nearest whole number | ||
| D Rates following remission-induction therapy with an anthracycline- or anthracenedione-based regimen. | ||
| Population incidenceB | 17.6 | 1.8 |
| Favorable cytogeneticsC | ||
| t(8;21) | 2% | 9% |
| inv 16 or t(16;16) | 1–3% | 10% |
| t(15;17) | 4% | 6–12% |
| Unfavorable cytogeneticsC | ||
| −7 | 8–9% | 3% |
| +8 | 6–10% | 4% |
| Complex | 18% | 7% |
| MDR1 expression | 71% | 35% |
| Secondary AML | 24–56% | 8% |
| Treatment-related mortalityD | 25–30% | 5–10% |
| Complete remissionD | 38–62% | 65–73% |
| Long-term survivalC | 5–15% | 30% |
Selected randomized studies defining remission induction therapy in older adults.
| Goal . | Remission Induction Agents . | Complete Remission . | Overall Survival . | Comments . |
|---|---|---|---|---|
| Abbreviations: M, mitoxantrone; A, Ara-C (cytosine arabinoside); D, daunorubicin; I, idarubicin; E, etoposide; T, thioguanine; P, PSC-833; GM-CSF, granulocyte macrophage colony stimulating factor; G-CSF, granulocyte colony stimulating factor; ND, not done | ||||
| 1 For comparison of DAT to ADE | ||||
| 2 For comparison of DAT to MAC | ||||
| 3 For comparison of ADE to MAC | ||||
| Compare Anthracyclines and Anthracenediones | ||||
| Lowenberg, 19982 | M (8 mg/m2/d) + A (100 mg/m2/d) vs. D (30 mg/m2/d) + A (100 mg/m2/d) | 47% vs. 38% P = 0.07 | 39 weeks (median) 9% (5 years) vs. 36 weeks (median) 6% (5 years) P = 0.23 | Early and post-induction death rates were similar for both arms. Patients receiving mitoxantrone had a significantly higher rate of severe infections (25.1% vs 18.6%) and a trend toward a longer duration of aplasia (22 vs 19 days). |
| AML Collaborative Group, 199819 | I (8–20 mg/m2/d) + A (100–200 mg/m2/d) vs. D (45–50 mg/m2/d) + A (100–200 mg/m2/d) | 51% vs. 46% P = ND | 33.6 weeks (median) vs. 29.9 weeks (median) P = 0.58 | Early induction failure tended to be higher with idarubicin, while late induction failure was lower. Myelosuppression was greater in patients receiving idarubicin. |
| Archimbaud, 199920 | I (8 mg/m2/d) + A (100 mg/m2/d) + E (100 mg/m2/d) vs. M (7 mg/m2/d) + A (100–200 mg/m2/d)+ E (100 mg/m2/d) | 45% vs. 50% P = 0.52 | 7 months (median) 21% (2 years) vs. 7 months (median) 21% (2 years) P = ND | No difference between groups in degree of myelosuppression or in early death rates. |
| Vary 7+3 Dose | ||||
| Buchner, 199721 | D (60 mg/m2/d) + A (100 mg/m2/d) vs. D (30 mg/m2/d) + A (100 mg/m2/d) | 54% vs. 42% P = 0.038 | 16% (5 years) vs. 10% (5 years) P = 0.11 | The 30 mg daunorubicin arm was closed prematurely due to higher response rates in the 60 mg arm, resulting in 42 patients receiving lower dose daunorubicin, and 130 patients receiving the higher dose. |
| Dillman, 199113 | D (45 mg/m2/d) + A (100 mg/m2/d) vs. D (45 mg/m2/d) + A (200 mg/m2/d) | 44% vs. 38% P = 0.68 | 11.0 weeks (median) vs. 9.6 weeks (median) P = 0.23 | This study has short overall survival times compared to other studies in this patient population. |
| Add Agents | ||||
| Goldstone, 200115 | D (50 mg/m2/d) + A (100 mg/m2 q 12 hours)+ T (100 mg/m2 q 12 hours) vs. D (50 mg/m2/d) + A (100 mg/m2 q 12 hours) + E (100 mg/m2/d) vs. M (12 mg/m2/d) + A (100 mg/m2/d | 62% vs. 50% P = 0.0021 vs. 55% P = 0.042 | 12% (5 years) vs. 8% (5 years) P = 0.021 vs. 10% (5 years) P = 0.1,2 0.23 | There were no significant differences in myelosuppression or other toxicities, neutrophils were slower to recover in the mitoxantrone arm. Patients receiving ADE had higher rates of induction death (26% compared to 16% for DAT and 17% for MAC) |
