Abstract
Remarkable progress has been made in the past decade in the treatment and in the understanding of the biology of childhood lymphoid and myeloid leukemias. With contemporary improved risk assessment, chemotherapy, hematopoietic stem cell transplantation and supportive care, approximately 80% of children with newly diagnosed acute lymphoblastic leukemia and 50% of those with myeloid neoplasm can be cured to date. Current emphasis is placed not only on increased cure rate but also on improved quality of life.
In Section I, Dr. Ching-Hon Pui describes certain clinical and biologic features that still have prognostic and therapeutic relevance in the context of contemporary treatment programs. He emphasizes that treatment failure in some patients is not due to intrinsic drug resistance of leukemic cells but is rather caused by suboptimal drug dosing due to host compliance, pharmacodynamics, and pharmacogenetics. Hence, measurement of minimal residual disease, which accounts for both the genetic (primary and secondary) features of leukemic lymphoblasts and pharmacogenomic variables of the host, is the most reliable prognostic indicator. Finally, he contends that with optimal risk-directed systemic and intrathecal therapy, cranial irradiation may be omitted in all patients, regardless of the presenting features.
In Section II, Dr. Martin Schrappe performs detailed analyses of the prognostic impact of presenting age, leukocyte count, sex, immunophenotype, genetic abnormality, early treatment response, and in vitro drug sensitivity/resistance in childhood acute lymphoblastic leukemia, based on the large database of the Berlin-Frankfurt-Münster consortium. He also succinctly summarizes the important treatment components resulting in the improved outcome of children and young adolescents with this disease. He describes the treatment approach that led to the improved outcome of adolescent patients, a finding that may be applied to young adults in the second and third decade of life. Finally, he believes that treatment reduction under well-controlled clinical trials is feasible in a subgroup of patients with excellent early treatment response as evidenced by minimal residual disease measurement during induction and consolidation therapy.
In Section III, Dr. Raul Ribeiro describes distinct morphologic and genetic subtypes of acute myeloid leukemia. The finding of essentially identical gene expression profiling by DNA microarray in certain specific genetic subtypes of childhood and adult acute myeloid leukemia suggests a shared leukemogenesis. He then describes the principles of treatment as well as the efficacy and toxicity of various forms of postremission therapy, emphasizing the need of tailoring therapy to both the disease and the age of the patient. Early results suggest that minimal residual disease measurement can also improve the risk assessment in acute myeloid leukemia, and that cranial irradiation can be omitted even in those with central-nervous-system leukemia at diagnosis.
In Section IV, Dr. Charlotte Niemeyer describes a new classification of myelodysplastic and myeloproliferative diseases in childhood, which has greatly facilitated the diagnosis of myelodysplastic syndromes and juvenile myelomonocytic leukemia. The recent discovery of somatic mutations in PTPN11 has improved the understanding of the pathobiology and the diagnosis of juvenile myelomonocytic leukemia. Together with the findings of mutations in RAS and NF1 in the other patients, she suggests that pathological activation of RAS-dependent pathways plays a central role in the leukemogenesis of this disease. She then describes the various treatment approaches for both juvenile myelomonocytic leukemia and myelodysplastic syndromes in the US and Europe, emphasizing the differences between childhood and adult cases for the latter group of diseases. She also raises some controversial issues regarding treatment that will require well-controlled international clinical trials to address.
I. Risk Assessment in Childhood and Adolescent Acute Lymphoblastic Leukemia
Ching-Hon Pui, MD*
Department of Hematology-Oncology, St. Jude Children’s Research Hospital, 332 N. Lauderdale, Memphis TN 38105-2794. This work was supported by grants (CA-21765, CA-51001, CA-36401, CA-78224, CA-71907, CA-60419, CA-71970, GM-61393, and GM-61374) from the National Institutes of Health, by a Center of Excellence grant from the State of Tennessee, and by the American Lebanese Syrian Associated Charities. Dr. Pui is the American Cancer Society F. M. Kirby Clinical Research Professor.
As the cure rate of acute lymphoblastic leukemia (ALL) in children and adolescents has reached 80%,1,2 stringent risk assessment has become an important prerequisite in the selection of therapy, ensuring that patients are neither overtreated nor undertreated. Although there is general agreement that certain clinical features, genetic abnormalities of leukemic cells, pharmacodynamics, pharmacogenetics, and early treatment response have important prognostic and therapeutic implications, no consensus on risk criteria or terminology has been reached. On the basis of prognostic factors, patients in most studies are assigned to one of three risk groups (e.g., standard-, high-, or very high–risk groups). Although remission induction treatment may be the same for all risk groups in some clinical trials, the intensity of postremission treatment differs according to the risk groups in all studies. Patients at high risk are typically treated with intensified postremission chemotherapy, and patients at very high risk are considered as candidates for allogeneic hematopoietic stem cell transplantation. The Children’s Oncology Group (United States) has advocated the inclusion of a fourth category (low risk) to permit those with a very low risk of relapse to receive treatment at reduced intensity.3 This review will briefly describe risk factors used for classification by various study groups or institutions. It should be noted that treatment efficacy is the most important determinant and can abolish the clinical significance of most, if at all, prognostic factors.
Clinical Factors
The prognostic impact of clinical features differs between B cell precursor and T cell ALL (Table 1 ). For patients with B cell precursor ALL, an age of 1 to 9 years and low leukocyte count (< 50 × 109/L) confer a favorable prognosis; patients with both features are generally considered to have standard-risk ALL.4 With improved treatment, the prognostic impact of these two factors has been attenuated substantially. Indeed, excellent treatment outcome has been reported for adolescents treated in a Children’s Cancer Group (CCG) study with “augmented Berlin-Frankfurt-Münster (BFM)” therapy.5 A recent update of the study indicated that this therapy was more beneficial for those younger than 13 years (J Nachman, personal communication). T cell ALL is generally considered to be high or very high risk, depending on the patients’ response to induction therapy. For T cell ALL, age and leukocyte count have little clinical significance; however, as discussed later, a leukocyte count > 100 × 109/L at diagnosis is an indication for more intensive central nervous system (CNS)-directed therapy.
Male sex has generally been associated with a poor prognosis.4 In an attempt to reduce the difference in treatment outcome between the two sexes, the duration of continuation treatment has been increased for boys but not for girls in some trials.6 However, the adverse prognostic impact of male sex has been abolished in clinical trials in which the overall 5-year event-free survival (EFS) rate is 80% or more.1,2 Black patients have continued to fare more poorly than white patients in recent US multi-institutional clinical trials. Notwithstanding this finding, our single-institution study showed that with equal access to effective treatment, black patients can have cure rates as great as those of white patients.2
Genetic Features of Leukemic Cells
The diverse clinical outcomes associated with the various subtypes of ALL can be attributed primarily to drug sensitivity or resistance of leukemic blasts that harbor specific genetic abnormalities.7 Favorable genetic abnormalities associated with B cell precursor ALL include hyperdiploidy (> 50 chromosomes) and the TEL-AML1 fusion. The exquisite sensitivity of hyperdiploid blasts to chemotherapy is correlated with their propensity to undergo spontaneous apoptosis when cultured in vitro and to accumulate high concentrations of methotrexate and its active polyglutamate metabolites after treatment.4 Hyperdiploid blasts typically have 3 to 4 copies of chromosome 21, which harbors a gene encoding the reduced folate transporter.7 The increased expression of this transporter, which is due to the increase in gene dosage, may account in part for the high level of accumulation of methotrexate polyglutamates in hyperdiploid blasts. ALL cells that express TEL-AML1 are highly sensitive to asparaginase in vitro for reasons that remain unclear.7 The favorable outcome associated with this genotype was observed only in clinical trials featuring intensive chemotherapy, especially asparaginase.4,7 Studies of the US Children’s Oncology Group have shown that trisomies 4, 10, and 17 are associated with an independently favorable prognosis; on the other hand, the U.K. Medical Research Council has found that trisomies 4 and 18 are indicators of a favorable prognosis.3 The underlying basis for these findings remains elusive. Although pre-B cell ALL with the t(1;19)/E2A-PBX1 fusion was associated with a poor prognosis when treatment consisted of standard antimetabolite-based regimens, intensive contemporary treatment has made it one of the most favorable genetic subtypes of childhood ALL, with the long-term EFS estimate now approaching 90%.7
Patients with the Philadelphia chromosome or the t(4;11)/MLL-AF4 fusion are considered to have very high risk ALL. There is a marked influence of age on the prognosis of patients with these genetic subtypes. In Philadelphia chromosome–positive ALL, the prognosis is generally dismal for adolescents, but is relatively favorable in children 1 to 9 years old with a low leukocyte count at diagnosis.8 In ALL with the MLL-AF4 fusion, infants younger than 1 year fare considerably worse than children 1 year of age or older.9 The basis for this age-related difference in prognosis may be related to secondary genetic events, the developmental stage of the targeted cell undergoing malignant transformation, the pharmacokinetic features of the patient, or a combination of these three factors.
Recently, gene expression profiling studies showed that almost all cases of T cell ALL can be grouped on the basis of involvement of one or more specific oncogenes: LYL1 plus LMO2, HOX11, TAL1 plus either LMO1 or LMO2, HOX11L2, and MLL-ENL.10 The pattern of gene expression in LYL1 plus LMO2–positive cells resembles that seen in cells during the pro-T stage of development; that of HOX11-positive cells resembles the pattern seen during the early cortical thymocyte stage, and that of cells positive for TAL1 plus either LMO1 or LMO2 resembles the pattern seen during the late thymocyte stage.10 A highly favorable prognosis was noted for patients with the HOX11 or MLL-ENL subtype.10 The prognostic significance of the HOX11L2 subtype depends on the type of treatment administered.7
Oncogenic events triggered by chromosomal rearrangements are not sufficient by themselves to cause leukemia and must cooperate with additional mutations that alter the normal proliferation and survival of cells to induce leukemia. Genes commonly involved in secondary mutations are FLT-3, which encodes a receptor tyrosine kinase important for the normal development of hematopoietic stem cells,11 and those involved in the interrelated pathways controlled by the tumor suppressor retinoblastoma protein (e.g., p16INK4a, p14ARF) and p53.7 Overexpression of HDM2 (a protein that binds to p53 and induces its degradation), mutations in p53, and silencing of the p53 transcriptional target p21CIP1 have each been associated with a dismal prognosis.7 In a genome-wide analysis of gene expression in drug-sensitive versus drug-resistant leukemic lymphoblasts, we recently identified 123 discriminating genes whose differential expression conferred drug resistance and inferior treatment outcome in two independent patient cohorts.12 This finding provides new insights into the basis of treatment failure and points to novel targets for developing strategies to overcome drug resistance.
Pharmacodynamic and Pharmacogenetic Factors
Host factors can exert a crucial influence on the effectiveness of treatment. It is well recognized that wide interpatient differences in drug disposition and pharmacologic effects can affect treatment outcome.13 For example, low systemic exposure to methotrexate and low-dose intensity of mercaptopurine have each been associated with poor treatment outcome.13 This finding indicates that treatment is unsuccessful in some patients because of inadequate doses of drugs, not because of drug resistance.
Recent studies have linked the genetic polymorphisms of several drug-metabolizing enzymes, transporters, receptors, or drug targets with treatment outcome.13,14 A homozygous or heterozygous deficiency of thiopurine methyltransferase, the enzyme that catalyzes the S-methylation (inactivation) of mercaptopurine, was associated with improved leukemia control but increased risk of therapy-related second neoplasms.13,14 Inactivating polymorphisms of several detoxifying enzymes (e.g., glutathione S-transferase, reduced nicotinamide adenine dinucleotide phosphate: quinone oxidoreductase) were associated with increased drug toxicity but superior leukemia control, presumably because of an increased dose-intensity of chemotherapy.13,14 On the other hand, homozygosity for a triple-tandem-repeat polymorphism of thymidylate synthase, an important target of methotrexate, was associated with increased enzyme expression and inferior treatment outcome;15 conceivably, patients with such mutations require an increased dose of methotrexate for optimal treatment response. Additional studies are needed to confirm these associations. In all likelihood, some of the associations are treatment-dependent.
Minimal Residual Leukemia
Because response to therapy is determined by many factors including the genetic features of leukemic lymphoblasts and pharmacogenetic variables of the host, measurement of response to therapy has consistently shown independent prognostic significance.4 Measurement of minimal residual disease (MRD) in bone marrow (i.e., flow cytometric detection of leukemic cell–associated immunophenotype or polymerase chain reaction [PCR] analysis of clonal antigen-receptor gene rearrangements) is much more specific and sensitive than morphologic examination of blast cells.4 In fact, in our recent study MRD determination rendered many other previously identified prognostic factors obsolete with the exception of the t(4;11)/MLL-AF4 fusion and the Philadelphia chromosome.16 Patients who achieve immunologic or molecular remission, which is defined as leukemic involvement of < 0.01% nucleated bone marrow cells on completion of induction therapy, have an outcome superior to that of patients who do not.4 Patients who are in morphologic remission but have an MRD level of 1% or more at the end of 6-week remission induction therapy have a very high relapse risk, which is similar to that of patients who require extended induction therapy to achieve morphologic remission, and may be considered candidates for allogeneic transplantation. Because tandem application of flow cytometry and PCR can be used to study MRD in almost all patients, the measurement of MRD is now applied as a part of the risk classification in the front-line clinical trial at St. Jude Children’s Research Hospital.17 An ongoing study by the Berlin-Frankfurt-Münster Group and the Italian Association of Pediatric Hematology and Oncology is testing whether the intensity of postremission therapy can be safely reduced for standard-risk patients who attain molecular remission. Although there are no data showing that intensification of therapy improved outcome of patients with high level of MRD at the end of induction, the results of “augmented Berlin-Frankfurt-Münster” therapy of Children’s Cancer Group would support this approach. In that study, intensification therapy significantly benefited patients with high-risk ALL and a slow response to initial induction therapy based on morphologic examination.5 Additional studies are needed to determine if MRD results are related to specific gene expression profile.
