Abstract
Recent advances resulting from the identification of the genes responsible for four inherited marrow failure syndromes, Fanconi anemia, dyskeratosis congenita, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome, are reviewed. The interpretation of genetic testing should be guided by an understanding of the limitations of such testing for each disorder. The possibility of an inherited basis for marrow failure must be considered for adults as well as children with aplastic anemia. Shared molecular themes are emerging from functional studies of the genes underlying the different inherited disorders. Genomic instability may result from impaired DNA repair in Fanconi anemia or telomere dysregulation in dyskeratosis congenita. Mutations affecting ribosome assembly or function are associated with Diamond-Blackfan anemia, dyskeratosis congenita, and Shwachman-Diamond syndrome. These findings raise new questions about the molecular mechanisms regulating hematopoiesis and leukemogenesis. Clinical implications arising from these molecular studies are explored.
The inherited marrow failure syndromes are characterized by impaired hematopoiesis and cancer predisposition. Most inherited marrow failure syndromes are also associated with a range of congenital anomalies. These rare diseases offer important insights into general mechanisms governing human development, hematopoiesis and tumorigenesis. First, the clinical features of four of the inherited marrow failure syndromes: Fanconi anemia (FA), dyskeratosis congenita (DC), Diamond-Blackfan anemia (DBA), and Shwachman-Diamond syndrome (SDS), are briefly reviewed. Emerging molecular themes arising from recent studies of these syndromes are discussed. Finally, clinical applications arising from our growing understanding of molecular pathogenesis are explored.
Clinical Features
In the past, the diagnosis of these diseases relied on the recognition of characteristic clinical features. With the advent of laboratory and genetic tests for many of these disorders, our understanding of the clinical spectrum of these disorders has broadened. Indeed, it is becoming increasingly apparent that patients lacking characteristic physical stigmata may still harbor an inherited marrow failure syndrome and develop marrow failure or malignancy. Clinical presentation is no longer confined to the pediatric population but may manifest in adults as well.
Fanconi anemia
FA is classically characterized by progressive marrow failure, congenital anomalies, and a predisposition to develop leukemias and solid tumors. Marrow failure often progresses to aplastic anemia. The leukemias are typically acute myelogenous leukemias (AML), though a few cases of acute lymphocytic leukemias (ALL) have also been reported. The solid tumors are typically squamous cell carcinomas, commonly involving the head and neck or female genital tract. Associated clinical findings may include short stature, skin pigment abnormalities (e.g., cafe au lait spots), radial ray anomalies, genitourinary abnormalities, and microphthalmia. Of note, a subset of patients may lack the characteristic physical stigmata of FA but can be diagnosed with chromosomal breakage testing (see below). In addition, patients may first present in adulthood with marrow failure or malignancy as the primary clinical manifestation of FA.
Dyskeratosis congenita
DC is classically characterized by the clinical triad of dystrophic nails, a reticular rash, and mucosal leukoplakia. Bone marrow failure is the usual cause of mortality. DC is associated with an increased risk of leukemias and solid tumors, particularly squamous cell carcinomas. Pulmonary fibrosis has also been described in these patients. Additional clinical features may be variably seen, and include short stature, dental abnormalities, esophageal stricture, premature hair loss, early graying, osteoporosis, liver cirrhosis, urinary tract anomalies, and hyperhidrosis. Of note, the characteristic clinical triad is typically absent early in life but manifests with age. The diagnosis may be obscured in patients presenting with aplastic anemia at a young age prior to the onset of skin, nail and mucosal findings. The clinical spectrum is highly variable, even within a given family.
Diamond-Blackfan anemia
DBA is classically characterized by reticulocytopenic anemia presenting in early infancy. The bone marrow typically shows red cell aplasia with a paucity of erythroid precursors. Other causes of pure red cell aplasia must be ruled out. The red cells are typically macrocytic. Red cell adenosine deaminase (ADA) activity is elevated in most, but not all, patients. Associated congenital anomalies, such as craniofacial anomalies, radial ray abnormalities, renal and cardiac defects, may also be seen. Hemoglobin levels may improve upon treatment with adrenocortical steroids. Spontaneous remission may occur in a subset of patients. An increased risk of AML and osteosarcomas and likely additional malignancies has been reported.1 Aplastic anemia has also been described in a few patients with DBA.