| Baer, 20025 | D (60 mg/m2/d) + A (100 mg/m2/d)+ E (100 mg/m2/d) vs. D (40 mg/m2/d) + A (100 mg/m2/d) + E (60 mg/m2/d) + P (10 mg/kg/d) | 46% vs. 39% P = 0.008 | 7 months (median) vs. 2 months (median) P = 0.48 | Because of concern about excessive mortality on the ADEP arm (25 deaths vs 12 on the ADE arm), it was closed early to to further accrual. Survival between the two arms was similar at one year. |
| Use Hematopoietic Growth Factors | ||||
| Stone, 19953 | D (45 mg/m2/d) + A (200 mg/m2/d) vs. D (45 mg/m2/d) + A (200 mg/m2/d) + GM-CSF (5 μg/kg/d) | 54% vs. 51% P = 0.61 | 10.8 months (median) vs. 8.4 months (median) P = 0.10 | Median duration of neutropenia was 15 days in the GM-CSF arm and 17 days in placebo arm (P = 0.02). The duration of hospitalization did not differ between the arms; nor did the rates of life-threatening infection, or persistent leukemia. |
| Godwin, 199814 | D (45 mg/m2/d) + A (200 mg/m2/d) vs. D (45 mg/m2/d) + A (200 mg/m2/d) + G-CSF | 50% vs. 41% P = 0.89 | 9 months (median) vs. 6 months (median) P = 0.71 | The duration of neutropenia was 15% shorter in the G-CSF arm compared to the placebo arm (P = 0.14). The duration of hospitalization did not differ between the arms; nor did the rates of life-threatening infection, or persistent leukemia. |
| Goal . | Remission Induction Agents . | Complete Remission . | Overall Survival . | Comments . |
|---|---|---|---|---|
| Abbreviations: M, mitoxantrone; A, Ara-C (cytosine arabinoside); D, daunorubicin; I, idarubicin; E, etoposide; T, thioguanine; P, PSC-833; GM-CSF, granulocyte macrophage colony stimulating factor; G-CSF, granulocyte colony stimulating factor; ND, not done | ||||
| 1 For comparison of DAT to ADE | ||||
| 2 For comparison of DAT to MAC | ||||
| 3 For comparison of ADE to MAC | ||||
| Compare Anthracyclines and Anthracenediones | ||||
| Lowenberg, 19982 | M (8 mg/m2/d) + A (100 mg/m2/d) vs. D (30 mg/m2/d) + A (100 mg/m2/d) | 47% vs. 38% P = 0.07 | 39 weeks (median) 9% (5 years) vs. 36 weeks (median) 6% (5 years) P = 0.23 | Early and post-induction death rates were similar for both arms. Patients receiving mitoxantrone had a significantly higher rate of severe infections (25.1% vs 18.6%) and a trend toward a longer duration of aplasia (22 vs 19 days). |
| AML Collaborative Group, 199819 | I (8–20 mg/m2/d) + A (100–200 mg/m2/d) vs. D (45–50 mg/m2/d) + A (100–200 mg/m2/d) | 51% vs. 46% P = ND | 33.6 weeks (median) vs. 29.9 weeks (median) P = 0.58 | Early induction failure tended to be higher with idarubicin, while late induction failure was lower. Myelosuppression was greater in patients receiving idarubicin. |
| Archimbaud, 199920 | I (8 mg/m2/d) + A (100 mg/m2/d) + E (100 mg/m2/d) vs. M (7 mg/m2/d) + A (100–200 mg/m2/d)+ E (100 mg/m2/d) | 45% vs. 50% P = 0.52 | 7 months (median) 21% (2 years) vs. 7 months (median) 21% (2 years) P = ND | No difference between groups in degree of myelosuppression or in early death rates. |
| Vary 7+3 Dose | ||||