Factors in Determining Intensity of CNS-Directed Therapy
All patients require CNS-directed therapy to prevent relapse. Although cranial irradiation is the most effective CNS-directed therapy, it can cause neurocognitive impairment, endocrinopathy, and cancer. Long-term survivors who received cranial irradiation have a 20% cumulative risk of second neoplasm at 30 years after initial treatment and continue to be at risk beyond that time point.18 Consequently, irradiated patients have a mortality rate that is greater than that of the general population; they also have higher-than-average unemployment rates. Most contemporary clinical trials have now limited the use of cranial irradiation to 10% to 20% of patients at particularly high risk of CNS relapse and reduced the radiation dose to 12 to 18 Gy.
Factors associated with an increased risk of CNS relapse include high-risk genetic features (e.g., the presence of a Philadelphia chromosome), a large leukemic cell burden (e.g., leukocyte count > 100 × 109/L), T cell ALL, male sex, and the presence of any amount of leukemic cells in cerebrospinal fluid (even leukemic cells introduced via traumatic lumbar puncture at diagnosis).19 In our recently completed clinical trial, early intensification of intrathecal therapy for patients with high-risk or very high-risk ALL, and the addition of cranial irradiation for 12% of patients at particularly high risk of CNS relapse (i.e., those with CNS-3 status or T cell ALL plus a leukocyte count > 100 × 109/L) reduced the CNS relapse rate to approximately 1% and boosted the 5-year EFS estimate to 81%.16 We contend that with the precise assessment of risk factors, the prevention of traumatic lumbar puncture (e.g., prevention by means of platelet transfusion for patients with thrombocytopenia and circulating leukemic cells, deep sedation of patients during the procedure, and the execution of the procedure by the most experienced clinicians),20 and the use of optimal intrathecal and systemic therapy, one could safely omit the use of cranial irradiation for all patients. This approach is now being tested in our ongoing clinical trial.
II. Treatment Strategies for Childhood and Adolescent ALL
Martin Schrappe, MD, PhD*
Chairman, ALL-BFM Study Group, Department of Pediatric Hematology and Oncology, Hannover Medical School, Carl-Neuberg-Str. 1, 30625 Hannover, Germany Supported by grants from the Deutsche Krebshilfe (Bonn, Germany). Acknowledgment: The continuous support of all members of the study group and in particular from the reference laboratories (J. Harbott, molecular genetics; W.-D. Ludwig, immunophenotyping) is highly appreciated. In particular, Alfred Reiter (MD, PhD) and Helmut Gradner (MD, PhD) have made major contributions. In the study center, the contributions of Anja Moericke (MD), Martin Zimmermann (PhD), Martin Stanulla (MD, MSc), André Schrauder (MD), Gunnar Cario (MD), Rita Beier (MD), Britta Buerger (MD), and Edelgard Odenwald has been most valuable for the ALL-BFM group. Karl Welte (MD, PhD) has always been most supportive of the group’s activities. Hansjoerg Riehm (MD, PhD) has pioneered the concept and has generated a large number of innovative concepts in the conduct of the ALL-BFM trials and has always stimulated important discussions.
Successful treatment of childhood ALL has become reality for 80% of affected children if modern polychemotherapy and adequate supportive care can be provided. The treatment burden remains high despite some adjustments such as elimination or reduction of CNS directed radiotherapy but precise assessment of early response to therapy is providing the tools for better risk-adapted treatment intensity. Large differences in treatment outcome for age subgroups of ALL have been described.1–,5 They often correspond to the prevailing cytogenetic subtypes in such subgroups of ALL.6 Large heterogeneity with regard to treatment response and subsequent outcome was demonstrated for all subgroups of childhood ALL, even within well-defined cytogenetic subsets such as Philadelphia chromosome positive ALL.7 Due to the heterogenous response and outcome within the pediatric ALL population, interest has grown to identify the potential biological and clinical reasons which may also apply to differences in treatment results between the second and third (and subsequent) decade(s) of life.8,9
Characteristics of Age Subgroups in Pediatric ALL Patients
Distribution of key prognostic factors such as age, initial white blood cell count (WBC), CNS involvement, and gender distribution is different by immunophenotypic subtype.10 Characteristic diagnostic findings in 3076 patients (age 0–18 years) enrolled in the two subsequent trials ALL-BFM 86 and 9011,12 are summarized in Table 2 , demonstrating the large differences not only between age subgroups but also between precursor-B cell (p-Bc) ALL and T cell ALL. There is a predominance of male patients in T cell ALL, in particular, beyond 10 years of age. By contrast, among p-Bc ALL the slight predominance of male patients is homogenously found in all age subgroups, except for infants (< 1 year).
Initial Determinants of Treatment Outcome in Age Subgroups
Gender
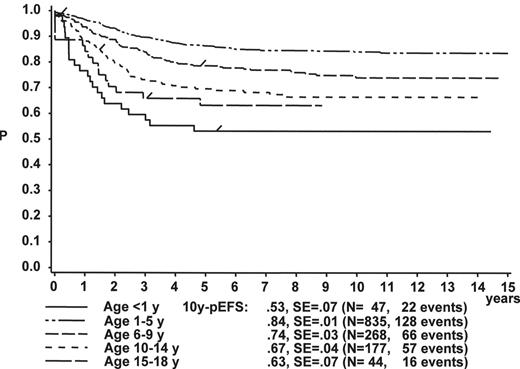
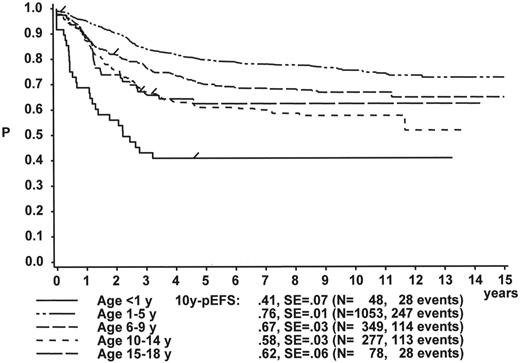
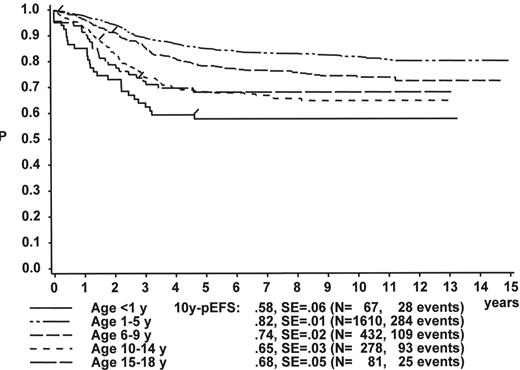
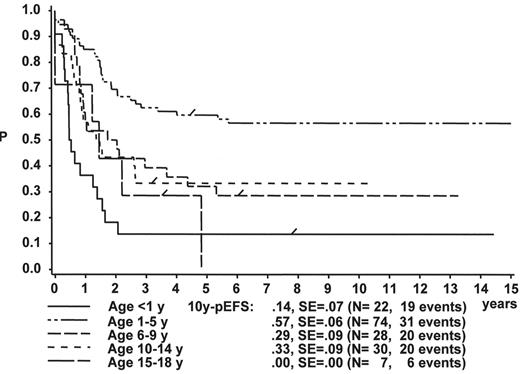
Figure 1 illustrates the large differences in long-term treatment results between age groups, but also indicates that in all age groups the probability for EFS at 10 years (10y-pEFS) for female patients (Figure 1A ) is approximately 10% higher than for male patients (Figure 1B ) except for patients 15–18 years of age. This coincides with early observations made by a consortium of adult hematologists when intensive chemotherapy derived from a pediatric protocol had been introduced for treatment of adult ALL: No difference in outcome was found according to gender. There, age (favorable if less than 35 years) and WBC (favorable if < CR achieved within 4 weeks) were found to be prognostically relevant.13
WBC
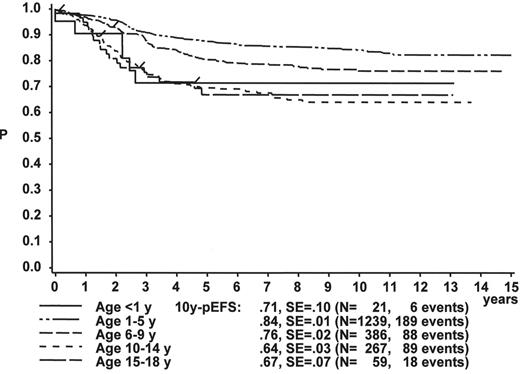
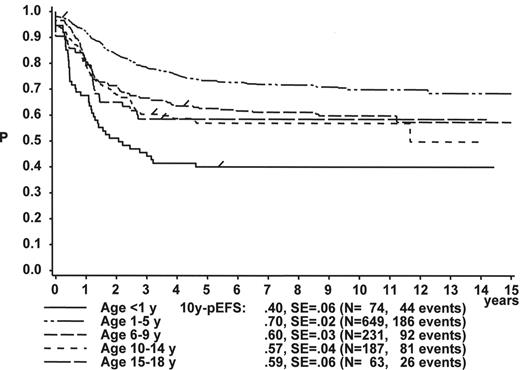
The initial WBC is a robust prognostic marker, but if stratified by age it partly loses its prognostic impact. Figure 2 demonstrates that even the prognostically most adverse group, which comprises patients < 1 year of age, can have an outcome that is statistically not different from the other age subgroups, if only patients with WBC of less than 20,000 are analyzed (Figure 2A ). If only patients with WBC ≥ 20,000 are analyzed, patients 1–5 years of age have by far the most favorable, and infants (< 1 year) have the worst treatment result (Figure 2B ). This is a reflection of the underlying predominant cytogenetic feature (TEL/AML-1 in the 1- to 5-year-old patients, MLL rearrangements in the infants) and of the associated in vitro and in vivo responsiveness.14– 16
Immunophenotype
Age-dependence of treatment outcome is evident for p-Bc ALL: By far the most favorable subset in p-Bc ALL is the group of patients 1–5 years old, in which 80% long-term EFS can be achieved. The outcome for patients 10–14 years of age with p-Bc ALL is not significantly different from that of the children older than 14 years, but worse than that of every other subgroup. Interestingly, no differences in EFS are found for the two age groups above 10 years of age for p-Bc ALL and T cell ALL, confirming earlier data.17 As shown in Table 2B , and published previously, the treatment outcome in T cell ALL is not different by age.10
Genetics
Structural and numerical chromosomal aberrations as much as the associated or other (cytogenetically cryptic) molecular genetic changes are diagnosed (in part) with age-dependent frequency.18,19 Recent refinements in technology have made it feasible to detect not only more but also subtle genetic aberrations.20–,22 Relevant genetic signatures and pathways are being detected by the use of microarray based gene expression profiling. Some investigations have shed new light on well-defined genetic subgroups of ALL, or have looked at treatment related genetic changes.23– 27
Some genetic aberrations in childhood ALL have prognostic impact that varies by age. Hyperdiploidy (> 50 chromosomes) is the single most frequent cytogenetic aberration in childhood ALL, comprising one quarter of all cases, but it is only a small subgroup in adult ALL (6% of all cases).6 By contrast, hypodiploidy (< 46 chromosomes) is found in approximately 5% of both pediatric and adult ALL patients.28 A good example for age-dependent incidence of genetic aberrations is the most frequent aberration in pediatric ALL, which is t(12;21) or its molecular counterpart TEL/AML1: It is predominant in precursor-B-cell ALL in patients 2–5y of age but very rare in adolescents or adults.29,30 It is associated with an excellent prognosis (long-term EFS at approx. 85%) and some particularities regarding drug uptake and chemosensitivity.14,31 After large heterogeneity with regard to treatment response and subsequent outcome was found for Philadelphia chromosome positive pediatric ALL,7 a large international metaanalysis revealed that there is an age-dependent effect on outcome in this cohort of ALL.32 In another, rather rare subgroup of pediatric ALL which comprises all patients with 11q23 rearrangements, an age-dependent prognostic impact of genetic subtypes was seen, but within some genetic subgroups also early treatment response was identified to be of prognostic significance.33
In the group of patients with p-Bc ALL who are both TEL/AML1 and BCR/ABL negative, patients above 10 years of age do significantly worse than younger children (data from trials ALL-BFM 90 and 95): 10y-pEFS for patients at 10–14y of age is 52.5% (SE 9.2%), 44.4% (SE 8.9%) for 15–18y old patients, but 72.1% (SE 3.7%) and 81.9% (SE 1.8%) for 6–9y and 1–5y patients, respectively.
Assessment of early treatment response
The persistence of blasts in peripheral blood (PB) or bone marrow (BM) after the first 7 or 14 days of treatment, even more specifically the subsequent failure to enter complete remission (CR), is highly predictive of disease recurrence.34–,36Table 2 demonstrates the large heterogeneity with regard to age subgroups and early response when the so-called prednisone response is used to assess the in vivo sensitivity of the disease. When compared to p-Bc ALL, a 3- to 7-fold higher proportion of T cell ALL patients (depending on age) is found to have more than 1000 blasts per μL after the 7 days prednisone prephase and one dose of intrathecal methotrexate (IT MTX) on day 1. The rate of resistance to induction is also slightly higher in T-ALL. While there appears to be only a small trend that the induction failure rate is higher in older children in particular with p-Bc ALL, data from adult series suggest that response to induction therapy decreases by age and varies by subtype: Hoelzer et al early on reported CR rates of 81% for patients up to 35 years of age, but of only 68% for patients older than 35 years. In that series, p-Bc ALL had lower CR rates than T cell ALL.13 A series of adult patients with pro-B ALL demonstrated CR rates of 75%.37 More recently, even higher CR rates were reported, but it remains to be seen if this translates also into a larger proportion of adult ALL patients staying in CR.38
Cytomorphological evaluation of early response certainly has some limitations: If the initial leukemic cell count in peripheral blood is very low it may be impossible to assess the prednisone or any other type of early response. It was possible, however, to demonstrate that among patients with < 1000 blasts on day 8 of therapy those with initially less than 1,000 blasts have a better prognosis than the remaining patients.39 In addition, combining both response evaluation in PB on day 8 and in the BM on day 15 of therapy characterized mainly two new subgroups: Patients with prednisone good response (PRED-GR) but M3 BM on day 15 did even worse than prednisone poor response (PRED-PR) patients with M1 BM d15; within the group of PRED-PR a M3 BM d15 was highly predictive of relapse.40 Determination of blasts in the BM can be difficult or even impossible if the cellularity is very low. Persisting lack of hematopoetic recovery in the BM after induction therapy even without the presence of leukemic blasts may indicate an early relapse. In contrast to AML, only absence of blasts (< 5%) is required for diagnosis of CR in the BM.
