Abstract
Heparin-induced thrombocytopenia, or HIT, can present in many ways, ranging from common—isolated thrombocytopenia, venous thromboembolism, acute limb ischemia—to less common but specific presentations—necrotizing skin lesions at heparin injection sites, post-bolus acute systemic reactions, and adrenal hemorrhagic necrosis (secondary to adrenal vein thrombosis). Many patients with HIT have mild or moderate thrombocytopenia: the median platelet count nadir is 60 × 109/L, and ranges from 15 to 150 × 109/L in 90% of patients, most of whom evince a 50% or greater fall in the platelet count. HIT that begins after stopping heparin (“delayed-onset HIT”) is increasingly recognized. Factors influencing risk of HIT include type of heparin (unfractionated heparin > low-molecular-weight heparin), type of patient (surgical > medical), and gender (female > male). Since timely diagnosis and treatment of HIT may reduce the risk of adverse outcomes, this review focuses on those clinical circumstances that should prompt the clinician to “think of HIT.” Coumarin anticoagulants such as warfarin are ineffective in acute HIT and can even be deleterious by predisposing to micro-thrombosis via protein C depletion (venous limb gangrene and skin necrosis syndromes). Thus, it is important to avoid or postpone coumarin while managing HIT hypercoagulability, focusing on agents that inhibit thrombin directly (lepirudin, argatroban) or that inhibit its generation (danaparoid, ?fondaparinux). Post-marketing experience suggests that standard dosing of lepirudin is too high; current recommendations are to avoid the initial lepirudin bolus and to begin with lower infusion rates, even in patients without overt renal dysfunction.
Heparin-induced thrombocytopenia (HIT) is an acquired, transient, prothrombotic disorder that, ironically, is caused by the anticoagulant heparin. Another irony is that HIT is a high-risk situation for coumarin-induced microthrombosis (“Coumadin necrosis”), at least 100 times greater than in non-HIT situations (5–10% vs 0.01%).1 I will summarize different ways that HIT can present, and thus trigger the clinician to “think of HIT.”
HIT is caused by platelet-activating antibodies of IgG class that recognize complexes of platelet factor 4 (PF4) and heparin (or certain other polyanions). It is strongly associated with thrombosis (odds ratio, 20 to 40)2 and is a hypercoagulability disorder (markedly elevated thrombin-antithrombin complexes).3 This reflects in vivo platelet activation (including formation of procoagulant, platelet-derived microparticles) and, possibly, activation of endothelium and monocytes (for review 4).
The frequency of HIT involves several factors (Table 1 ).5–7 These include heparin type (unfractionated heparin [UFH] > low-molecular-weight heparin [LMWH] >? fondaparinux), patient type (surgical > medical > pregnancy/neonatal), and gender (female > male).5–7 The role of heparin dose is less clear, but even heparin flushes can cause HIT antibody formation or even clinical HIT on rare occasions.5 The most common scenario for HIT is 1 to 2 weeks of antithrombotic prophylaxis with UFH in a postoperative patient (frequency, 1–5%). This is a situation in which frequent platelet count monitoring is recommended.8
Thrombocytopenia for many non-HIT reasons is common in the first days after starting heparin, especially in postoperative or critically ill patients. Physicians can become inured to the common coexistence of heparin and low platelets, and may not appreciate the import of a platelet count fall that begins or recurs a few days later.
Classic Presentation
A highly characteristic clinical profile for HIT is summarized by the 4 T’s Clinical Scoring System: Thrombocy-topenia plus Thrombosis plus Timing (in relation to heparin use) in the absence of oTher explanations.9 The classic picture of arterial thrombosis—especially of lower limb arteries—has evolved to the recognition that even more patients with HIT have symptomatic venous thromboembolism (deep-vein thrombosis [DVT], pulmonary embolism).5
How Should Thrombocytopenia in HIT Be Defined?