Shwachman-Diamond syndrome
SDS is characterized by exocrine pancreatic insufficiency and bone marrow failure. Neutropenia is the most common manifestation of marrow failure, but anemia or thrombocytopenia may also occur. The pancreatic acini are largely replaced by fatty tissue with relative sparing of the pancreatic ducts and islets. Pancreatic insufficiency typically manifests in early infancy with steatorrhea and failure to thrive. Serum levels of trypsinogen or pancreatic isoamylase are generally low. With increasing age, pancreatic function improves in over half of patients, which may render the diagnosis elusive. Cytopenias may be intermittent, further obscuring the diagnosis. Patients with SDS are at increased risk of developing aplastic anemia and AML. To date, solid tumors have not been reported in patients with SDS.
Molecular Pathogenesis
Genomic instability and Fanconi anemia
The hallmark of FA is the inability to repair DNA damage induced by DNA crosslinking agents such as mitomycin C or diepoxybutane. When exposed to these agents, cells from FA patients manifest chromosomal breaks and fusions with characteristic radial forms (Figure 1 ) consistent with an underlying genomic instability. Complex cytogenetic abnormalities are typically associated with the leukemias arising in FA patients. Ongoing molecular studies support a role for the FA pathway in DNA repair.
FA is a recessive disorder with both autosomal and X-linked patterns of inheritance. Twelve different FA complementation groups have been identified to date, and the responsible genes for 11 of these groups have been identified (Table 1 ) (reviewed in 2,3). Around 84% of patients fall within the subtypes A, C, or G, with the majority of patients comprising subtype A. Many of the FA genes encode novel proteins of unknown function. The identification of the FANCD1 gene as the BRCA2 tumor suppressor gene involved in homologous recombination repair provided a direct link between the FA pathway and DNA repair. The FANCJ/BACH1/BRIP1 gene encodes a helicase, which is an enzyme that unwinds DNA. The FANCM (Hef) gene shares regions of homology with both helicases and endonucleases, though these functions have yet to be directly demonstrated. FANCM may function in DNA translocation. The FANCL/PHF9/POG gene shares homology with other E3 ubiquitin ligases.
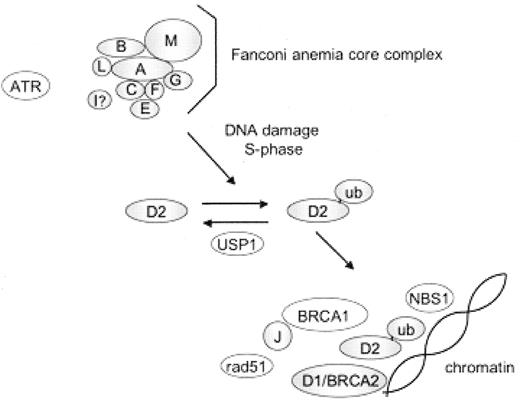
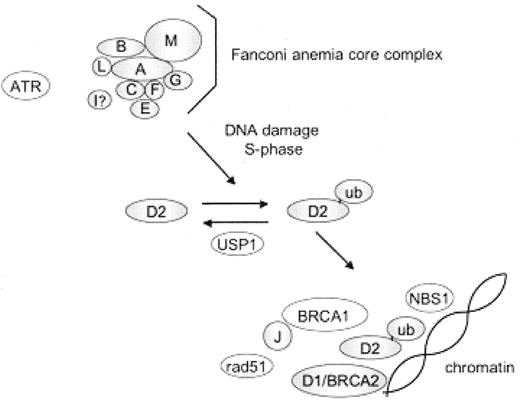
Eight of the FA proteins (A, B, C, E, F, G, L, and M) associate in a nuclear core complex and together with the Fanconi anemia I protein are required for monoubiquitination of the Fanconi anemia D2 (FANCD2) protein (Figure 2 ).3 Monoubiquitinated FANCD2 carries a ubiquitin protein covalently linked via post-translational modification to a specific FANCD2 lysine residue (K561). Abrogation of any of the nuclear complex components disrupts FANCD2 monoubiquitination. Although the Bloom syndrome helicase, BLM, associates with the FA core complex,4 BLM is not required for FANCD2 monoubiquitination.5 FANCD2 is monoubiquitinated during S phase of the cell cycle and in response to DNA damaging agents such as mitomycin C or radiation (ultraviolet or ionizing). The monoubiquitinated FANCD2 protein localizes to chromatin, where it associates with other DNA repair proteins such as FANCD1/BRCA2, BRCA1, RAD51, and NBS1. In addition to functioning to promote ubiquitination of FANCD2, the FA core complex is required for translocation of ubiquitinated FANCD2 to chromatin and for resistance to interstrand crosslinking agents.6 Indeed, the FA core complex proteins FANCA, FANCC, and FANCG also associate with chromatin particularly in response to DNA damage.7 The precise molecular function of the FA pathway in DNA repair remains unclear.