In Vivo Resistance to Therapy: Prognostic Implications for Heterogeneous Treatment Results in Children and Adolescents
Early in vivo treatment resistance is probably the most important prognostic factor in childhood ALL. 6 It reflects drug resistance that is a genetically imprinted feature of the leukemic cell, but it also comprises all mechanisms of the host related to uptake, metabolism and excretion of the antileukemic drugs. Host factors are also responsible for the side effects of treatment that can have an indirect effect on the dose intensity. For some genes, the impact of such genetic polymorphisms responsible for variation in treatment response or treatment tolerance has been clearly determined.41–,43 It has not yet been determined why a higher proportion of adolescent patients (as compared to the younger patients) have an intrinsic treatment resistance if determined by early blast reduction in PB or BM. The data on age-dependent outcome in pediatric Ph+ ALL suggest that secondary (induced?) genetic events having occurred during growth and maturation may contribute to this observation, not so much the treatment performed.32
Most study groups use one or the other early in vivo response analysis for stratification, usually in combination with relevant initial findings such as age, WBC, immunophenotype, and cytogenetics.34 In vitro evaluation of resistance at least for some essential antileukemic drugs has certainly also robust predictive power.44 An age-dependent resistance profile has been shown for most antileukemic agents.45 Usually, in vitro drug resistance correlates well with in vivo resistance to antileukemic agents.46,47 No study has, however, demonstrated that adaptation of clinical therapy to such an in vitro resistance profile allowed to overcome the inferior outcome of older patients. Certain resistance profiles in genetic subtypes of ALL may be related to corresponding functional genetic changes.25,48
Monitoring of Therapy: A Guide for Risk-Adapted Treatment Intensity?
One limitation of all available initial (diagnostic) or early (response) risk parameters is the lack of their predictive value: The majority of relapses occur in patients who have an average or even above average prognosis based on their presenting features and their response to early therapy.4 In trial ALL-BFM 90, 90% of the patients have been in the standard risk and intermediate risk groups. Despite the favorable outcome of these two risk groups, two thirds of all recurrences were registered there. Therefore, a more precise assessment of response to chemotherapy was mandatory. This appears feasible by now as the technologies for the detection of MRD have improved and standardized.49– 53
The first ALL trials in which MRD was prospectively evaluated tried to determine the prognostic impact of MRD detection at consecutive time points in ALL therapy. Information on MRD in age subgroups is limited. Comparisons between trials are difficult due to the different techniques applied and due to the limited information on quantifiable range and levels of detection. Also time and composition of therapy up to postinduction remission control is different. As shown in Table 3 there is a trend that a higher proportion of older children (≥ 10 years) is not yet MRD negative after induction therapy, as compared to the younger children. There is a large variation in response to induction therapy not only by age, but also between p-Bc ALL and T-cell ALL.54,55 This implies, however, that certain levels of MRD derived from different patient subsets after induction or at some later time point do not have the same prognostic implications. This was clearly demonstrated in a comparison of homogenously treated patients with p-BC and T cell ALL.56 Currently several pediatric and adult ALL trials utilize monitoring of MRD for treatment adjustments, mainly in order to identify patients at increased risk for relapse. Prospective monitoring of MRD revealed, however, that there are also subgroups of pediatric ALL in which well-controlled treatment reduction may be feasible.52,57
Treatment Approaches in Well-Defined ALL Subsets
Treatment itself has a major impact on outcome of all ALL subgroups. Compared to results from the 1980s contemporary treatment programs have shown improved results in children diagnosed in the second decade of life. Table 4 summarizes recent data from large cooperative groups. The underlying mechanisms for inferior outcome in particular for p-Bc ALL in adolescents is not known.58 In part, it may be related to a higher rate of severe complications, even though the data from the two large BFM trials do not provide a clear picture in this regard: As shown in Table 2 , a higher rate of early fatal complications has not been observed. Also the treatment related mortality is not much different by age. There is certainly a higher number of recurrences in the older patients (Table 2 ). Underdosing in particular in large and heavy teenagers may play a role as much as different pharmacokinetics in age subgroups.59,60 In addition to the choice and overall cumulative dosage of drugs (e.g., cumulative dose of L-asparaginase) the dose intensity for adolescent patients is probably more strictly enforced in pediatric centers and may contribute to the superior treatment results as observed in an important French study.9 Within the BFM data set from a more recent trial (ALL-BFM 95), the median time to finish the intensive treatment phases was never differing by more than two days between age subgroups, except for the infant group in which clearly more time was required to accomplish the full therapy. Thus, it appears that these minor treatment variations do not explain the larger outcome differences seen in p-Bc ALL.
Treatment of ALL Patients with Adequate Early Response
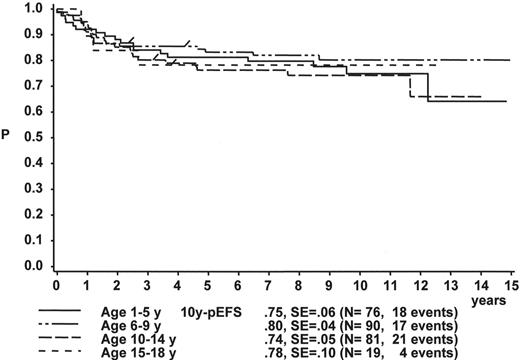
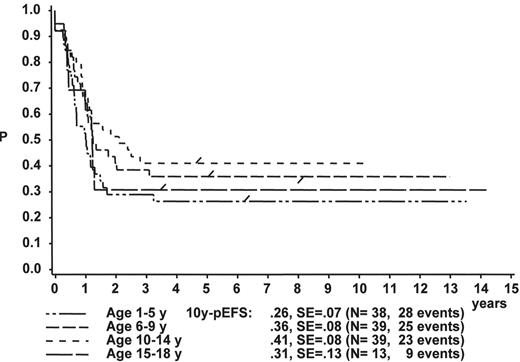
When the age subgroups as described in Table 2 are analyzed according to their initial response to prednisone and IT MTX, a distinct difference between p-Bc- and T cell ALL appears: In p-Bc ALL, the outcome of patients who had been “good responders” to prednisone (PRED-GR) varies by age (Figure 3A ), while, in contrast, all T-ALL patients with adequate early response have a rather similar outcome, independent of their age (Figure 3C ). The same homogeneity by age applies to T-ALL patients with prednisone poor-response (PRED-PR) (Figure 3D ), however, not to p-Bc ALL with PRED-PR (Figure 3B ). If early response in p-Bc ALL has been inadequate, the treatment outcome for all age groups in p-Bc ALL has been rather dismal except for the largest subset of patients being 1–5 years old.
Treatment of ALL Patients with Inadequate Early Response
A comparison of the treatment results generated in p-Bc ALL and T-ALL patients with adequate early response (blast count at day 8 in PB < 1000 per μL) reveals that current BFM therapy provides an equal chance of EFS in all age subsets of T-ALL. These results are actually more favorable than those of the corresponding age groups of p-Bc ALL. It is more difficult, however, to treat the T-ALL patients with intrinsic treatment resistance (indicated by PRED-PR). This subset comprises one third of all T-ALL patients. In trial ALL-BFM 90, outcome for such high-risk patients was impaired due to changes in therapy which had larger than expected consequences in particular for high risk T-ALL: Nine rotational cycles of short, very intensive treatment pulses replaced all other intensive postinduction treatment elements including the well-established BFM delayed reintensification, called “Protocol II.”12 This element has been shown to be effective for high risk as much as for standard risk ALL.11,61 This modification drastically reduced the overall dose of alkylating agents, which most likely has contributed to the adverse effect. The Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) group demonstrated that the application of two reintensification elements (Protocol II ×2) was able to improve outcome for this difficult patient subgroup mainly characterized by inadequate early response.62 Outcome for high-risk patients defined by slow early response measured by blast count in the bone marrow on day 15 (in which, however, patients with induction failure and a certain number of T-ALL patients with lymphomatous features had been excluded) was greatly improved by intensifying and repeating postinduction treatment elements in particular for patients less than 10 years old.58
Essential Treatment Elements
Remission induction
The primary question in ALL induction therapy over the last few years has been the choice of the right corticosteroid. Several large study groups are randomizing dexamethasone (DEX) versus prednisone. Some investigators have experienced severe toxicity with DEX.63 Severity of side effects certainly depends also on other components of induction therapy.64 Dose of corticosteroids may be able to overcome resistance.65 In low-risk ALL patients treated on CCG-1922 patients were randomly treated with DEX or prednisone throughout therapy except for delayed reintensification in which all patients received DEX. Even though it is not possible to assess if upfront DEX or the overall use of DEX was advantageous there was a significant benefit of DEX in that trial.66
Extracompartment therapy and preventive cranial radiotherapy (pCRT)
Over the last 15 years several trials have shown that reduction or even elimination of cranial radiotherapy in treatment of childhood ALL is feasible.1,3,4,5,67–,69 In T cell ALL with high WBC count elimination of pCRT was not successful.70,71 The question remains whether very intensive intrathecal medication may also cause some late effects, and it may not be necessary in certain subsets of patients. Interestingly, some trials have suggested that the extended use of IT MTX in non-irradiated patients may have reduced the rate of systemic relapses.67,72 For patients with initial CNS involvement or traumatic lumbar puncture, more intensive CNS-directed therapy may be warranted as these patients are at increased risk of systemic and CNS relapse.73,74
Delayed intensification (DI)
This component of ALL therapy has contributed significantly to improved outcome in standard and high-risk ALL. Numerous studies, mainly from BFM and CCG, have confirmed this observation and do not need to be listed here. It appears that at least some adult ALL trials use no comparable intensive reinduction,9 or it is being used with no DEX, the impact of which can certainly not be assessed.37
Allogeneic hematopoietic stem cell transplantation
In CR1, the indication for allogeneic hematopoietic stem cell transplantation (hSCT) should be carefully selected and adapted to the current results with chemotherapy.62,75 Certain subsets of ALL such as Ph+ ALL appear to have a benefit.32 For high-risk ALL patients large trials are being performed which will solve some issues.76 On the other hand, better management in supportive care, but also the more refined HLA-typing resulting in the selection of better matched unrelated donors, will have a positive impact on hSCT results in high-risk ALL.77
Maintenance therapy
There has been a large metaanalysis with respect to this issue.78 It is difficult to interpret as the composition of maintenance therapy varies tremendously. Some groups (such as BFM) do not use any intrathecal medication or corticosteroid pulses during maintenance while others repeatedly apply such medications. With respect to duration there is overall agreement that shortening maintenance therapy to less than 24 months (total treatment duration) has never really been successful.
Conclusion
For treatment of ALL, pediatric protocols have been shown to be very successful. Composition and dose intensity cannot simply be applied in an identical way in adolescent and adult patients. Some pediatric protocols already now have reached a limit with regard to acute and delayed toxicity. Application of such protocols in adolescent patients has improved prognosis but also caused severe impairment.79 Combined information from both adult and pediatric ALL trials may provide a rational basis for common prospective treatment approaches better adapted to the specific features for ALL patients in the second and third decade of life.
III. Acute Myeloid Leukemia in Children and Adolescents
Raul C. Ribeiro, MD,*Bassem I. Razzouk, MD, Ching-Hon Pui, MD, and Jeffrey E. Rubnitz, MD
St. Jude Children’s Research Hospital, Dept. of Hematology/Oncology, 332 N. Lauderdale St., Memphis TN 38105 Acknowledgments: The author is indebted to Sharon Naron and Margaret Carbaugh for expert editorial assistance. This work was in part supported by grants CA21765 and CA20180 from the National Cancer Institute, by a Center of Excellence grant from the State of Tennessee, and by the American Lebanese Syrian Associated Charities (ALSAC).
Biology and Epidemiology
Hematopoiesis is a complex process regulated by the coordinated expression of several transcription factors, which are activated or inhibited as hematopoiesis proceeds. The dysregulated expression of transcription factors and their resulting functional imbalance is believed to be required for malignant transformation.1 Because hematopoiesis in vertebrates is intensely active during fetal development and the first few years of life, it is not surprising that leukemia is the most common childhood malignancy. Genetic analysis of pediatric acute myeloid leukemia (AML), which occurs in approximately 1 of 130,000 individuals younger than 20 years of age each year, provides clues to both normal hematopoiesis and leukemogenesis. The genetic abnormalities found in leukemic cells have allowed identification of many of the affected genes as crucial contributors to hematopoietic lineage commitment (Table 5 ). There is increasing evidence that AML results from more than one genetic aberration.
Diagnosis and Classification
A combination of morphologic, immunophenotypic, and cytogenetic studies is usually required to establish the diagnosis of AML. Immunophenotype is important, particularly in establishing a diagnosis of acute megakaryoblastic leukemia (AMKL), myeloid leukemia with minimal differentiation, and myeloid/lymphoid (mixed, biphenotypic) leukemia. Recently, the World Health Organization (WHO), in conjunction with the Society for Hematopathology and the European Association of Hematopathology, has proposed a new classification for hematopoietic neoplasms.2 The WHO classification defines subsets of AML on the basis of both morphologic and cytogenetic characteristics. Although the current AML classification schemes, including the WHO scheme, have been developed for adult AML, the concepts underlying these classifications can be applied to pediatric AML. Recent gene expression profiling studies demonstrated distinct expression signatures for each of the known prognostic subtypes of AML. Importantly, some of the pediatric AML subtype specific expression signatures were essentially the same as those of selected adult AML cases, suggesting a shared leukemogenesis.3
Characteristics of Selected Subtypes of Pediatric AML
There are considerable differences in clinical and biological features and in response to and tolerance of therapy by age group.4 Therefore, to improve the outcome of pediatric patients with AML will require that therapy be tailored to both the disease and the age of the patient.