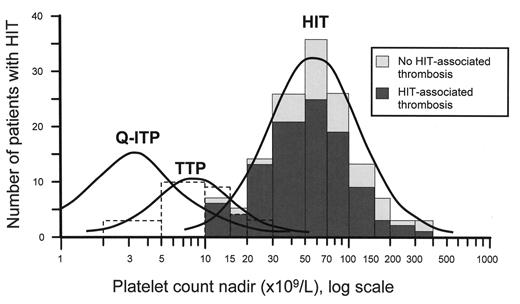
The standard definition of thrombocytopenia is a platelet count less than 150 × 109/L. This definition, which is based on the platelet count distribution of a normal population, is reasonably useful when assessing whether a stable out-patient could have a disorder affecting platelets. However, it is not as helpful for the dynamic circumstances of hospitalized inpatients. For example, consider a patient with a baseline platelet count of 155 × 109/L whose platelet count subsequently declines to 145 × 109/L; despite meeting the definition for thrombocytopenia, it is unlikely there is a major platelet disorder present. This might explain why older studies of HIT often defined thrombocytopenia as a platelet count fall to below 100 × 109/L, since at least a 33% drop in platelet count was required (assuming a normal baseline) to meet the threshold.
As noted, HIT is more common in surgical patients.5–7 Since platelet counts typically rise from postoperative day 2 to reach a peak 2 weeks after surgery, this means that HIT can complicate postoperative thrombocytosis.10,11 Thus, defining thrombocytopenia as a proportional (relative) platelet count fall of 50% or more from the postoperative peak has greater sensitivity for detecting HIT than using an absolute platelet count threshold, without loss of specificity.10,11 Some investigators use lower platelet count falls (40%11 or even 30%12) to define thrombocytopenia. The appropriate “baseline” platelet count is not the preoperative (pre-heparin) value, but rather the peak postoperative count that precedes the fall indicating HIT.10
Miscellaneous Sequelae of HIT
Some clinical features are relatively specific for HIT, including skin lesions at heparin injection sites,15,16 acute systemic reactions after intravenous bolus heparin,15,17 and adrenal hemorrhagic necrosis.15,18
Heparin-induced skin lesions occur in about 10% to 20% of patients who develop HIT in association with subcutaneous UFH or LMWH injections.15,16 Both necrotizing and non-necrotizing lesions (erythematous plaques) are reported. Not all patients who develop heparin-induced skin lesions develop thrombocytopenia, but among those who do, arterial thrombosis is especially common.
Giving an intravenous heparin bolus to a patient with circulating HIT antibodies can trigger abrupt onset of an acute systemic reaction 5 to 30 minutes later (or up to 2 hours after subcutaneous UFH or LMWH injection).15,17 Various inflammatory (fever, chills, flushing), cardiorespiratory (tachycardia, hypertension, tachypnea, dyspnea, cardiac or respiratory arrest), neurologic (pounding headache, transient global amnesia), and/or gastrointestinal (large-volume diarrhea) signs and symptoms can occur. An abrupt platelet count drop accompanies these reactions.
Adrenal hemorrhagic necrosis occurs in 3 to 5% of HIT patients, and is characterized by abdominal or flank pain; when necrosis is bilateral, death from adrenal crisis can result, which is preventable with timely use of corticosteroids.15,18 The pathogenesis is adrenal vein thrombosis, with secondary hemorrhagic infarction.