| Buchner, 199721 | D (60 mg/m2/d) + A (100 mg/m2/d) vs. D (30 mg/m2/d) + A (100 mg/m2/d) | 54% vs. 42% P = 0.038 | 16% (5 years) vs. 10% (5 years) P = 0.11 | The 30 mg daunorubicin arm was closed prematurely due to higher response rates in the 60 mg arm, resulting in 42 patients receiving lower dose daunorubicin, and 130 patients receiving the higher dose. |
| Dillman, 199113 | D (45 mg/m2/d) + A (100 mg/m2/d) vs. D (45 mg/m2/d) + A (200 mg/m2/d) | 44% vs. 38% P = 0.68 | 11.0 weeks (median) vs. 9.6 weeks (median) P = 0.23 | This study has short overall survival times compared to other studies in this patient population. |
| Add Agents | ||||
| Goldstone, 200115 | D (50 mg/m2/d) + A (100 mg/m2 q 12 hours)+ T (100 mg/m2 q 12 hours) vs. D (50 mg/m2/d) + A (100 mg/m2 q 12 hours) + E (100 mg/m2/d) vs. M (12 mg/m2/d) + A (100 mg/m2/d | 62% vs. 50% P = 0.0021 vs. 55% P = 0.042 | 12% (5 years) vs. 8% (5 years) P = 0.021 vs. 10% (5 years) P = 0.1,2 0.23 | There were no significant differences in myelosuppression or other toxicities, neutrophils were slower to recover in the mitoxantrone arm. Patients receiving ADE had higher rates of induction death (26% compared to 16% for DAT and 17% for MAC) |
| Baer, 20025 | D (60 mg/m2/d) + A (100 mg/m2/d)+ E (100 mg/m2/d) vs. D (40 mg/m2/d) + A (100 mg/m2/d) + E (60 mg/m2/d) + P (10 mg/kg/d) | 46% vs. 39% P = 0.008 | 7 months (median) vs. 2 months (median) P = 0.48 | Because of concern about excessive mortality on the ADEP arm (25 deaths vs 12 on the ADE arm), it was closed early to to further accrual. Survival between the two arms was similar at one year. |
| Use Hematopoietic Growth Factors | ||||
| Stone, 19953 | D (45 mg/m2/d) + A (200 mg/m2/d) vs. D (45 mg/m2/d) + A (200 mg/m2/d) + GM-CSF (5 μg/kg/d) | 54% vs. 51% P = 0.61 | 10.8 months (median) vs. 8.4 months (median) P = 0.10 | Median duration of neutropenia was 15 days in the GM-CSF arm and 17 days in placebo arm (P = 0.02). The duration of hospitalization did not differ between the arms; nor did the rates of life-threatening infection, or persistent leukemia. |
| Godwin, 199814 | D (45 mg/m2/d) + A (200 mg/m2/d) vs. D (45 mg/m2/d) + A (200 mg/m2/d) + G-CSF | 50% vs. 41% P = 0.89 | 9 months (median) vs. 6 months (median) P = 0.71 | The duration of neutropenia was 15% shorter in the G-CSF arm compared to the placebo arm (P = 0.14). The duration of hospitalization did not differ between the arms; nor did the rates of life-threatening infection, or persistent leukemia. |
Disease-free survival (DFS) from complete response (CR) for four prognostic cytogenetic risk groups—EORTC/GIMEMA trial comparing sibling allogeneic versus autologous HCT for consolidation.
The cytogenetic risk groups are good risk (1A), normal (1B) and bad/very bad risk (1C). 1D captures the data for patients in whom cytogenetics were unknown. Reprinted with permission from Blood. 2003;102,1237.
Disease-free survival (DFS) from complete response (CR) for four prognostic cytogenetic risk groups—EORTC/GIMEMA trial comparing sibling allogeneic versus autologous HCT for consolidation.
The cytogenetic risk groups are good risk (1A), normal (1B) and bad/very bad risk (1C). 1D captures the data for patients in whom cytogenetics were unknown. Reprinted with permission from Blood. 2003;102,1237.