Acute promyelocytic leukemia
The most common genetic abnormality of acute promyelocytic leukemia (APL) is the reciprocal translocation between the PML gene at chromosome band 15q22 and RARα at 17q21. The introduction of all-trans-retinoic acid (ATRA) as front-line therapy for APL has changed the prognosis of this disease in both adults and children. Molecular testing for the PML-RARα fusion is a useful tool to monitor response and identify the need for changes in therapy. Persistence or reappearance of the fusion transcript after completion of therapy can be used to predict disease relapse. With current treatments, approximately 25% of children with APL experience relapse and another 5% die of complications during the early phases of treatment.
Because most pediatric APL protocols include cumulative doses of anthracyclines ≥ 400 mg/m2, which can be cardiotoxic,5 particularly for children who may require hematopoietic stem cell transplantation, there have been efforts to develop strategies to limit the potential toxicity of anthracyclines. Cardiotoxicity can be prevented by reducing the cumulative dosage, modifying the administration schedule to lower the peak drug levels, using an alternative anthracycline (liposomal preparations), or using a cardioprotectant. A randomized study of the use of dexrazoxane as a cardioprotectant after administration of doxorubicin in children with ALL showed a significant reduction in the number of patients with elevated troponin T levels in the dexrazoxane arm with no reduction of EFS.6
Strategies to reduce anthracycline-induced cardiotoxicity will have to be evaluated in pediatric APL. Further, the optimal dosage, schedule of administration, and pharmacologic monitoring of ATRA have not been determined for children. Whether early introduction of arsenic trioxide or the anti-CD33 antibody-calicheamicin conjugate (gemtuzumab ozogamicin) can improve outcome remains to be determined. Finally, prognostic factors can differ according to age.7
AML with the t(8;21)(q22;q22)
Although the in utero origin of AML with the t(8;21) and AML1-ETO fusion was recently reported,8 the disease is rarely diagnosed before 3 years of age; it is also rare in individuals > 60 years of age. The AML1 gene encodes the CBFα2 protein, a component of the core-binding factor (CBF) heterodimer, which plays a crucial role in regulating transcription of several genes. Intensive chemotherapy regimens that include high-dose cytarabine have been associated with improved outcome of this subtype in adults and children. Interestingly, the persistent presence of the AML1-ETO fusion is not always indicative of disease activity. Two recent Medical Research Council (MRC) treatment protocols have yielded 5-year survival rates exceeding 80% in children with this subtype of AML.9 Loss of a sex chromosome was associated with a particularly good prognosis.9
AML with the inv(16) or t(16;16)(p13;q22)
These genetic abnormalities result in the fusion of the CBFβ gene on chromosome band 16q22 with the MYH11 gene located at 16p13. The molecular defect in the inv(16) disrupts the CBF heterodimer subunits in much the same way that the t(8;21) abnormality does. However, these two subtypes of AML have different clinical and biological features, early mortality rates, and responses to treatment. The rate of early mortality in AML with the CBFβ-MYH11 fusion can be as high as 10%, due to multiple organ failure resulting from the release of leukemic cell contents.10,11 Persistence of the CBFβ-MYH11 fusion after induction and consolidation therapy is associated with a high risk of relapse. There is still controversy as to whether a high cumulative dose of cytarabine is crucial for the cure of this subtype of AML. Recent studies by French12 and St. Jude13 investigators suggest that it is not. However, a Cancer and Leukemia Group B study found that repeated courses of high-dose cytarabine benefit patients with this subtype of AML.14 Contemporary studies at St. Jude Children’s Research Hospital have not used multiple courses of high-dose cytarabine, but they have produced a 5-year EFS estimate of 76% for patients who have AML with the inv(16).15 In the latter study, the addition of etoposide and cladribine to the cytarabine-anthracycline combination may have contributed to improved results.
AML with the t(9;11)(p21-22;q23)
Rearrangements of the 11q23 chromosomal region occur in approximately 15% of the cases of AML. The majority of these abnormalities result from a reciprocal translocation between the MLL gene at band 11q23, and one of more than 50 partner genes. Approximately half of the cases have the t(9;11) translocation. Results of recent studies at our institution (St. Jude)16 and by the Nordic AML group17 have shown that children with AML and the t(9;11) have a significantly better outcome than do those with AML and other 11q23 abnormalities. In the St. Jude study, the 5-year EFS estimate was 65% ± 11% (SE) for patients with AML and the t(9;11) and 24% ± 9% (SE) for those with AML and other 11q23 abnormalities. This result was not corroborated in the MRC trials9; this disparity is likely to reflect the influence of the different treatment strategies used. The use of relatively high dosage intensity of etoposide and cladribine may have contributed to the improved results in the St. Jude studies.
Acute megakaryoblastic leukemia
AMKL (FAB M7) is truly a disorder of young children. The median age of patients with this diagnosis is approximately 2 years; the disease is rarely diagnosed in children more than 3 years of age. It accounts for approximately 12%–15% cases of primary childhood AML and 50% of the cases of AML in children with Down syndrome. AMKL can also occur as a transient phenomenon in newborns with Down syndrome. Bone lesions and extramedullary involvement are fairly common in AMKL. Often, a diagnosis of AML M7 is difficult to establish. Aspirated bone marrow samples are frequently hypocellular and are usually “dry taps” that do not provide sufficient bone marrow for testing. Cytogenetic studies frequently show multiple karyotypic anomalies. In approximately 2% of the cases of AMKL, a specific translocation—the t(1;22)(p13;q13)—results in the fusion of RBM15 on chromosome band 1p13 and MKL1 on band 22q13. Virtually all patients with Down syndrome and transient myeloproliferative disorder (TMD) or AMKL have had acquired mutations in the GATA-1 gene in their leukemic cells.18 The prognosis of children with AMKL has been considered poor. In our experience, only those who have received bone marrow transplants have been long-term survivors.19 More recent reports suggest that intensive chemotherapy is effective in AMKL.9,20 Further studies are necessary to determine prognostic factors within the AMKL subtypes.
Principles of Treatment
Despite several strategies to increase the intensity of therapy, the overall survival rate has reached a plateau at approximately 60%, suggesting that further intensification of therapy per se will not substantially improve survival rates. It appears that the “one-size-fits-all” treatment strategy will not further improve current AML survival rates. The merit of individualized therapy for AML has been clearly demonstrated in the treatment of APL. The introduction of ATRA and the tailored use of specific treatment components (increased dose-intensity of anthracycline during induction and consolidation; use of ATRA plus 6-mercaptopurine and methotrexate during maintenance) has resulted in reproducible 5-year survival rates exceeding 80%.7 Because only a limited number of compounds are active against specific subtypes of AML, it is currently impossible to apply the individualized treatment to the vast majority of AML cases.
Remission induction
The combination of cytarabine and daunorubicin continues to form the backbone of AML induction therapy. A third drug, usually etoposide or 6-thioguanine, has usually been added to this combination. A randomized study showed no differences in the rate of complete remission or overall survival between pediatric patients with AML who received etoposide and those who received 6-thioguanine.21 With two courses of this 3-drug combination and adequate supportive care, more than 90% of the children experience complete remission; the rate of resistant disease in newly diagnosed primary AML is approximately 5%, and early mortality should not exceed 2%. Dose intensity during induction therapy with these three drugs has varied across several large pediatric AML studies (Table 6 ). Various strategies have been used to intensify remission induction, with the goal of improving long-term survival rather than increasing complete remission rates. These strategies have generally substituted daunorubicin with idarubicin22 or mitoxantrone,23 increased the dosage of cytarabine,20,24 or reduced the intervals between the initial cycles of chemotherapy (“intensive timing”).25 The rates of complete remission obtained by using these methods have been similar to, or in some cases worse (due to toxicity) than, those obtained with cytarabine and daunorubicin plus 6-thioguanine or etoposide. In general, these studies have shown that the quality or “depth” of remission affects the likelihood of long-term disease-free survival, although death due to toxicity during induction can offset the expected benefits of some of these strategies. An alternative method to intensify remission induction would be to administer drugs with newer mechanisms of action to selected groups of patients early in treatment. For example, patients with residual disease after remission induction, who are at particularly high risk of relapse, could be selected to receive drugs such as anti-CD33 antibody-calicheamicin conjugate (gemtuzumab ozogamicin).
Postremission therapy
Several courses of intensive postremission chemotherapy combining non-crossresistant agents, administered every 4 to 6 weeks, have been shown to improve disease-free survival. Intermediate- or high-dose cytarabine has been extensively used in contemporary treatment programs, either alone or more commonly in combination with L-asparaginase, mitoxantrone, amsacrine, or etoposide. No randomized studies have been conducted to compare the efficacy of different post-remission combinations used in pediatric AML; best results are observed when multiple dose-intensive cytarabine-containing regimens are used. The number of courses prescribed has also varied among studies. A recently completed randomized study by the MRC group (AML-12)9 revealed that two postremission courses were as effective as three. Although prolonged nonmyeloablative maintenance chemotherapy given after 2 to 3 courses of intensive chemotherapy has not been investigated in a prospective randomized fashion, there is no evidence that it would improve the outcome of pediatric AML. In fact, French investigators have suggested that it may have a deleterious effect on overall survival by decreasing the success of salvage therapy.23
Allogeneic and autologous hematopoietic stem cell transplantation (HSCT) have been extensively used in the treatment of pediatric AML in first complete remission. In most studies, the availability of HLA-matched sibling donors determined whether patients underwent HSCT as postremission treatment. Hence, there have been no true randomized and stratified studies comparing the efficacy of allogeneic HSCT with that of intensive chemotherapy. Allogeneic HSCT is recommended for all patients with a matched sibling by some investigators because it is thought to be more effective than intensive cycles of post-remission chemotherapy. The relapse rate is generally lower among patients who have received transplants than among those treated with intensive post-remission chemotherapy, particularly in early protocols that prescribed less effective chemotherapy regimens. However, overall survival rates have not been substantially different between these two groups due to the higher mortality rate associated with transplantation and the better salvage rate for patients treated with chemotherapy. More importantly, certain subgroups of AML have been found to be highly curable with contemporary chemotherapy; therefore, allogeneic HSCT could, in fact, reduce the rate and quality of survival for these individuals. Moreover, long-term complications develop more often in patients, particularly young children, who undergo HSCT than in those treated with intensive chemotherapy. These observations have fueled an intense debate on the indications for allogeneic HSCT in pediatric patients with AML. At St. Jude, allogeneic HSCT during first remission is not recommended for patients with favorable prognostic markers: (inv16), t(8;21), t(9;11), t(15;17), age < 1 year, or constitutional trisomy 21. Sibling matched-donor HSCT is recommended for all other patients with AML. Unrelated matched-donor HSCT is reserved for patients with unfavorable AML (Table 7 ).
The efficacy of autologous HSCT has been studied in several contemporary pediatric AML trials.21,24– 26 In the MRC AML10 trial, children with AML were randomly assigned to undergo autologous HSCT or observation only after receiving 4 courses of chemotherapy. Although the relapse rate was significantly higher in the observation arm, the overall survival rate was similar in the two arms. Because autologous HSCT has failed to substantially improve outcome, it is not used in our current AML trial.
CNS-directed therapy
The incidence of CNS involvement at diagnosis of AML ranges from 5% to 30%, depending on the criteria. Factors associated with CNS leukemia include hyperleukocytosis (leukostasis), monocytic leukemia (FAB M4 or M5), and young age. For patients without CNS involvement, CNS prophylaxis therapy has varied from intrathecal chemotherapy (cytarabine, methotrexate, or cytarabine, methotrexate, and hydrocortisone) given alone or combined with cranial radiotherapy. There has been no study comparing the efficacy of intrathecal cytarabine or methotrexate with that of triple therapy (methotrexate, cytarabine, and hydrocortisone). However, the triple intrathecal regimen has been associated with low rates of CNS leukemia relapse in clinical trials,21,26 even when the majority of the patients do not receive cranial radiotherapy. For patients with CNS disease at diagnosis of AML, intrathecal chemotherapy plus cranial radiotherapy has usually been recommended. A recent St. Jude study27 revealed that CNS disease is not an adverse prognostic factor in AML and that patients with CNS leukemia at diagnosis can be cured without the use of cranial irradiation.
Prognostic Factors
The prognostic importance of many clinical and biological features has varied across contemporary clinical trials. Recently, response to therapy, as measured by PCR or flow-cytometric techniques, has been used to assess individual patients’ risk of relapse of AML. In approximately 90% of patients with AML, flow-cytometric techniques allow the detection of 1 AML cell or less among 1000 non-leukemic cells (0.1% residual leukemia). Patients who have persistent disease at a level of 0.1% or higher after two courses of intensive chemotherapy have a very poor prognosis.28 However, whether more sensitive methods can improve the risk assessment remains to be determined. Currently, various combinations of clinical and biologic features as well as treatment responses have been used in risk classification (Table 7 ).
Refractory or Relapsed Acute Myeloid Leukemia
Children with refractory or relapsed AML constitute a formidable management challenge. With current treatment strategies, about 5% of children with newly diagnosed AML have overt morphologic evidence of leukemia (≥ 5% blasts), and another 20% have substantial minimal residual leukemia (0.1%–4% blasts), at the end of two cycles of induction therapy. Moreover, about 30% to 40% of children who achieve “solid” remission eventually experience relapse. Hematological relapse is the most common form of treatment failure.29 Relapse occurs at various times after diagnosis, usually within the first year, and after various treatment modalities, including HSCT. Thus, overall, about 50% of children with AML require contingency treatment strategies to deal with either resistant or relapsed disease. A recent report by French investigators30 illustrates the array of therapies prescribed to patients who relapse after treatment on a front-line protocol. The variety of therapies is not surprising, as some patients may experience relapse soon after allogeneic HSCT, often while still having transplant-associated complications; others may relapse several months or years after diagnosis. For the former group, adoptive immunotherapy with donor lymphocytes has induced durable remission in few cases, but the overall prognosis is very poor. Hence, palliative care is a reasonable consideration for these patients. For the latter group of patients, salvage therapy offers a realistic expectation of cure. Several combinations that include two or more of the agents cytarabine, mitoxantrone, etoposide, idarubicin, and fludarabine have typically been used to induce complete remission, at rates that range from 50% to 80%. In the subset of AML that expresses CD33, gemtuzumab ozogamicin alone or in combination with other agents can be considered. Allogeneic or autologous HSCT is usually prescribed for patients who achieve remission, yielding a long-term disease-free survival estimate of about 30% to 40%. These findings raise the possibility that early identification of patients at high risk of relapse and prescription of novel treatment strategies might reduce the rate of relapse. In the St. Jude AML2002 protocol, patients who have persistent disease after one or two courses of induction chemotherapy receive gemtuzumab ozogamicin, either alone or combined with conventional chemotherapy, followed by bone marrow transplantation. It remains to be determined whether this strategy will improve the outcome of childhood AML. Given the dismal prognosis of relapsed AML, new agents or therapeutic strategies must be expeditiously incorporated into national and international collaborative treatment protocols as they become available.