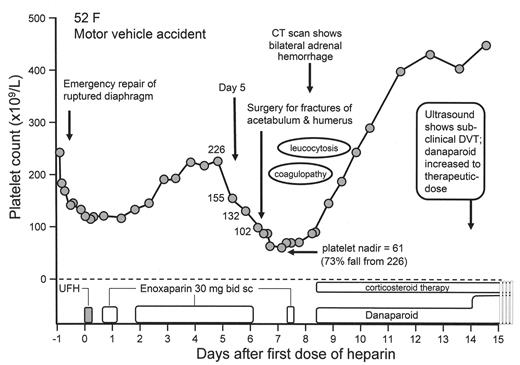
Figure 2 shows the clinical course of a patient who developed thrombocytopenia and bilateral adrenal hemorrhage during LMWH use. A superficial analysis might suggest HIT to be unlikely—after all, hemorrhage is not a feature of HIT, LMWH is not a common cause of HIT, and these events occurred in a post-trauma patient who had undergone two major operations. Yet, when one considers that the patient received a dose of UFH, that the timing of onset of thrombocytopenia was precisely 5 days after this single UFH injection, that a large platelet count fall occurred otherwise inexplicably on the day before the second operation, and that adrenal hemorrhage is a manifestation of adrenal vein thrombosis, then it is clear that the patient has a high pretest probability for HIT.
Coumarin-induced Microthrombosis
There are two forms of coumarin-induced necrosis, the classic syndrome of skin necrosis (non-acral dermal and sub-dermal necrosis) and venous limb gangrene (acral necrosis of a limb, usually one affected by DVT).1 Coumarin-induced necrosis complicating HIT is much more likely to manifest as venous limb gangrene.3 The pathogenesis is a profound disturbance in procoagulant-anticoagulant balance, namely interaction of HIT-associated hypercoagulability (increased thrombin generation) and coumarin-induced compromise of the protein C natural anticoagulant pathway. Micro-thrombosis results from impaired ability of protein C to downregulate thrombin in the microcirculation.
Delayed-onset HIT and/or Delayed Platelet Count Recovery from HIT
Sometimes, HIT resembles an autoimmune disorder, either because the onset of thrombocytopenia begins after heparin has been stopped (“delayed-onset HIT”), and/or the thrombocytopenia persists for a week or more despite stopping all heparin15,20,21 (the median time to platelet count recovery is 4 days, and 90% of patients recover to a platelet count of > 150 × 109/L within 1 week15). Patients with delayed-onset HIT may return to hospital because of thrombosis, at which time thrombocytopenia is recognized, and the inciting role of the recent heparin exposure appreciated. Patients with delayed-onset HIT have strong positive testing for HIT antibodies (usually > 1.5 units by ELISA), including many whose serum can activate platelets even in the absence of added heparin.20 High platelet-associated PF4 levels could also be relevant to the pathogenesis of delayed-onset HIT.22 In patients with very slow platelet count recovery that causes the diagnosis to be questioned, serologic evidence of persisting high levels of antibodies supports a diagnosis of HIT. On exceptionally rare occasions, an illness clinically and serologically identical to HIT can arise without preceding exposure to heparin (“spontaneous HIT”).23
Thrombocytopenia without Thrombosis but Timing Consistent with HIT
About half of all patients with HIT are recognized because of thrombocytopenia rather than thrombosis.24 Such patients with “isolated HIT” nevertheless have a high risk of subsequent symptomatic thrombosis if the heparin is simply stopped or replaced by coumarin. Thrombotic event-rates range from 36% (positive ELISA with optical density > 1.0 units) to 52% (positive platelet serotonin release assay).24,25
The likelihood of HIT (pending availability of HIT antibody test results) depends on the timing and magnitude of the platelet count fall, as well as whether there are other plausible explanations for the thrombocytopenia.9 To provide some perspective, consider the patient who develops a 50% or greater fall in the platelet count beginning between day 5 and 10 of UFH thromboprophylaxis after orthopedic surgery. In one study, about 75% of patients with this clinical profile had HIT, whether or not thrombosis was already apparent at the time of thrombocytopenia.10 In contrast, if a thrombocytopenic patient is in the intensive care unit, and thrombocytopenia in the absence of thrombosis is evaluated without considering its temporal relationship to heparin, then the probability of HIT is less than 1 in 100. This assertion is based upon the reported frequencies of thrombocytopenia in the ICU (30% to 50%) compared with the reported frequency of serologically confirmed HIT in critically ill patients (0.3% to 0.5%).26