The FA pathway interacts with additional DNA repair pathways involved in tumor suppression.2,3 Monoubiquitinated FANCD2 co-localizes with the tumor suppressor proteins FANCD1/BRCA2, BRCA1, and NBS1 in chromatin-bound foci. The ataxia-telangiectasia protein, ATM kinase, phosphorylates FANCD2 to induce an S-phase cell cycle checkpoint in response to radiation. This checkpoint is important to arrest DNA synthesis under conditions of DNA damage. In addition, the ATR/CHK1 pathway has been implicated in the activation of the FA pathway in response to DNA damage.
Genomic instability and dyskeratosis congenita
Dyskeratosis congenita may be inherited in an X-linked recessive, autosomal dominant, or autosomal recessive form. The X-linked recessive form is usually more severe with an earlier clinical onset than the autosomal dominant form. Cutaneous manifestations may be mild or missing in the autosomal dominant form. The clinical phenotype may vary widely, even within a given family. The gene for the autosomal recessive form has not yet been identified.
Mutations in DKC1, which encodes the protein dyskerin, are associated with the X-linked form of DC.8 Most DKC1 mutations are missense mutations. Dyskerin is an evolutionarily conserved protein that is expressed throughout all tissues. Dyskerin associates with the box H/ACA class of RNAs, which includes the telomerase RNA, and is important for their nuclear accumulation and stability. Dyskerin also shares structural similarities to pseudouridine synthases.
In support of a role for telomerase dysfunction in DC, patients with the autosomal dominant form of DC harbor mutations in the RNA component of telomerase (TERC)9,10 or the telomerase reverse transcriptase (TERT).11 Data indicate that the clinical phenotype results from haplo-insufficiency of TERC or TERT rather than from dominant-negative effects. Patients with DC generally exhibit markedly shortened telomeres.12 Family members with autosomal dominant DC manifest “disease anticipation” with progressive disease severity and earlier clinical onset over successive generations in the context of inheriting both progressively shorter telomeres in association with a mutated copy of TERC or TERT from generation to generation.13,14
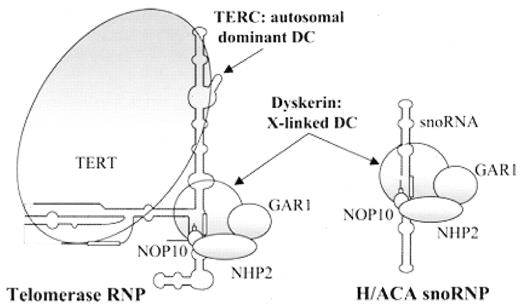
Telomeres stabilize the chromosome ends to prevent their shortening during replication, to distinguish chromosome ends from DNA damage-induced breaks, and to inhibit end-to-end fusions. Telomeres are comprised of 6-basepair repeated sequences (TTAGGG) bound by specific telomere-associated proteins. The telomeres are maintained by the telomerase enzyme, which is composed of an RNA component (TERC) complexed with a reverse transcriptase (TERT) as well as additional proteins including dyskerin, GAR1, NHP2, and NOP10 (Figure 3 ). The TERC RNA aligns with the 3′ end of the chromosomal telomeric repeats and serves as a template for the TERT polymerase to replicate the 3′ chromosomal terminus.