IV. Juvenile Myelomonocytic Leukemia and Myelodysplastic Syndrome
Charlotte M. Niemeyer, MD*
Division of Pediatric Hematology and Oncology, Department of Pediatrics and Adolescent Medicine, University Hospital, Mathildenstrasse 1, 79106 Freiburg, Germany Acknowledgments: I am grateful to all members of EWOG-MDS, especially Henrik Hasle, Franco Locatelli and Gabrielle Kardos for their collaborations. Marco Tartaglia, Bruce Gelb, Mignon Loh, Kevin Shannon and Christian Kratz are acknowledged for their contribution concerning the molecular analysis of PTPN11.
A new classification of myelodysplastic and myeloproliferative diseases in childhood has been widely accepted, and has greatly facilitated the diagnosis of myelodysplastic syndrome (MDS) and juvenile myelomonocytic leukemia (JMML).1,2 In addition, the discovery of somatic mutations in PTPN11 in about a third of the patients with JMML3 has not only improved our understanding of the pathophysiology of this unique disorder but has helped to establish the diagnosis early in the clinical course. This section reviews diagnostic procedures, as well as recent advances in molecular genetics and treatment strategies in JMML and MDS in childhood.
Juvenile Myelomonocytic Leukemia
JMML is a clonal hematopoietic disorder of early childhood characterized by excessive proliferation of cells of the monocytic and granulocytic lineages. Children present at a median age of 1.8 years with pallor, fever, infection, skin bleeding and cough.4 There is generally marked splenomegaly and moderate hepatomegaly. Occasionally, spleen size is normal at diagnosis, but it rapidly increases thereafter. Diagnostic guidelines helping distinguishing JMML from other disorders with myeloproliferative features have been proposed (Table 8 ).5
Hematological features
Microscopy of the peripheral blood smear is the first important step in establishing the diagnosis of JMML. Almost all cases show a striking monocytosis, often with dysplastic features. An absolute monocyte count exceeding 1 × 109/L is required for the diagnosis. Immature granulocytic precursors and nucleated red cells are usually evident. The blast cell percentage in blood is less than 2% on average and rarely exceeds 20%. Leukocytosis is less pronounced compared to Philadelphia chromosome-positive chronic myeloid leukemia (CML), with a median white blood cell count (WBC) of 33 × 109/L. Occasionally, WBC is below 10 × 109/L, particularly in children with monosomy 7.
Bone marrow findings in JMML are by themselves not diagnostic but rather consistent with the diagnosis. The aspirate shows a high cell number with predominance of granulocytic cells at all stages of maturation, except for the few cases in which the erythroid series predominates. Monocytosis in bone marrow is generally less impressive than that in blood. The marrow blast count is moderately elevated, but never reaches the level seen in acute leukemia. Megakaryocytes are reduced in number or absent in about two thirds of cases, and thrombocytopenia is generally the most prominent hematological feature.
Classification
To account for myelodysplasia observed in some cases, the WHO included JMML in the category of myelodysplastic/myeloproliferative disorders.2 The entity incorporates those leukemias previously referred to as juvenile chronic myeloid leukemia (JCML) and chronic myelomonocytic leukemia (CMML) of infancy. Cases previously included in the infantile monosomy 7 syndrome are incorporated in this category. Approximately 25% of JMML patients have monosomy 7 and 10% have other chromosomal abnormalities, but the majority (65%) has a normal karyotype.4
Cellular and molecular studies
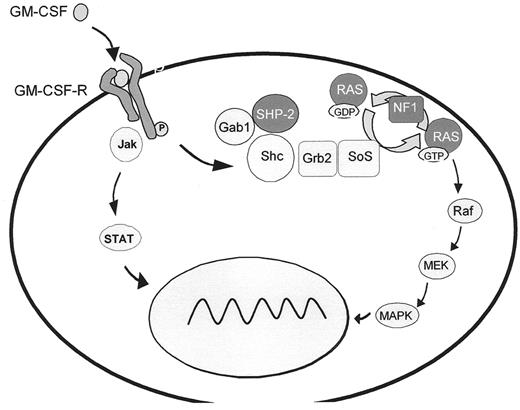
A hallmark of JMML is hypersensitivity of myeloid progenitor cells to GM-CSF in culture, a finding which has become an important diagnostic criterion.6 Studies on human leukemic cells and mouse models provide evidence that GM-CSF hypersensitivity is due to a selective inability to downregulate RAS-dependent signaling pathways. In approximately 25% of JMML cases, the pathological inactivation of the RAS/MAPK cascade results from oncogenic RAS mutations3 (Figure 4 ) that affect GTP hydrolysis, leading to the accumulation of RAS in the GTP-bound active conformation. It has recently been demonstrated that oncogenic RAS is sufficient to initiate a rapidly fatal myeloproliferative disorder in mice associated with hyperproliferation, tissue infiltration and aberrant responses to growth factors.7
JMML has also been reported in children with neurofibromatosis type 1 (NF1), an autosomal dominant disorder resulting from germline loss-of-function mutations of the NF1 tumor suppressor gene. About 25–30% of JMML cases either carry the clinical diagnosis of NF1 or are known to harbor NF1 gene mutations.4,8 The proliferative advantage of the leukemic cells results from “second hits,” the somatic loss or inactivation of the normal NF1 allele.8,9 Since the NF1 gene product, neurofibromin, is a negative modulator of RAS function, this loss is associated with RAS hyperactivity.
The most common molecular events in JMML, noted in about 35% of patients, are somatic mutations in PTPN11, the gene encoding SHP-2.3 SHP-2 is a member of a small subfamily of cytoplasmic src-homology 2 (SH2) domain-containing protein tyrosine phosphatases. It is required for hematopoietic cell development and participates in signal transduction of a number of cytokines, mediated at least in part by activation of the RAS/MAPK cascade. SHP-2 contains two tandem SH2 domains at the N-terminus and a catalytic protein tyrosine phosphatase (PTP) domain at the C-terminus. In its inactive state, PTPase activity is repressed by inhibition of the enzymatic cleft by the N-terminal SH2 domain. Binding of the SH2 domain to phosphorylated tyrosine residues induces a conformational shift that relieves the inhibitory interaction between the SH2 domain and the catalytic PTP domain. Virtually all PTPN11 mutations identified in JMML are missense mutations in the N-terminal SH2 (exon 3, 90%) or PTP (exon 13, 10%) interacting surfaces, predicting gain-of-function in SHP-2 through preferential occupation of the activated state.3,10 Mutations in PTPN11, RAS and NF1 are largely mutually exclusive in JMML, again suggesting that pathological activation of RAS dependent pathways plays a central role in the pathophysiology of the disease.
PTPN11 has also been identified as the disease gene in 50% of patients with Noonan syndrome (NS), a relatively common heterogenous disorder characterized by short stature, facial dysmorphia, cardiac and skeletal defects. It has been speculated that gain-of-function in the NS-related SHP-2 mutants may be insufficient to perturb hematopoiesis, while somatic JMML-related SHP-2 mutants have stronger gain-of-function, resulting in pronounced dysregulation with embryonic death when inherited through the germline.3 A JMML-like disease can occasionally be noted in children with NS with germline mutations in part altering the same codons as in non-syndromic JMML.3,10 The functional consequences of these mutants may be intermediate between those of the isolated NS and somatic JMML-related mutants; further studies will have to address this question. Because the JMML-like disorder in NS most often spontaneously resolves,11 and stigmata of NS may be subtle, germline mutations have to be excluded in all JMML patients harboring PTPN11 mutations in their leukemic cells.
Hematopoietic stem cell transplantation
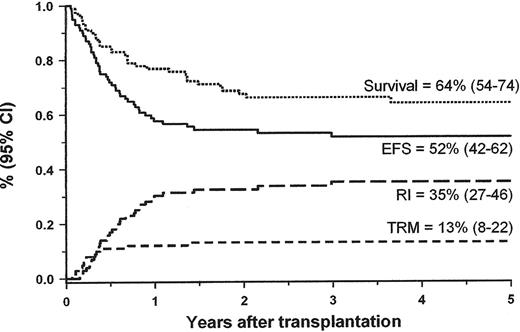
Although JMML rarely converts into blast crisis, it is a rapidly fatal disorder for most children if left untreated. The median survival time without allogeneic stem cell transplantation (HSCT) is about 1 year. Low platelet count, age above 2 years at diagnosis and high HbF at diagnosis are the main predictors of short survival.4,12 Long-term survival has only been achieved with HSCT. Recent data from the European Working Group of MDS in Childhood (EWOG-MDS),13 the Japanese study group14 and single-center experiences indicated that approximately half of the children with JMML can be cured with HSCT. EWOG-MDS reported the outcome of 100 children with JMML given unmanipulated HSCT following a preparative regimen consisting of three alkylating agents, busulfan, cycophosphamide and melphalan (Figure 5 ). The probability of EFS at 5 years after HSCT for the 48 children transplanted from a matched-related donor (MRD) was similar to that obtained in 52 children given an HSCT from a matched unrelated donor (MUD) (55% and 49%, respectively).
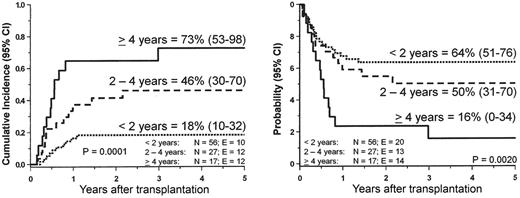
Disease recurrence remains the major cause of treatment failure. Median time from HSCT to relapse is 4–6 months with only few patients relapsing more than 1 year after transplantation. In the multivariate analysis of the EWOG-MDS study, only older age predicted leukemia relapse (Figure 6 ). Karyotype, splenectomy before HSCT, and spleen size at time of the allograft had no impact on post-transplant outcome.13
There is clear evidence that graft-versus-leukemia effect plays an important role in curing children with JMML with HSCT. Re-emerging donor cells and frank hematologic relapse have been successfully eradicated by reduction of ongoing immunosuppressive therapy. Reducing the intensity and duration of graft-versus-host disease prophylaxis may significantly contribute to successful leukemia control. However, unlike BCR-ABL positive CML, donor lymphocyte infusion in JMML relapse has largely been unsuccessful.15 Despite aggressive re-emergence of the malignant clone and short interval between the first and second HSCT, a substantial proportion of children can be cured after a second HSCT.16
Treatment other than hematopoietic stem cell transplantation
The role of antileukemic therapy prior to HSCT is currently uncertain. Comparative evaluation of the efficacy of different clinical protocols and great variety of antineoplastic drugs applied is hampered by the lack of uniform criteria for response. The current JMML study of the Children’s Oncology Group (COG) prescribes cytoreductive therapy consisting of fludarabine and high-dose cytarabine concomitantly with 13-cis retinoic acid prior to HSCT, while most patients in Europe traditionally receive mercaptopurine or no therapy. Therapeutic strategies targeting individual components of the GM-CSF–RAS pathway include the administration of the GM-CSF analog E21R and the farnesyltransferase inhibitor R115772 currently being studied in an up-front Phase II window by the COG. The benefit of these strategies as well as the question whether intensive, moderate or no chemotherapy is most appropriate prior to HSCT will have to be studied in well-controlled international trials.
Like the use of pretransplant chemotherapy, there is significant controversy over the role of splenectomy. The spleen is an important site in JMML and may contribute to its evolution. Some investigators favor splenectomy because it can relieve the child from an often enormous abdominal mass, may improve platelet counts and reduces tumor burden. In addition, splenectomy significantly improved lengths of survival in children who did not receive an HSCT (author’s unpublished data). Others argue that there is currently no evidence that splenectomy in these young children significantly hastens engraftment or reduces relapse rate following HSCT.
Myelodysplastic Syndromes
MDS are clonal disorders characterized by ineffective hematopoiesis and subsequent frequent development of AML. Like JMML, pediatric MDS is an uncommon disorder accounting for less than 5% of hematopoietic neoplasia in childhood. The new WHO classification incorporating both morphology and genetic changes defines 5 subgroups based on the review of adult cases.2 In addition, it eliminates the FAB subtype of refractory anemia with excess blasts in transformation (RAEB-T) by reducing the threshold of blasts required to make the diagnosis of AML to 20%.
There are a number of differences between adult and pediatric MDS. In children, refractory anemia (RA) with ringed sideroblasts and MDS associated with del(5q) chromosome are exceedingly rare. In addition, the importance of multilineage dysplasia in RA is unknown. Anemia is generally the main presenting symptom in adults with RA, but in childhood cases, neutropenia and thrombocytopenia are frequently observed.17 Therefore, “refractory cytopenia” (RC) was felt to be a more suitable term for pediatric MDS without excess blasts. Furthermore, in children there are no data to indicate whether a blast threshold of 20% is better than the traditional 30% to distinguish MDS from de novo AML. To accommodate these characteristics of pediatric MDS, a simple classification scheme based on morphological features and conforming with the WHO suggestions was proposed.1 It recognizes three diagnostic groups: RC (BM blasts < 5%), RA with excess blasts (RAEB) (BM blasts 5%–20%) and RAEB-T (BM blasts 20%–30%). Auer rods are no longer a discriminator for classification. The dysplastic prodrome of AML in Down syndrome is classified within myeloid leukemia in Down syndrome1 and excluded from population-based studies of MDS.12
The genetic changes predisposing children to MDS remain largely obscure. The presumed underlying mechanism may also give rise to subtle phenotypic abnormalities noted in many children with MDS. There is a also need to specify “secondary” MDS following chemo- or radiation therapy, congenital bone marrow failure, or aplastic anemia and that in familial disease.1
Monosomy 7 is the most common chromosomal change in malignant cells of children with MDS (Table 9 ) and often occurs as a sole abnormality. The frequency of the different karyotypic alterations varies among MDS subtypes.12 Reported differences in incidence between studies may be primarily due to the different composition of study populations.