Thrombosis without Thrombocytopenia but Timing Consistent with HIT
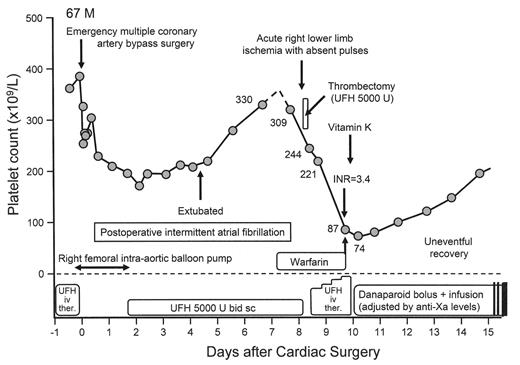
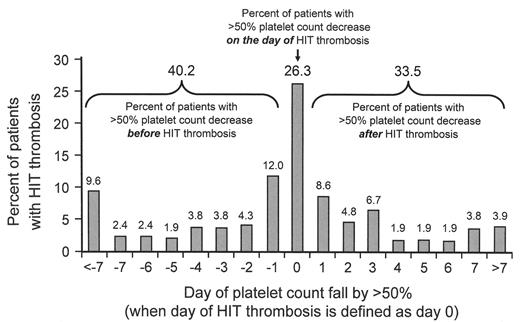
About half of all patients with HIT already have symptomatic HIT-associated thrombosis at the time of diagnosis.12,24 One reason is that thrombosis can precede the onset of thrombocytopenia, as illustrated in Figure 3A . That this clinical profile is not uncommon was shown in a recent study12 where 60% of patients with HIT-associated thrombosis developed thrombocytopenia either on or after the day that HIT-associated thrombosis occurred (Figure 3B ). Thus, a thrombotic event that occurs 5 to 14 days (occasionally later) following initiation of heparin could represent HIT-associated thrombosis, even if the platelet count is normal. Sometimes, the thrombocytopenia is “unmasked” only once therapeutic-dose heparin is given to treat the thrombosis that on platelet count grounds did not initially appear to suggest the diagnosis of HIT (Figure 3A ).
What is the likelihood that a thrombotic event that occurs during, or soon after, heparin treatment could represent the initial presentation of HIT? Levine and colleagues27 performed a meta-analysis of studies of heparin-treated patients in which data on frequencies of venous thromboembolism (VTE) and HIT occurrence were available. They concluded that about 1 in 8 patients with post-UFH thrombosis had HIT, whereas the frequency was less than 1 in 100 if preceding therapy had been with LMWH. Another study28 investigating 140 patients with acute coronary syndrome who had been in hospital within the previous 6 months found that 4.3% had detectable platelet-activating anti-PF4/heparin antibodies (whether any patients subsequently developed HIT upon further heparin use was not reported). Thus, it is possible that a patient recently treated with heparin (particularly UFH) may have “latent HIT” if he (or she) presents with symptomatic thrombosis 5 to 30 days (rarely later) after heparin use, even if the platelet count is normal. How might such a patient be managed, given that rapid testing for HIT antibodies is not widely performed? In theory, the use of the non-heparin anticoagulant, fondaparinux, could be appropriate. This agent is approved for VTE prophylaxis and treatment, and is effective for treating acute coronary syndrome.29 Moreover, fondaparinux does not cross-react with HIT antibodies.30 Thus, its use in the setting of “post-heparin thrombosis” should avoid the potential to “unmask” latent HIT. However, use of an approved drug for HIT is the mainstay of therapy if the platelet count indicates HIT.
Treatment of HIT
Table 2 lists six treatment principles for strongly-suspected (or confirmed) HIT. Stopping heparin does not prevent onset, progression, or recurrence of thrombosis, and in theory could even increase risk of HIT-associated thrombosis (absence of anticoagulant effect). Thus, it is important to administer a rapidly acting non-heparin anticoagulant when HIT is the likely diagnosis, even before results of HIT antibody testing are available.8 Treatment should continue until complete platelet count recovery has occurred, postponing overlapping warfarin until substantial platelet count recovery is evident (v.i.).8 Three to 6 months of warfarin is often given for patients with HIT-associated thrombosis (HIT is a transient disorder and thus is not a reason for longer duration of warfarin therapy than otherwise indicated based upon the type of thrombosis).