Telomerase is highly expressed in tissues with a high replicative state, such as hematopoietic cells, germ cells, and tissue stem cells. The clinical phenotype of DC mirrors those tissues where there is a high rate of cell turnover and telomerase is highly expressed. Telomerase is also present in many cancer cells. When telomerase levels are low or absent, as is the case in most somatic tissues, telomeres progressively shorten with each cell division. When a critically short telomere length is reached, a checkpoint is triggered causing the cells to stop dividing and senesce to prevent chromosomal rearrangements. Thus, progressive telomere shortening limits the replicative capacity of most somatic tissues.
Telomerase RNA-null mice (mTR−/−)15,16 are viable and manifest successive telomere shortening over subsequent generations. Telomere shortening is accompanied by hair greying, alopecia, and genetic instability.16 Impaired hematopoietic recovery following 5-FU treatment is observed.16 Later generations of mTR−/− mice develop early tumors that manifest a high incidence of cytogenetic abnormalities.15,16
Mutations abrogating Dkc1 expression resulted in embryonic lethality in mouse models. Mice carrying a targeting vector integrated downstream and in opposite orientation to the Dkc1 gene demonstrated diminished dyskerin expression levels, presumably from transcription interference.17 These Dkc1m mice showed early phenotypic abnormalities, including severe anemia, lymphopenia and decreased marrow cellularity. Bone marrow colony-forming assays showed diminished CFU-E and CFU-preB colonies, but no difference in proliferation rate measured by [3H]-thymidine incorporation. A high incidence of tumors involving the lung, mammary gland, and kidney was observed. Interestingly, although the clinical manifestations were apparent in early generations of Dkc1m mice, significant telomere shortening was not seen until later generations, suggesting that additional functions of dyskerin, such as ribosome modification (discussed below), may also play an important role in the clinical spectrum of DC.
Ribosomal dysfunction in dyskeratosis congenita
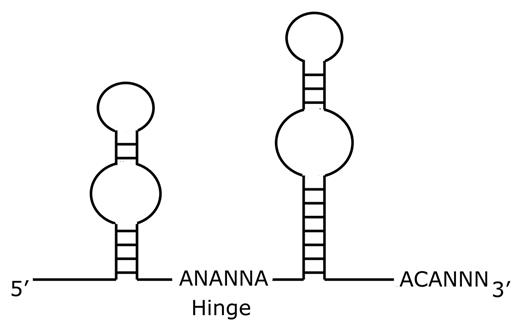
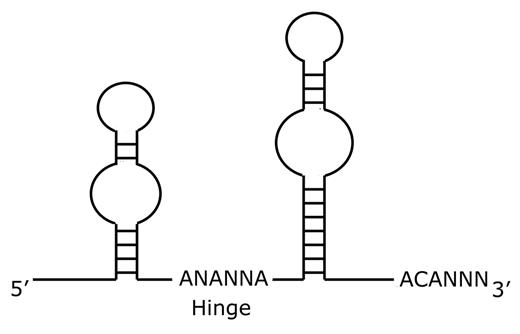
Since dyskerin associates with other box H/ACA RNA species in addition to the TERC RNA, additional effects of dyskerin mutations may also contribute to the pathobiology of DC. Box H/ACA RNAs18 complex with proteins to form ribonucleoparticles (RNPs). Box H/ACA RNAs share a common secondary structure comprised of two hairpin stem-loop structures separated by a hinge region and followed by a tail containing the sequence ACA located three residues from the 3′ terminus (Figure 4 ). The nomenclature reflects the hinge region (box H) and the conserved ACA triplet sequence. Several classes of box H/ACA RNAs have been identified. The small nucleolar RNA (snoRNA) class of box H/ACA RNAs function in ribosomal RNA (rRNA) pseudouridylation and rRNA maturation. The telomerase RNA (TERC or hTR) associates in the telomerase complex to maintain telomeres. The U17/E1 RNAs function in rRNA processing. The small Cajal body RNAs (scaRNA) function in pre-mRNA splicing. There are additional orphan box H/ACA RNAs whose functions are not yet understood. The different classes of box H/ACA RNAs associate with the same four highly conserved core proteins: dyskerin (NAP57, CBF5p), GAR1, NHP2, and NOP10.18 Studies in yeast indicate that all four core proteins are essential.