Refractory cytopenia
MDS with less than 5% blasts in the bone marrow is particularly difficult to diagnose, because dysplasia of hematopoietic cells is frequently observed in association with infections, metabolic disorders, nutritional deficiencies, and a variety of other diseases. In the absence of a cytogenetic marker, the clinical course will have to be carefully evaluated before a diagnosis of RC can be established. RC is the most common subtype of childhood MDS accounting for about half of the cases.12 In contrast to adult MDS, bone marrow cellularity in RC is often reduced. In the ongoing study of the EWOG-MDS 98, about 75% of patients with RC have a hypocellular biopsy specimen (unpublished data). This observation together with a low rate of leukemic transformation raises the question whether some of these children have an unrecognized congenital disorder with dysplasia and marrow failure rather than acquired MDS. Differentiating hypoplastic RC from acquired aplastic anemia and congenital bone marrow failure disorders remains an intriguing challenge.
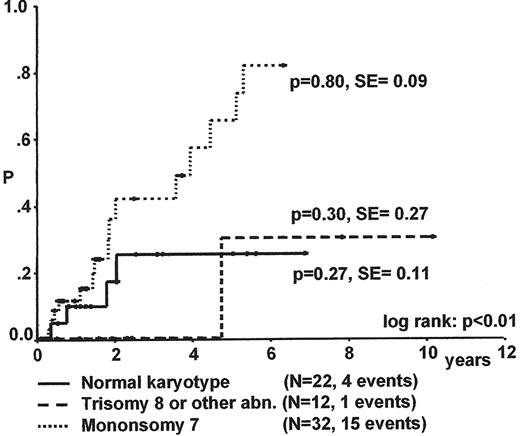
Karyotype is the most important factor for progression to advanced MDS and survival (Figure 7 ).17 The median time to progression for children with RC and monosomy 7 is less than 2 years. Spontaneous disappearance of monosomy 7 and cytopenia has been noted in some infants, but remains a rare event. In contrast to monosomy 7, patients with trisomy 8 and other karyotypes may experience a long stable course of their disease.
HSCT from an MRD or MUD is the treatment of choice for patients with monosomy 7 early in the course of their disease. In view of the low transplant-related mortality observed in patients transplanted from a sibling donor, HSCT can also be recommended for all other patients if a suitable MRD is available. An expectant approach with careful observation may be reasonable for patients without an MRD in the absence of transfusion requirements, severe cytopenia or infections. However, with the recent treatment results of HSCT with MUD nearly comparable to those of grafts from an MRD, early referral for HSCT prior to prolonged cytopenia or disease progression has been recommended.
Initiating events of MDS are thought to occur at a genetic level of the hematopoietic stem cell. However, early bone marrow failure can result at least in part from T cell–mediated suppression of hematopoiesis. Studies in adult MDS demonstrated that about 30% of RA patients have clinically relevant responses to immunosuppressive therapy. Whether immunosuppression can result in sustained responses in a substantial number of children with RC is currently unknown.
Refractory cytopenia with excess blasts and excess blasts in transformation
There is consensus that the relationship between MDS and de novo AML is better defined by biological and clinical behavior than by blast count.1,2 Consequently, myeloid disease with low blast count and cytogenetic abnormalities typically associated with de novo AML is classified as AML; protocols of AML trials should allow inclusion of these patients. Because monosomy 7 is the only chromosomal abnormality strongly suggestive of MDS, children presenting with a low blast count and other chromosomal aberrations or normal karyotype have to be followed closely before a diagnosis of MDS can be established. Disease with rapid increase in marrow blasts or organ infiltration should be considered de novo AML, which is by far the more common disorder. To allow for the interface between MDS and de novo AML, RAEB-T was retained in the pediatric classification until new data becomes available.
The most appropriate therapy for children with RAEB or RAEB-T is unknown. Although most investigators agree that HSCT can improve survival, the importance of cytoreductive therapy prior to grafting remains controversial. In the US and Great Britain, children with RAEB and RAEB-T are generally included in pediatric AML trials.18,19 Data from EWOG-MDS indicate that intensive chemotherapy prior to HSCT will not improve survival.20 In this study of advanced primary MDS, the probability of survival was about 50% and not influenced by marrow blast percentage at the time of transplantation. Well-controlled international clinical trials will have to be designed to resolve the issues on pre-HSCT remission induction therapy, optimal preparative regimen and stem cell source in the future.
Prognostic factors in childhood acute lymphoblastic leukemia (ALL).
| Leukemia Subtype . | Favorable Prognostic Factors . | Unfavorable Prognostic Factors . |
|---|---|---|
| *This rearrangement occurs more often in B cell precursor ALL among which it is an unfavorable feature, especially in infant cases. | ||
| B cell precursor | Hyperdiploidy (> 50 chromosomes); TEL-AML1 fusion; Trisomies 4,10, and 17 | Poor early response; MLL rearrangement in infants; Philadelphia chromosome; Leukocyte count > 50 × 109/L; Age > 10 years at diagnosis |
| T cell | HOX11 overexpression; t(11;19) with MLL-ENL fusion* | Poor early response; Low dose-intensity chemotherapy |
| Leukemia Subtype . | Favorable Prognostic Factors . | Unfavorable Prognostic Factors . |
|---|---|---|
| *This rearrangement occurs more often in B cell precursor ALL among which it is an unfavorable feature, especially in infant cases. | ||
| B cell precursor | Hyperdiploidy (> 50 chromosomes); TEL-AML1 fusion; Trisomies 4,10, and 17 | Poor early response; MLL rearrangement in infants; Philadelphia chromosome; Leukocyte count > 50 × 109/L; Age > 10 years at diagnosis |
| T cell | HOX11 overexpression; t(11;19) with MLL-ENL fusion* | Poor early response; Low dose-intensity chemotherapy |
Patient characteristics, response, and treatment results in age subgroups of (A) precursor B-cell (p-Bc) acute lymphoblastic leukemia (ALL) and (B) T cell ALL.*
| . | . | Age (years) . | . | ||
|---|---|---|---|---|---|
| Feature . | < 1 . | 1–5 . | 6–9 . | 10–14 . | 15–18 . |
| * Combined data from trials ALL-BFM 86 (n = 998) and ALL-BFM 90 (n = 2178); data are given in %. In 100 patients (3.1%) the immunophenotype was not available. | |||||
| PRED-PR: Prednisone poor-response, defined by ≥ 1000 leukemic blasts in peripheral blood after 7 days of prednisone and one dose of intrathecal methotrexate on day 1.12,80 In T cell ALL, only 3 patients were < 1 year old; therefore, data are not listed. | |||||
| CR: complete remission in the bone marrow (less than 5% blasts) after 6 weeks of induction therapy; no CR: no remission achieved, died due to disease. | |||||
| Abbreviations: CNS, central nervous system; pEFS, probability for event-free survival | |||||
| A. p-Bc ALL | |||||
| Total no. of patients | 90 | 1709 | 464 | 318 | 89 |
| Male | 50 | 54.6 | 52.4 | 54.7 | 53.9 |
| WBC (per μL) | |||||
| < 10,000 | 12.2 | 49.7 | 60.8 | 54.4 | 46.1 |
| 10–19,999 | 7.8 | 18.4 | 11.4 | 15.4 | 13.5 |
| 20–99,999 | 21.1 | 25.4 | 21.3 | 21.1 | 31.5 |
| ≥ 100,000 | 58.9 | 6.5 | 6.4 | 9.2 | 9.0 |
| CNS involvement | 11.1 | 1.4 | 0.4 | 1.3 | 4.5 |
| t(9;22) | – | 1.3 | 4.9 | 5.5 | 4.1 |
| PRED PR | 24.4 | 4.3 | 6.0 | 9.4 | 7.9 |
| Early death | 2.2 | 0.6 | 0.4 | 1.6 | 5.6 |
| No CR | 3.3 | 0.1 | 0.2 | 1.3 | 1.1 |
| Relapse | 45.6 | 15.8 | 25.4 | 29.9 | 28.1 |
| Death in CR | 2.2 | 1.1 | 1.3 | 3.8 | 0 |
| 10y-pEFS (SE) | 46.3 (5.3) | 80.4 (1.0) | 70.8 (2.2) | 60.6 (2.9) | 63.7 (5.3) |
| B. T Cell ALL | |||||
| Total no. of patients | 117 | 132 | 122 | 32 | |
| Male | 71.8 | 69.7 | 77.9 | 90.6 | |
| WBC (per μL) | |||||
| < 10,000 | 7.7 | 14.4 | 17.2 | 12.5 | |
| 10–19,999 | 6.8 | 12.9 | 9.0 | 3.1 | |
| 20–99,999 | 38.5 | 40.2 | 36.9 | 50 | |
| ≥ 100,000 | 47.0 | 32.6 | 36.9 | 34.4 | |
| CNS involvement | 10.3 | 9.1 | 7.4 | 12.5 | |
| PRED-PR | 32.5 | 29.5 | 32.0 | 40.6 | |
| Early death | 2.6 | 1.5 | – | – | |
| No CR | 2.6 | 2.3 | 2.5 | 3.1 | |
| Relapse | 22.2 | 26.5 | 31.1 | 37.5 | |
| Death in CR | 6.8 | 1.5 | 0 | 0 | |
| 10y-pEFS (SE) | 58.3 (4.8) | 66.1 (4.2) | 63.9 (4.5) | 58.5 (8.8) | |
| . | . | Age (years) . | . | ||
|---|---|---|---|---|---|
| Feature . | < 1 . | 1–5 . | 6–9 . | 10–14 . | 15–18 . |
| * Combined data from trials ALL-BFM 86 (n = 998) and ALL-BFM 90 (n = 2178); data are given in %. In 100 patients (3.1%) the immunophenotype was not available. | |||||
| PRED-PR: Prednisone poor-response, defined by ≥ 1000 leukemic blasts in peripheral blood after 7 days of prednisone and one dose of intrathecal methotrexate on day 1.12,80 In T cell ALL, only 3 patients were < 1 year old; therefore, data are not listed. | |||||
| CR: complete remission in the bone marrow (less than 5% blasts) after 6 weeks of induction therapy; no CR: no remission achieved, died due to disease. | |||||
| Abbreviations: CNS, central nervous system; pEFS, probability for event-free survival | |||||
| A. p-Bc ALL | |||||
| Total no. of patients | 90 | 1709 | 464 | 318 | 89 |
| Male | 50 | 54.6 | 52.4 | 54.7 | 53.9 |
| WBC (per μL) | |||||
| < 10,000 | 12.2 | 49.7 | 60.8 | 54.4 | 46.1 |
| 10–19,999 | 7.8 | 18.4 | 11.4 | 15.4 | 13.5 |
| 20–99,999 | 21.1 | 25.4 | 21.3 | 21.1 | 31.5 |
| ≥ 100,000 | 58.9 | 6.5 | 6.4 | 9.2 | 9.0 |
| CNS involvement | 11.1 | 1.4 | 0.4 | 1.3 | 4.5 |
| t(9;22) | – | 1.3 | 4.9 | 5.5 | 4.1 |
| PRED PR | 24.4 | 4.3 | 6.0 | 9.4 | 7.9 |
| Early death | 2.2 | 0.6 | 0.4 | 1.6 | 5.6 |
| No CR | 3.3 | 0.1 | 0.2 | 1.3 | 1.1 |
| Relapse | 45.6 | 15.8 | 25.4 | 29.9 | 28.1 |
| Death in CR | 2.2 | 1.1 | 1.3 | 3.8 | 0 |
| 10y-pEFS (SE) | 46.3 (5.3) | 80.4 (1.0) | 70.8 (2.2) | 60.6 (2.9) | 63.7 (5.3) |
| B. T Cell ALL | |||||
| Total no. of patients | 117 | 132 | 122 | 32 | |
| Male | 71.8 | 69.7 | 77.9 | 90.6 | |
| WBC (per μL) | |||||
| < 10,000 | 7.7 | 14.4 | 17.2 | 12.5 | |
| 10–19,999 | 6.8 | 12.9 | 9.0 | 3.1 | |
| 20–99,999 | 38.5 | 40.2 | 36.9 | 50 | |
| ≥ 100,000 | 47.0 | 32.6 | 36.9 | 34.4 | |
| CNS involvement | 10.3 | 9.1 | 7.4 | 12.5 | |
| PRED-PR | 32.5 | 29.5 | 32.0 | 40.6 | |
| Early death | 2.6 | 1.5 | – | – | |
| No CR | 2.6 | 2.3 | 2.5 | 3.1 | |
| Relapse | 22.2 | 26.5 | 31.1 | 37.5 | |
| Death in CR | 6.8 | 1.5 | 0 | 0 | |
| 10y-pEFS (SE) | 58.3 (4.8) | 66.1 (4.2) | 63.9 (4.5) | 58.5 (8.8) | |
Post-induction minimal residual disease (MRD) patterns by age and subtype.