Warfarin is ineffective and can even be deleterious in patients with HIT. Coumarins are ineffective for treating hypercoagulability states such as HIT, cancer-associated DIC, or sepsis-associated thrombosis, as they do not inhibit thrombin generation (they impair synthesis, rather than inhibit the action, of the vitamin K–dependent factors). Indeed, coumarin use can lead to microthrombosis due to impairment of the protein C anticoagulant pathway.3 But there is a further problem with coumarins: their use may lead to underdosing of direct thrombin inhibitor therapy (DTI).31 This is because coumarins prolong the activated partial thromboplastin time (aPTT), which is how DTIs are most commonly monitored. Thus, vitamin K is recommended when HIT is diagnosed after coumarin has been administered.8,31 Once HIT hypercoagulability has subsided—as assessed by platelet count recovery—it is usually safe to begin warfarin. It is prudent to postpone warfarin until the platelet count has risen substantially (preferably, > 150 × 109/L), and to begin warfarin only as overlapping therapy (for at least 5 days) with an agent that inhibits thrombin or its generation.
Major bleeding is a risk of DTIs, especially with high drug levels. Recent studies indicate that lepirudin accumulation is common with standard (package insert) dosing even if renal impairment is minor. Secondary analysis of three prospective studies of lepirudin found high bleeding rates if the serum creatinine levels were minimally elevated.32 Retrospective studies show that use of lower doses than those recommended by the manufacturer are associated with less bleeding and without compromised efficacy.33,34 Thus, current trends are to avoid the initial lepirudin bolus, and to start with a lower infusion rate (0.05 to 0.10 mg/kg/hr). Monitoring of the aPTT should continue at 4-hour intervals until it is clear that the aPTT is within the therapeutic range and stable (no longer rising). Particularly in critically ill patients, trends to lower argatroban dosing are also reported.35
Postponement of warfarin is especially important during treatment with argatroban. Higher molar concentrations of argatroban, compared with lepirudin (about 20-fold), required to reach therapeutic effect (judged roughly by twofold prolongation of aPTT) is the explanation for the disproportionate prolongation of the INR by argatroban.36 INR prolongation can cause confusion during DTI–warfarin overlap. The reported efficacy of argatroban (hazard ratio of 0.33 to 0.39, assessed using a thrombotic end point37) could underestimate its true efficacy, given that the prospective trials were performed prior to recognition of the danger of warfarin, and duration of argatroban use was relatively brief (mean, 6 to 7 days).2
An additional treatment option in non-U.S. jurisdictions is danaparoid, a mixture of non-heparin anticoagulant glycosaminoglycans. Unlike historically controlled studies of DTIs, in which major bleeding rates were higher with DTIs than controls, bleeding rates with danaparoid were lower than in controls.38 Besides this favorable therapeutic index, other advantages of danaparoid include availability of both prophylactic and therapeutic dosing regimens, availability of direct measurements of drug levels (anti–factor Xa levels), lack of INR prolongation, and a long half-life (avoids rebound hypercoagulability if the drug is abruptly stopped). A potential negative is in vivo cross-reactivity of HIT antibodies with danaparoid, but this is uncommon, and assessing for in vitro crossreactivity prior to use of danaparoid is not recommended.8
Fondaparinux shares many of these advantages of danaparoid, without the potential disadvantage of HIT antibody crossreactivity. However, insufficient data on treatment outcomes in well-characterized HIT patients and uncertainty regarding optimal dosing prevent any firm recommendations.
Is HIT Overdiagnosed?