Dyskerin, the protein mutated in dyskeratosis congenita, shares homology with pseudouridine synthases. Mutations of the yeast homolog CBF5P reduce rRNA pseudouridylation and impair growth.19 In patients with X-linked DC, mutations in DKC1 are typically missense mutations. Although the mutations are located in scattered regions of the DKC1 gene, analysis of the crystal structure of the yeast homolog CBF5 revealed that the mutations clustered around a common spacial region of the folded protein structure (the PUA domain) that is predicted to play a role in binding H/ACA RNAs.20
The bulge in the hairpin of snoRNAs basepairs with complementary sequences flanking a uridine in the RNA species targeted for pseudouridylation. Pseudouridylation is a post-transcriptional modification resulting in a different isomer of uridine at specific residues of RNA molecules. Pseudouridylation has been postulated to affect rRNA secondary structure and binding. Pseudouridylation of rRNA is seen throughout evolution and tends to occur in functionally important regions of the rRNA. Pseudouridylation of specific regions of rRNA is essential for ribosome function and growth in yeast.21 A defect in translation initiated by internal ribosome entry site sequences (IRES) has been observed in Dkc1m mice and in cells from X-linked DC patients wherein rRNA pseudouridylation was diminished.22 Patients with autosomal dominant forms of DC, who harbor mutations affecting telomerase function but not ribosome function, typically manifest milder clinical phenotypes than do patients with DKC1 mutations. It has been proposed that ribosomal dysfunction might affect clinical severity in the context of telomerase dysfunction. In support of this model, some DC patient–derived point mutations in DKC1 have been demonstrated to result in different effects on telomerase activity versus pseudouridylation.10,23
Ribosomal dysfunction in Diamond-Blackfan anemia
Around 20–25% of patients with DBA harbor heterozygous mutations in the gene for the ribosomal protein RPS19.24 The disease likely results from haploinsufficiency of RPS19 expression rather than from a dominant negative effect.25 An autosomal dominant pattern of inheritance may be seen in families, but many cases appear to arise spontaneously. RPS19 is a component of the small 40S eukaryotic ribosomal subunit and localizes to the nucleolus, the major cellular site of rRNA transcription and ribosome biogenesis. The eukaryotic ribosome is composed of many more proteins than the prokaryotic ribosome, and there is no prokaryotic ortholog for RPS19. The roles of many of the ribosomal proteins are not well understood in higher eukaryotes. Most such studies have been performed in yeast.
Yeast have two RPS19 orthologs. Yeast carrying deletions of both copies of the RPS19 gene are nonviable. Deletion of either RPS19 gene results in decreased growth with diminished production of the 40S ribosomal subunit. Yeast RPS19 is required for maturation of the 3′ end of the 18S rRNA. Deletion of RPS19 leads to accumulation of aberrant pre-40S rRNA species that accumulate in the nucleus. Introduction of patient-derived RPS19 point mutations into yeast leads to similar defects in 18S rRNA maturation and 40S ribosomal subunit assembly.26 Additional potential nonribosomal functions for RPS19 are currently under investigation.27
Ribosomal dysfunction in Shwachman-Diamond syndrome
Around 90% of patients who meet the clinical criteria for Shwachman-Diamond syndrome harbor mutations in the SBDS gene.28 SBDS is highly conserved throughout evolution and appears to be ubiquitously expressed throughout all tissues. The majority of SBDS mutations appear to arise from a gene conversion event with its adjacent pseudogene. To date, no patients homozygous for two null SBDS alleles have been reported, suggesting that complete abrogation of SBDS function is likely to be lethal. In support of this hypothesis, mice lacking both sbds alleles exhibit early embryonic lethality.29 The SBDS protein is localized throughout the cell, but shuttles into the nucleolus, the major cellular site of ribosome biogenesis, in a cell cycle–dependent manner.30 Based on inferences from orthologs, SBDS protein has been proposed to function in RNA metabolism.28 Studies in yeast further suggest that SBDS may function in rRNA maturation.31–34 The role of ribosome dysfunction in SDS remains to be ascertained.