| Trial . | Method Sensitivity . | Age Group . | n . | % Pts MRD Neg.*; Day of CR Control . | Ref. . |
|---|---|---|---|---|---|
| # in precursor B cell (p-Bc) ALL 64%, in T-ALL 10% | |||||
| * usually defined as being below the level of sensitivity | |||||
| + median MRD levels at day 29 were significantly higher in patients 1–<2y old, 6–9y old, or ≥10y old as compared to pts 2–5y old | |||||
| Abbreviations: EORTC, European Organisation for Research and Treatment of Cancer; SJCRH, St Jude’s Cancer Research Hospital; I-BFM-SG, International Berlin-Frankfurt-Münster Study Group; NOPHO, Nordic Society for Pediatric Hematology; TCR, T cell receptor; MRD, minimal residual disease; CR, complete response; PCR, polymerase chain reaction; T ALL, T cell acute lymphoblastic leukemia | |||||
| EORTC 58881 | TCR/IgH PCR 5 × 10−5 | up to 15y | 151 | 58 #; d 35 | 81 |
| SJCRH | Flow 1 × 10−4 | 1–9y ≥ 10y | 104 55 | 80.8; d 46 67.3; d 46 | 82 |
| I-BFM-SG MRD group | TCR/IgH PCR 1 × 10−4 | 1–9y 10–18y | 142 26 | 45.1;d 33 27.0; d 33 | updated from 83 |
| NOPHO | TCR/IgH PCR 1 × 10−4 | 1–15y | 100 | 40+; d 29 | 55 |
| Trial . | Method Sensitivity . | Age Group . | n . | % Pts MRD Neg.*; Day of CR Control . | Ref. . |
|---|---|---|---|---|---|
| # in precursor B cell (p-Bc) ALL 64%, in T-ALL 10% | |||||
| * usually defined as being below the level of sensitivity | |||||
| + median MRD levels at day 29 were significantly higher in patients 1–<2y old, 6–9y old, or ≥10y old as compared to pts 2–5y old | |||||
| Abbreviations: EORTC, European Organisation for Research and Treatment of Cancer; SJCRH, St Jude’s Cancer Research Hospital; I-BFM-SG, International Berlin-Frankfurt-Münster Study Group; NOPHO, Nordic Society for Pediatric Hematology; TCR, T cell receptor; MRD, minimal residual disease; CR, complete response; PCR, polymerase chain reaction; T ALL, T cell acute lymphoblastic leukemia | |||||
| EORTC 58881 | TCR/IgH PCR 5 × 10−5 | up to 15y | 151 | 58 #; d 35 | 81 |
| SJCRH | Flow 1 × 10−4 | 1–9y ≥ 10y | 104 55 | 80.8; d 46 67.3; d 46 | 82 |
| I-BFM-SG MRD group | TCR/IgH PCR 1 × 10−4 | 1–9y 10–18y | 142 26 | 45.1;d 33 27.0; d 33 | updated from 83 |
| NOPHO | TCR/IgH PCR 1 × 10−4 | 1–15y | 100 | 40+; d 29 | 55 |
Outcome comparison from various trials for teenage acute lymphoblastic leukemia (ALL) patients (≥10y).
| Trial . | Age Range . | n . | % of Total Study Population . | 5y-pEFS (SE) . | Ref. . |
|---|---|---|---|---|---|
| Abbreviations: pEFS, probability for event-free survival; AIEOP, Associazione Italiana Ematologia Oncologia Pediatrica; BFM, Berlin-Frankfurt-Münster; CCG, Children’s Cancer Group; COALL, Cooperative Study Group for Childhood Acute Lymphoblastic Leukemia; DFCI, Dana-Farber Cancer Institute; EORTC-CLCG, European Organisation for Research and Treatment of Cancer–Children Cooperative Leukemia Group; SJCRH, St Jude’s Cancer Research Hospital; NOPHO, Nordic Society for Pediatric Hematology;TCCSG, Tokyo Children’s Cancer Study Group; UKALL, United Kingdom Acute Lymphoblastic Leukemia | |||||
| AIEOP 91 | 10–17y | 190 | 15.9 | 52.5 (3.6) | 1 |
| BFM 90 | 10–18y | 386 | 17.7 | 64.4 (2.5) | 3 |
| CCG 1800 series | ≥ 10y | 1107 | 21.6 | 66.0 (2.0) | 2 |
| COALL 92 | 10–18y | 104 | 19.3 | 70.5 (4.6) | 84 |
| DFCI 91-01 | 10–18y | 69 | 18.3 | 79.0 (5.0) | 85 |
| EORTC-CLCG 58881 | 10–18y | 361 | 17.5 | 58.2 (2.9) | 4 |
| NOPHO 92-98 | 10–15y | 179 | 15.7 | 72.3 (3.6) | 5 |
| SJCRH 13A | 10–18y | 43 | 26.1 | 55.7 (7.6) | 68 |
| TCCSG L92-13 | ≥ 10y | 83 | 23.9 | 57.0 (5.8) | 86 |
| UKALL XI | 10–14y | 316 | 15.1 | 62 (2.9) | 87 |
| Trial . | Age Range . | n . | % of Total Study Population . | 5y-pEFS (SE) . | Ref. . |
|---|---|---|---|---|---|
| Abbreviations: pEFS, probability for event-free survival; AIEOP, Associazione Italiana Ematologia Oncologia Pediatrica; BFM, Berlin-Frankfurt-Münster; CCG, Children’s Cancer Group; COALL, Cooperative Study Group for Childhood Acute Lymphoblastic Leukemia; DFCI, Dana-Farber Cancer Institute; EORTC-CLCG, European Organisation for Research and Treatment of Cancer–Children Cooperative Leukemia Group; SJCRH, St Jude’s Cancer Research Hospital; NOPHO, Nordic Society for Pediatric Hematology;TCCSG, Tokyo Children’s Cancer Study Group; UKALL, United Kingdom Acute Lymphoblastic Leukemia | |||||
| AIEOP 91 | 10–17y | 190 | 15.9 | 52.5 (3.6) | 1 |
| BFM 90 | 10–18y | 386 | 17.7 | 64.4 (2.5) | 3 |
| CCG 1800 series | ≥ 10y | 1107 | 21.6 | 66.0 (2.0) | 2 |
| COALL 92 | 10–18y | 104 | 19.3 | 70.5 (4.6) | 84 |
| DFCI 91-01 | 10–18y | 69 | 18.3 | 79.0 (5.0) | 85 |
| EORTC-CLCG 58881 | 10–18y | 361 | 17.5 | 58.2 (2.9) | 4 |
| NOPHO 92-98 | 10–15y | 179 | 15.7 | 72.3 (3.6) | 5 |
| SJCRH 13A | 10–18y | 43 | 26.1 | 55.7 (7.6) | 68 |
| TCCSG L92-13 | ≥ 10y | 83 | 23.9 | 57.0 (5.8) | 86 |
| UKALL XI | 10–14y | 316 | 15.1 | 62 (2.9) | 87 |
Main genetic abnormalities associated with pediatric acute myeloid leukemia (AML).
| Karyotype . | Genetic Lesion . | Predominant FAB type . | Frequency (%) . |
|---|---|---|---|
| *Sole chromosomal abnormality | |||
| †Sole chromosomal abnormality in non-Down syndrome | |||
| t(8;21)(q22;q22) | AML1-ETO | M2, eosinophilia | 12–15 |
| 11q23 abnormalities | MLL-several | M4, M5 | 10–15 |
| t(9;11)(p21-22;q23) | MLL-AF9 | M5 | 6–8 |
| t(15;17)(q22;q12-21) | PML-RARα | M3, M3v | 8–10 |
| Inv16(p13q22)/t(16;16)(p13;q22) | CBFβ-MYH11 | M4Eo | 6–8 |
| t(10;11)(p13;q21) | AF10-CALM | Diverse | 2 |
| t(6;9)(p23;q34) | DEK-CAN | M2, basophilia | 1 |
| t(8;16)(p11;p13)/inv(8)(p11q13) | MOZ-CBP/TIF2 | M4, M5 | 1 |
| t(1;22)(p13;q13) | RBM15-MKL1 | M7 | < 1 |
| t(3;5)(q25;q34) | NPM-MFL1 | M2 | < 1 |
| t(9;22)(q34;q11) | BCR-ABL | M1 | < 1 |
| t(11;20)(p15;q11) | NUP98-TOP1 | – | < 1 |
| Monosomy 7 or 7q deletions | Dysplasia | 2 | |
| Trisomy 8* | Diverse | 2 | |
| Trisomy 21† | M7 | 2 | |
| Complex (≥ 3 abnormalities) | Diverse | 10–15 | |
| Normal | Diverse | 20–25 | |
| Karyotype . | Genetic Lesion . | Predominant FAB type . | Frequency (%) . |
|---|---|---|---|
| *Sole chromosomal abnormality | |||
| †Sole chromosomal abnormality in non-Down syndrome | |||
| t(8;21)(q22;q22) | AML1-ETO | M2, eosinophilia | 12–15 |
| 11q23 abnormalities | MLL-several | M4, M5 | 10–15 |
| t(9;11)(p21-22;q23) | MLL-AF9 | M5 | 6–8 |
| t(15;17)(q22;q12-21) | PML-RARα | M3, M3v | 8–10 |
| Inv16(p13q22)/t(16;16)(p13;q22) | CBFβ-MYH11 | M4Eo | 6–8 |
| t(10;11)(p13;q21) | AF10-CALM | Diverse | 2 |
| t(6;9)(p23;q34) | DEK-CAN | M2, basophilia | 1 |
| t(8;16)(p11;p13)/inv(8)(p11q13) | MOZ-CBP/TIF2 | M4, M5 | 1 |
| t(1;22)(p13;q13) | RBM15-MKL1 | M7 | < 1 |
| t(3;5)(q25;q34) | NPM-MFL1 | M2 | < 1 |
| t(9;22)(q34;q11) | BCR-ABL | M1 | < 1 |
| t(11;20)(p15;q11) | NUP98-TOP1 | – | < 1 |
| Monosomy 7 or 7q deletions | Dysplasia | 2 | |
| Trisomy 8* | Diverse | 2 | |
| Trisomy 21† | M7 | 2 | |
| Complex (≥ 3 abnormalities) | Diverse | 10–15 | |
| Normal | Diverse | 20–25 | |
Dose intensity of cytarabine, daunorubicin, and etoposide in selected acute myeloid leukemia (AML) trials.
| . | . | . | . | Cumulative Dose (mg/m2) . | ||||
|---|---|---|---|---|---|---|---|---|
| Study . | Number of Patients . | Number of Cycles . | Estimated Time to Assess Remission (weeks) . | Cytarabine . | Daunorubicin . | Etoposide . | CR Rate (%) . | Overall Survival Rate . |
| Some patients received idarubicin (12 mg of idarubicin = 60 mg of daunorubicin) and mitoxantrone (10 mg of mitoxantrone = 40 mg of daunorubicin). | ||||||||
| Abbreviations: POG, Pediatric Oncology Group; AML-91, acute myeloid leukemia-91 study (St. Jude Children’s Research Hospital); MRC-10/12, Medical Research Council-10/12 study; BFM-93 and Oncology-93 study; ANZCCSG-AML2, Australian and New Zealand Children’s Cancer Study Group AML-2 study; BFM, Berlin-Frankfurt-Munster-93 study; LAME89/91, Leucamie Aique Myeloide Enfant-89/90 study; NOPHO, Nordic Society for Paediatric Haematology | ||||||||
| POG 882124 | 649 | 2 | 6–8 | 18,700 | 135 | — | 85 | 3-yr, 42% |
| CCG-289125 (intensive timing) | 295 | 4 | 16–17 | 3200 | 320 | 1600 | 75 | 3-yr, 51% |
| AML-9126 | 73 | 3–4 | 8–12 | 1250–3750 | 90–270 | 400–1200 | 78 | 5-yr, 53% |
| MRC-1021 | 341 | 2 | 8–10 | 1800 | 300 | 0–1000 | 83 | 7-yr, 56% |
| MRC-129 | 564 | 2 | 8–10 | 1800 | 288–300* | 1000 | 92 | 5-yr, 68% |
| BFM-9322 | 471 | 4 | 16–22 | 21,200–39,200 | 264–380* | 950 | 82 | 5-yr, 60% |
| LAME-89/9123 | 268 | 2 | 6–8 | 1400–2000 | 225–315* | — | 90 | 6-yr, 60% |
| NOPHO-9317 | 219 | 2–322 | 8–6 | 1600–13,300 | 150–195* | 800–900 | 91 | 7-yr, 64% |
| ANZCCSG20 (AML–2) | 167 | 2 | 8–0 | 12,700–18,700 | 150 | — | 91 | 5-yr, 55% |
| . | . | . | . | Cumulative Dose (mg/m2) . | ||||
|---|---|---|---|---|---|---|---|---|
| Study . | Number of Patients . | Number of Cycles . | Estimated Time to Assess Remission (weeks) . | Cytarabine . | Daunorubicin . | Etoposide . | CR Rate (%) . | Overall Survival Rate . |
| Some patients received idarubicin (12 mg of idarubicin = 60 mg of daunorubicin) and mitoxantrone (10 mg of mitoxantrone = 40 mg of daunorubicin). | ||||||||
| Abbreviations: POG, Pediatric Oncology Group; AML-91, acute myeloid leukemia-91 study (St. Jude Children’s Research Hospital); MRC-10/12, Medical Research Council-10/12 study; BFM-93 and Oncology-93 study; ANZCCSG-AML2, Australian and New Zealand Children’s Cancer Study Group AML-2 study; BFM, Berlin-Frankfurt-Munster-93 study; LAME89/91, Leucamie Aique Myeloide Enfant-89/90 study; NOPHO, Nordic Society for Paediatric Haematology | ||||||||
| POG 882124 | 649 | 2 | 6–8 | 18,700 | 135 | — | 85 | 3-yr, 42% |
| CCG-289125 (intensive timing) | 295 | 4 | 16–17 | 3200 | 320 | 1600 | 75 | 3-yr, 51% |
| AML-9126 | 73 | 3–4 | 8–12 | 1250–3750 | 90–270 | 400–1200 | 78 | 5-yr, 53% |
| MRC-1021 | 341 | 2 | 8–10 | 1800 | 300 | 0–1000 | 83 | 7-yr, 56% |
| MRC-129 | 564 | 2 | 8–10 | 1800 | 288–300* | 1000 | 92 | 5-yr, 68% |
| BFM-9322 | 471 | 4 | 16–22 | 21,200–39,200 | 264–380* | 950 | 82 | 5-yr, 60% |
| LAME-89/9123 | 268 | 2 | 6–8 | 1400–2000 | 225–315* | — | 90 | 6-yr, 60% |
| NOPHO-9317 | 219 | 2–322 | 8–6 | 1600–13,300 | 150–195* | 800–900 | 91 | 7-yr, 64% |
| ANZCCSG20 (AML–2) | 167 | 2 | 8–0 | 12,700–18,700 | 150 | — | 91 | 5-yr, 55% |
Prognostic factors in pediatric acute myeloid leukemia (AML).