This review, which focuses on pointing out those clinical features that should prompt the clinician to “think of HIT,” could be thought to imply that HIT may be underdiagnosed. And, indeed, there may be occasions when an atypical presentation does result in failure to diagnose. However, there is increasing evidence that HIT may be overdiagnosed. This is because the vast majority of anti-PF4/heparin immune responses—especially when detected by the anti-PF4/heparin ELISA—do not result in thrombocytopenia, thrombosis, or other clinical sequelae.39 Thus, testing for antibodies in low probability situations for HIT raises the possibility that detecting non-pathogenic anti-PF4/heparin antibodies will lead to a false diagnosis of HIT when in fact the thrombocytopenia is explained by something else. Recent studies have estimated the potential for overdiagnosis by about 100%; in other words, for every 2 patients who are thought to have HIT because clinical “suspicion” is supported by a positive ELISA, only one really has HIT, based upon assessment of pretest probability and the presence of platelet-activating antibodies.9,40 This underscores the importance of performing diagnostic testing with more specific assays in situations when the pretest probability of HIT is not high, or if the results of the ELISA are not convincing (e.g., absorbance <1.0 units). Use of ELISAs aimed at detecting only IgG class antibodies will improve diagnostic specificity.39,40
Platelet count nadirs in heparin-induced thrombocytopenia (HIT), quinine-induced immune thrombocytopenic purpura (Q-ITP), and thrombotic thrombocytopenic purpura (TTP) with absent ADAMTS-13 activity.
The data for HIT, including the breakdown between patients with and without HIT-associated thrombosis, are published elsewhere (and reprinted with permission).13 The curve for Q-ITP is estimated from published data,14 and the data for TTP were kindly provided by Dr. James George (with permission).
Platelet count nadirs in heparin-induced thrombocytopenia (HIT), quinine-induced immune thrombocytopenic purpura (Q-ITP), and thrombotic thrombocytopenic purpura (TTP) with absent ADAMTS-13 activity.
The data for HIT, including the breakdown between patients with and without HIT-associated thrombosis, are published elsewhere (and reprinted with permission).13 The curve for Q-ITP is estimated from published data,14 and the data for TTP were kindly provided by Dr. James George (with permission).
Bilateral adrenal hemorrhagic infarction complicating heparin-induced thrombocytopenia (HIT).
The patient, a 52-year-old female with serious injuries sustained in a motor vehicle accident, had a high pretest probability for HIT, based upon clinical events that occurred during the first 10 hospital days. The pretest probability score (4 T’s clinical scoring system) was 8 (the maximum score), based upon 55% platelet count fall (2 points) that began on day 5 of heparin therapy and that preceded the second operation (2 points), with thrombotic manifestations (bilateral adrenal infarction and deep-vein thrombosis; 2 points) without other apparent explanation (2 points). The clinical diagnosis was supported by strong positive testing for HIT antibodies (99% serotonin release; normal < 10%; 2.245 optical density units in an in-house anti-PF4/heparin ELISA that detects IgG class antibodies; normal < 0.450 units). Coagulopathy (elevated INR) and leukocytosis were attributed to HIT and the early stages of adrenal crisis, respectively, and both abnormalities resolved with danaparoid anticoagulation and corticosteroids. The patient was switched from prophylactic-dose to therapeutic-dose danaparoid when routine duplex ultrasound detected a subclinical DVT involving a small area of the left femoral and popliteal veins.
Abbreviations: bid, twice-daily; DVT, deep-vein thrombosis; sc, subcutaneous; UFH, unfractionated heparin.
Bilateral adrenal hemorrhagic infarction complicating heparin-induced thrombocytopenia (HIT).