Bone Marrow Failure
Genomic instability and marrow failure
It is currently surmised that defects in telomerase likely result in premature senescence and apoptosis of the rapidly cycling hematopoietic stem cells to result in marrow failure for patients with DC. The molecular mechanisms underlying marrow failure in FA patients remains unclear. While it is possible that the hematopoietic compartment of FA patients might be particularly sensitive to endogenous and exogenous genotoxic stressors, it is striking that marrow failure is not a typical feature of most other chromosomal instability syndromes, such as ataxia-telangiectasia. FA proteins, FANCC in particular, possess additional functions that might additionally contribute to the pathogenesis of marrow failure.35 Cells from FA patients are hypersensitive to apoptotic stimuli, and this may result in premature loss of hematopoietic stem cells. It is not clear whether this heightened apoptosis represents a primary defect of inappropriate apoptosis causing marrow failure or a secondary checkpoint response to delete damaged cells that cannot achieve proper DNA repair. The distinction between these two possibilities carries profound clinical implications. Loss or inhibition of apoptotic checkpoints might promote the survival of cells that have acquired oncogenic mutations.
Ribosomal dysfunction and marrow failure
It remains unclear how haploinsufficiency for a ribosomal protein manifests primarily as a defect in erythropoiesis in DBA. It has also been postulated that, in theory, differential expression levels of specific ribosomal proteins might render some ribosomal proteins limiting for ribosome assembly in certain tissues.36 As with all marrow failure syndromes, increased levels of apoptosis have been observed in marrows of DBA patients, though whether this represents a direct effect of ribosomal insufficiency or a secondary response to eliminate damaged or stressed cells is yet unclear. It has been proposed that diminished translation of mRNAs for anti-apoptotic factors might contribute to marrow failure in DC.22
Another possibility that has yet to be explored is a model wherein insufficiency of one ribosomal protein might lead to a relative excess of other ribosomal proteins. The equimolar generation of ribosomal proteins appears to be a highly regulated process in the cell systems examined to date. Given the orderly sequential assembly of ribosomal proteins onto the maturing ribosomal RNAs, it is possible that a block in one step of ribosome synthesis might lead to inappropriate accumulation of unassembled ribosomal proteins or precursor complexes that could either be directly toxic to the cell or might trigger a checkpoint response. Such a situation would be reminiscent of that seen in the thalassemias, where erythroid demise results in part from excessive accumulation of free alpha or beta globin protein in the setting of the relative paucity of the partner globin protein. Since red cells contain high concentrations of ribosomes to permit high levels of production of hemoglobin, it is possible in this model that red cells are particularly sensitive to limiting amounts of specific ribosomal proteins. Such a model might also apply to the situation in DBA, where potential defects in rRNA maturation might result in inappropriate accumulation of rRNA precursors, unassembled ribosomal proteins or RNP intermediates to trigger a checkpoint response leading to the observed increase in apoptosis of erythroid precursors. Additional potential extra-ribosomal functions for RPS19 are under investigation. Further studies are needed to elucidate the mechanisms promoting marrow failure.