| *Intermediate prognosis group in some studies |
| Abbreviations: FAB, French-British-American classification; FLT3, FMS-like class III tyrosine kinase receptor gene; MLL, mixed-lineage leukemia gene |
| Favorable |
| AML with the t(15;17)(q22;q12-21) |
| AML with the inv(16)(p13q22) or t(16;16)(p13;q22) |
| AML with the t(8;21)(q22;q22)* |
| AML with the t(9;11)(p22;q23)* |
| AML in children with Down syndrome |
| AML in children under 1 year of age* |
| Intermediate or undefined |
| Acute megakaryoblastic leukemia |
| AML with other nonrandom chromosome abnormalities |
| AML with normal karyotype |
| FLT3 mutations/internal tandem duplications |
| MLL amplification |
| Expression of ATP-binding cassette (ABC) transporters |
| CCAAT/enhancer binding protein alpha gene mutations |
| Overexpression of ecotropic viral integration site-1 (EVI-1) |
| Unfavorable |
| AML with monosomy 7 or −7(q) |
| AML with the t(6;9)(p23;q34) |
| AML with complex karyotype |
| AML evolving from myelodysplastic syndrome |
| Persistent leukemia after 2 courses of intensive chemotherapy |
| Therapy-related AML |
| *Intermediate prognosis group in some studies |
| Abbreviations: FAB, French-British-American classification; FLT3, FMS-like class III tyrosine kinase receptor gene; MLL, mixed-lineage leukemia gene |
| Favorable |
| AML with the t(15;17)(q22;q12-21) |
| AML with the inv(16)(p13q22) or t(16;16)(p13;q22) |
| AML with the t(8;21)(q22;q22)* |
| AML with the t(9;11)(p22;q23)* |
| AML in children with Down syndrome |
| AML in children under 1 year of age* |
| Intermediate or undefined |
| Acute megakaryoblastic leukemia |
| AML with other nonrandom chromosome abnormalities |
| AML with normal karyotype |
| FLT3 mutations/internal tandem duplications |
| MLL amplification |
| Expression of ATP-binding cassette (ABC) transporters |
| CCAAT/enhancer binding protein alpha gene mutations |
| Overexpression of ecotropic viral integration site-1 (EVI-1) |
| Unfavorable |
| AML with monosomy 7 or −7(q) |
| AML with the t(6;9)(p23;q34) |
| AML with complex karyotype |
| AML evolving from myelodysplastic syndrome |
| Persistent leukemia after 2 courses of intensive chemotherapy |
| Therapy-related AML |
Diagnostic guidelines for juvenile myelomonocytic leukemia (JMML) (adapted from5).
| Suggestive clinical features | Hepatosplenomegaly |
| Lymphadenopathy | |
| Pallor | |
| Fever | |
| Skin rash | |
| Laboratory criteria | No Ph chromosome, no BCR-ABL rearrangement |
| Minimal criteria (all 3 must be fulfilled) | Peripheral blood monocyte count > 1 × 109/L Bone marrow blast < 20% |
| Criteria for definite diagnosis (at least 2 must be fulfilled) | Hemoglobin F increased for age |
| Myeloid precursors on peripheral blood smear | |
| White blood cell count > 10 × 109/L | |
| Clonal abnormality | |
| Granulocyte-macrophage colony-stimulating factor (GM- CSF) hypersensitivity of myeloid progenitors in vitro |
| Suggestive clinical features | Hepatosplenomegaly |
| Lymphadenopathy | |
| Pallor | |
| Fever | |
| Skin rash | |
| Laboratory criteria | No Ph chromosome, no BCR-ABL rearrangement |
| Minimal criteria (all 3 must be fulfilled) | Peripheral blood monocyte count > 1 × 109/L Bone marrow blast < 20% |
| Criteria for definite diagnosis (at least 2 must be fulfilled) | Hemoglobin F increased for age |
| Myeloid precursors on peripheral blood smear | |
| White blood cell count > 10 × 109/L | |
| Clonal abnormality | |
| Granulocyte-macrophage colony-stimulating factor (GM- CSF) hypersensitivity of myeloid progenitors in vitro |
Results of cytogenetics of patients with primary myelodysplastic syndrome (MDS) and those with MDS secondary to chemotherapy or radiation therapy in study EWOG-MDS 98.*
| . | Primary MDS (%) . | |||
|---|---|---|---|---|
| Karyotype . | All (n = 199) . | RC (n = 105) . | RAEB/RAEB-T (n = 94) . | MDS Secondary to Chemotherapy or Radiation Therapy (%) (n = 44) . |
| * MDS secondary to acquired or congenital bone marrow failure disorders and familial MDS were excluded from the analysis. EWOG-MDS unpublished data. | ||||
| Abbreviations: RC, refractory cytopenia; RAEB, refractory anemia with excess blasts; RAEB-T, RAEB in transformation; EWOG-MDS, European Working Group of Myelodysplasia in Childhood | ||||
| Complex (≥ 3 abnormalities) | 6 | 3 | 9 | 36 |
| Monosomy 7 | 24 | 17 | 32 | 23 |
| Trisomy 8 | 4 | 3 | 5 | 0 |
| Other abnormalities | 9 | 5 | 14 | 23 |
| Normal karyotype | 57 | 72 | 40 | 18 |
| . | Primary MDS (%) . | |||
|---|---|---|---|---|
| Karyotype . | All (n = 199) . | RC (n = 105) . | RAEB/RAEB-T (n = 94) . | MDS Secondary to Chemotherapy or Radiation Therapy (%) (n = 44) . |
| * MDS secondary to acquired or congenital bone marrow failure disorders and familial MDS were excluded from the analysis. EWOG-MDS unpublished data. | ||||
| Abbreviations: RC, refractory cytopenia; RAEB, refractory anemia with excess blasts; RAEB-T, RAEB in transformation; EWOG-MDS, European Working Group of Myelodysplasia in Childhood | ||||
| Complex (≥ 3 abnormalities) | 6 | 3 | 9 | 36 |
| Monosomy 7 | 24 | 17 | 32 | 23 |
| Trisomy 8 | 4 | 3 | 5 | 0 |
| Other abnormalities | 9 | 5 | 14 | 23 |
| Normal karyotype | 57 | 72 | 40 | 18 |
Treatment results in age subgroups by gender (ALL-BFM 86 and 90).
A: 10y-probability for event-free survival (pEFS) in female ALL patients (SE, standard error)
Log-rank: P < 0.01, except for: < 1y vs 10–14y: P = 0.04; 6–9y vs 10–14y: P = 0.03; 6–9y vs. 15–18y: P = 0.02; 10–14y vs. 15–18y, and < 1y vs 15–18y: P = n.s.
B: 10y-pEFS in male ALL patients
Log-rank: P < 0.01, except for: 6–9y vs 10–14y: P = 0.01; 6–9y and 10–14y vs 15–18y: P = n.s.
Treatment results in age subgroups by gender (ALL-BFM 86 and 90).
A: 10y-probability for event-free survival (pEFS) in female ALL patients (SE, standard error)
Log-rank: P < 0.01, except for: < 1y vs 10–14y: P = 0.04; 6–9y vs 10–14y: P = 0.03; 6–9y vs. 15–18y: P = 0.02; 10–14y vs. 15–18y, and < 1y vs 15–18y: P = n.s.
B: 10y-pEFS in male ALL patients
Log-rank: P < 0.01, except for: 6–9y vs 10–14y: P = 0.01; 6–9y and 10–14y vs 15–18y: P = n.s.
Treatment results in age subgroups by initial white blood cell count (WBC) (ALL-BFM 86 and 90).
A: 10y-probability for event-free survival (pEFS) in patients with initial WBC < 20,000
Log-rank: P < 0.01, except for all comparisons with infant acute lymphoblastic leukemia (ALL) (< 1y); 6–9y vs 15–18y: P = 0.03; 10–14y vs 15–18y: P = n.s.
B: 10y-pEFS in patients with initial WBC ≥ 20,000
Log-rank: P < 0.01 for all comparisons, except for: <1y vs 15–18y: P = 0.04; 6–9y vs. 10–14y or 15–18y: P = n.s.; 10–14y vs 15–18y: P = n.s.
Treatment results in age subgroups by initial white blood cell count (WBC) (ALL-BFM 86 and 90).
A: 10y-probability for event-free survival (pEFS) in patients with initial WBC < 20,000
Log-rank: P < 0.01, except for all comparisons with infant acute lymphoblastic leukemia (ALL) (< 1y); 6–9y vs 15–18y: P = 0.03; 10–14y vs 15–18y: P = n.s.
B: 10y-pEFS in patients with initial WBC ≥ 20,000
Log-rank: P < 0.01 for all comparisons, except for: <1y vs 15–18y: P = 0.04; 6–9y vs. 10–14y or 15–18y: P = n.s.; 10–14y vs 15–18y: P = n.s.
Treatment results in precursor B cell acute lymphoblastic leukemia (p-Bc-ALL) and T cell ALL by early response (ALL-BFM 86 and 90).
A: 10y-probability for event-free survival (pEFS) in p-Bc ALL with < 1,000 leukemic cells at day 8 (prednisone good response)
Log-rank: P < 0.01 for all comparisons, except for: 6–9y vs 15–18y: P = 0.04; < 1y vs. 10–14y or 15–18y, 10–14y vs 15–18y: P = n.s.
B: 10y-pEFS in p-Bc ALL with ≥ 1,000 leukemic cells at day 8 (prednisone poor response)
Log-rank: P < 0.01 for all comparisons, except for: < 1y vs 6–9y: P = 0.02; < 1 vs. 10–14y: P = 0.04; < 1y vs 15–18y, 6–9y vs 10–14y, 6–9y vs 15–18y, and 10–14y vs 15–18y: P = n.s.
C: 10y-pEFS in T-cell ALL with < 1,000 leukemic cells at day 8 (prednisone good response)
Log-rank: P = n.s.
D: 10y-pEFS in T-cell ALL with ≥ 1,000 leukemic cells at day 8 (prednisone poor response)
Log-rank: P = n.s.
Treatment results in precursor B cell acute lymphoblastic leukemia (p-Bc-ALL) and T cell ALL by early response (ALL-BFM 86 and 90).
A: 10y-probability for event-free survival (pEFS) in p-Bc ALL with < 1,000 leukemic cells at day 8 (prednisone good response)
Log-rank: P < 0.01 for all comparisons, except for: 6–9y vs 15–18y: P = 0.04; < 1y vs. 10–14y or 15–18y, 10–14y vs 15–18y: P = n.s.
B: 10y-pEFS in p-Bc ALL with ≥ 1,000 leukemic cells at day 8 (prednisone poor response)
Log-rank: P < 0.01 for all comparisons, except for: < 1y vs 6–9y: P = 0.02; < 1 vs. 10–14y: P = 0.04; < 1y vs 15–18y, 6–9y vs 10–14y, 6–9y vs 15–18y, and 10–14y vs 15–18y: P = n.s.
C: 10y-pEFS in T-cell ALL with < 1,000 leukemic cells at day 8 (prednisone good response)
Log-rank: P = n.s.
D: 10y-pEFS in T-cell ALL with ≥ 1,000 leukemic cells at day 8 (prednisone poor response)
Log-rank: P = n.s.
Model outlining the roles of SHP-2, RAS, and NF1 in the granulocyte-macrophage colony-stimulating factor (GM-CSF) signal transduction pathway. In juvenile myelomonocytic leukemia (JMML), molecular alterations have been demonstrated inPTPN11(the gene encoding SHP-2),RASandNF1in approximately 35%, 25% and 25% of patients, respectively.
Model outlining the roles of SHP-2, RAS, and NF1 in the granulocyte-macrophage colony-stimulating factor (GM-CSF) signal transduction pathway. In juvenile myelomonocytic leukemia (JMML), molecular alterations have been demonstrated inPTPN11(the gene encoding SHP-2),RASandNF1in approximately 35%, 25% and 25% of patients, respectively.
Overall survival, event-free survival (EFS), relapse incidence (RI) and transplant-related mortality (TRM) of 100 children with juvenile myelomonocytic leukemia (JMML) transplanted after a conditioning regimen including busulfan, cyclophosphamide and melphalan from a matched related (n = 48) or a matched unrelated (n = 52) donor.13
Overall survival, event-free survival (EFS), relapse incidence (RI) and transplant-related mortality (TRM) of 100 children with juvenile myelomonocytic leukemia (JMML) transplanted after a conditioning regimen including busulfan, cyclophosphamide and melphalan from a matched related (n = 48) or a matched unrelated (n = 52) donor.13
Relapse incidence (left side) and event-free survival (right side) by age at diagnosis of 100 children with juvenile myelomonocytic leukemia (JMML) transplanted after a conditioning regimen including busulfan, cyclophosphamide and melphalan from an matched related (n = 48) or a matched unrelated (n = 52) donor.13
Relapse incidence (left side) and event-free survival (right side) by age at diagnosis of 100 children with juvenile myelomonocytic leukemia (JMML) transplanted after a conditioning regimen including busulfan, cyclophosphamide and melphalan from an matched related (n = 48) or a matched unrelated (n = 52) donor.13
Cumulative incidence of progression to advanced myelodysplastic syndrome (MDS) for patients with refractory anemia and either normal karyotype, monosomy 7, or trisomy 8 or other abnormalities at the time of diagnosis. Patients who had received stem cell transplantation were censored at time of transplantation.17
Cumulative incidence of progression to advanced myelodysplastic syndrome (MDS) for patients with refractory anemia and either normal karyotype, monosomy 7, or trisomy 8 or other abnormalities at the time of diagnosis. Patients who had received stem cell transplantation were censored at time of transplantation.17