The patient, a 52-year-old female with serious injuries sustained in a motor vehicle accident, had a high pretest probability for HIT, based upon clinical events that occurred during the first 10 hospital days. The pretest probability score (4 T’s clinical scoring system) was 8 (the maximum score), based upon 55% platelet count fall (2 points) that began on day 5 of heparin therapy and that preceded the second operation (2 points), with thrombotic manifestations (bilateral adrenal infarction and deep-vein thrombosis; 2 points) without other apparent explanation (2 points). The clinical diagnosis was supported by strong positive testing for HIT antibodies (99% serotonin release; normal < 10%; 2.245 optical density units in an in-house anti-PF4/heparin ELISA that detects IgG class antibodies; normal < 0.450 units). Coagulopathy (elevated INR) and leukocytosis were attributed to HIT and the early stages of adrenal crisis, respectively, and both abnormalities resolved with danaparoid anticoagulation and corticosteroids. The patient was switched from prophylactic-dose to therapeutic-dose danaparoid when routine duplex ultrasound detected a subclinical DVT involving a small area of the left femoral and popliteal veins.
Abbreviations: bid, twice-daily; DVT, deep-vein thrombosis; sc, subcutaneous; UFH, unfractionated heparin.
A 67-year-old male developed acute right lower limb ischemia with absent pulses 8 days after emergency coronary artery bypass surgery.
The platelet count (309 × 109/L) had fallen only minimally (from 330 × 109/L) and the limb ischemia was attributed to either cardiac embolism (secondary to postoperative atrial fibrillation) or local right femoral injury (secondary to recent use of an intra-aortic balloon pump). After limb-salvaging thrombectomy (with intraoperative use of UFH), the patient received postoperative therapeutic-dose UFH monitored by the aPTT. Progressive decline in the platelet count by 78% to 74 × 109/L prompted the diagnosis of HIT, at which time the UFH was switched to danaparoid. Furthermore, vitamin K was given to reverse warfarin anticoagulation. Interestingly, a cool and painful left foot improved rapidly following treatment with vitamin K and danaparoid (not shown on the graph).
Abbreviations: bid, twice-daily; iv ther., intravenous therapeutic-dose; sc, subcutaneous; U, units; UFH, unfractionated heparin.
A 67-year-old male developed acute right lower limb ischemia with absent pulses 8 days after emergency coronary artery bypass surgery.
The platelet count (309 × 109/L) had fallen only minimally (from 330 × 109/L) and the limb ischemia was attributed to either cardiac embolism (secondary to postoperative atrial fibrillation) or local right femoral injury (secondary to recent use of an intra-aortic balloon pump). After limb-salvaging thrombectomy (with intraoperative use of UFH), the patient received postoperative therapeutic-dose UFH monitored by the aPTT. Progressive decline in the platelet count by 78% to 74 × 109/L prompted the diagnosis of HIT, at which time the UFH was switched to danaparoid. Furthermore, vitamin K was given to reverse warfarin anticoagulation. Interestingly, a cool and painful left foot improved rapidly following treatment with vitamin K and danaparoid (not shown on the graph).
Abbreviations: bid, twice-daily; iv ther., intravenous therapeutic-dose; sc, subcutaneous; U, units; UFH, unfractionated heparin.
Relation of onset of platelet count decrease and onset of HIT-associated thrombosis.
The data summarize 209 patients with HIT-associated thrombosis. About one quarter (26.3%) of patients develop thrombosis on the same day that the thrombocytopenia occurs (defined arbitrarily as the day the platelet count has fallen by more than 50%), and in 33.5% the platelet count reached thrombocytopenia levels only after the occurrence of thrombosis.
Reprinted, with modifications, with permission.12
Relation of onset of platelet count decrease and onset of HIT-associated thrombosis.
The data summarize 209 patients with HIT-associated thrombosis. About one quarter (26.3%) of patients develop thrombosis on the same day that the thrombocytopenia occurs (defined arbitrarily as the day the platelet count has fallen by more than 50%), and in 33.5% the platelet count reached thrombocytopenia levels only after the occurrence of thrombosis.
Reprinted, with modifications, with permission.12
Professor, Department of Pathology and Molecular Medicine, and Department of Medicine, Michael G. DeGroote School of Medicine, McMaster University, Hamilton, Ontario, Canada; Associate Head, Transfusion Medicine, Hamilton Regional Laboratory Medicine Program; Hematologist, Hamilton Health Sciences (General Site)