Malignancy
Genomic instability and malignancy
Multiple lines of evidence support a role for genomic instability in tumorigenesis.37 FA patients manifest cytogenetic abnormalities consistent with an underlying defect in DNA repair. The protein products of many DNA repair genes interact with the FA pathway (Figure 2 ). Indeed, the FANCD1 gene is identical to the DNA repair gene BRCA2, which is a tumor suppressor. Cancers arising in FA patients typically harbor complex cytogenetic abnormalities, consistent with an underlying genomic unstability.38 Genes causing chromosomal instability syndromes, such as ataxia-telangiectasia and xeroderma pigmentosa, also confer cancer predisposition. One model whereby genomic instability has been postulated to promote tumorigenesis is through the facilitated acquisition of oncogenic mutations as well as mutations promoting inappropriate survival of premalignant cells.
Telomere loss results in the generation of unprotected DNA ends that are unstable. These exposed DNA ends are prone to exonucleolytic degradation or may undergo end-to-end fusion with other chromosomes. The resulting dicentric chromosomes do not experience proper chromosomal segregation and are prone to additional chromosomal breakage during mitosis. Aneuploidy and end-to-end chromosomal fusions were observed in the telomerase knock-out mouse models. These events may facilitate the acquisition of genetic mutations predisposing to cancer formation.
Ribosomal dysfunction and malignancy
Evidence supports a correlation between oncogenesis and upregulation of ribosome biogenesis and translation, though a direct oncogenic role for ribosome dysfunction has yet to be ascertained.39 Dysregulation of specific subsets of mRNAs, such as increased translation of growth-promoting mRNAs, has been observed in response to oncogenic stimuli. The tumor suppressor protein, Arf, inhibits the production of rRNA,40 while the oncogenic protein, nucleophosmin, promotes rRNA biosynthesis.41 An intriguing mutagenesis screen for tumor suppressors in zebrafish revealed a high incidence of heterogyzous mutations in ribosomal protein genes among the zebrafish lines exhibiting tumor predisposition.42 Whether these ribosomal protein mutations are directly oncogenic remains to be determined.
Extra-ribosomal functions for specific ribosomal proteins are becoming increasingly apparent. For example, ribosomal protein L11 binds and inhibits HDM2, resulting in stabilization and activation of p53.43 Ribosomal protein RPL26 binds the 5′ untranslated region of p53 mRNA after DNA damage to upregulate p53 expression.44 The large ribosomal component L13a is phosphorylated in response to gamma interferon and binds to the 3′ untranslated region of the ceruloplasmin mRNA to inhibit its translation.45 Thus, possible oncogenic roles for specific ribosomal proteins may include functions beyond that of the ribosome.
Molecular Studies: Diagnostic Implications
With the identification of some of the genes underlying these disorders, genetic testing for the purposes of diagnosis has become possible (Table 2 ). The role of genetic testing in clinical management is an evolving field, and results must be considered critically. Interpretation of newly identified sequence alterations in the absence of functional data must be tempered with caution since they may not necessarily represent pathogenic mutations, but rather may constitute rare polymorphisms. The absence of apparent mutations should be evaluated critically and within the context of the limitations of the experimental assays utilized. For example, an exon-directed sequencing approach may miss potential deletions, inversions, or promotor mutations. For the SBDS gene, extended gene conversion events may be missed if the affected region is not spanned by the standard exon-directed primers. Gene identification is still ongoing, so the absence of mutations in currently known genes does not necessarily preclude a given diagnosis if the patient fits clinical criteria.
The availability of a functional test for FA has greatly facilitated diagnosis for this disease. Increased chromosomal breakage in response to the interstrand crosslinking agents mitomycin C or diepoxybutane is the diagnostic hallmark of FA. Somatic mosaicism, a state in which a somatic cell clone has undergone genotypic reversion to a non-Fanconi phenotype, may be seen in hematopoietic cells, thus obscuring the diagnosis. In such cases, the diagnosis may be ascertained by assessing a different tissue, typically skin fibroblasts. It is currently unknown whether somatic mosaicism might confound diagnosis in other inherited marrow failure syndromes. Patients with Nijmegen’s breakage syndrome may exhibit increased mitomycin C–induced chromosomal breakage, leading to confusion with FA. Loss of FANCD2 monoubiquitination is a useful adjunct diagnostic screen but is currently only available on a research basis. A limitation to this approach is that a rare subset of patients with subtypes D1 and J, where the affected FA proteins function distal to FANCD2 monoubiquitination, would be missed. Flow cytometry to assess cell cycle arrest at G2/M has been reported as a diagnostic test for FA. Advantages include the ability to rapidly screen large numbers of cells and relative ease of the assay. The sensitivity and specificity of this assay for the different FA subtypes remains to be ascertained.
Since patients with DC generally have shorter telomeres compared with age-matched controls, telomere length analysis is currently under investigation as a diagnostic screen for DC. The sensitivity and specificity of telomere length analysis for diagnostic purposes remains to be ascertained. The optimal cell population to be assayed for diagnostic purposes also remains to be determined. This test is currently only available on a research basis.
A careful patient history, including family history of any physical anomalies, hematologic abnormalities, and cancer predisposition, as well as a thorough physical exam to assess for clinical stigmata of the inherited marrow failure syndromes together with supportive laboratory tests, are critically important to the diagnostic workup of these syndromes and may aid in the choice of genetic tests pursued. The results of genetic testing should be interpreted carefully within the context of the entire clinical picture of the patient and the patient’s family.
Molecular Studies: Therapeutic Implications
Recently, attention has focused on the potential role of acquired FA gene mutations or epigenetic silencing in the somatic cells within the non-FA population.46 Studies indicate that acquired somatic defects in FA gene expression, either through gene mutation or epigenetic silencing, might contribute to tumorigenesis in a subset of non-FA patients.2,3 Since cells deficient in DNA repair are generally hypersensitive to chemotherapeutic agents and to radiation, agents that block DNA repair pathways such as the FA pathway in tumors might be useful therapeutic agents to influence drug or radiation sensitivity of tumors.46
Fanconi anemia chromosomal breakage test.
Diepoxybutane-induced chromosomal breakage in a metaphase lymphocyte from a patient with Fanconi anemia. Chromosomes with breaks and fusions are indicated with arrows. Courtesy of Lisa Moreau, Dana Farber Cancer Institute, Boston, MA.
Fanconi anemia chromosomal breakage test.
Diepoxybutane-induced chromosomal breakage in a metaphase lymphocyte from a patient with Fanconi anemia. Chromosomes with breaks and fusions are indicated with arrows. Courtesy of Lisa Moreau, Dana Farber Cancer Institute, Boston, MA.
The Fanconi anemia pathway.
FA proteins (A, B, C, D1, D2, E, F, G, I, J, L, M) are depicted with shaded circles. Additional DNA repair proteins interacting with the FA pathway are denoted in open circles. Following activation of the FA complex by DNA damage or the cell cycle, the D2 protein is monoubiquitinated. Monoubiquitinated D2 protein translocates to chromatin where it co-localizes with additional DNA repair proteins.
The Fanconi anemia pathway.
FA proteins (A, B, C, D1, D2, E, F, G, I, J, L, M) are depicted with shaded circles. Additional DNA repair proteins interacting with the FA pathway are denoted in open circles. Following activation of the FA complex by DNA damage or the cell cycle, the D2 protein is monoubiquitinated. Monoubiquitinated D2 protein translocates to chromatin where it co-localizes with additional DNA repair proteins.
Telomerase and H/ACA small nucleolar ribonucleoprotein complexes.
Reproduced with publisher permission from
Abbreviations: sno, small nucleolar; RNP, ribonucleoprotein.
Telomerase and H/ACA small nucleolar ribonucleoprotein complexes.
Reproduced with publisher permission from
Abbreviations: sno, small nucleolar; RNP, ribonucleoprotein.
Schematic diagram of box H/ACA RNA structure.
H/ACA RNAs are characterized by two hairpin stem-loop structures separated by a hinge region and followed by a tail containing the sequence ACA located three residues from the 3′ terminus.
Schematic diagram of box H/ACA RNA structure.
H/ACA RNAs are characterized by two hairpin stem-loop structures separated by a hinge region and followed by a tail containing the sequence ACA located three residues from the 3′ terminus.
Acknowledgments: The author would like to thank David Nathan and Monica Bessler for helpful discussions.