Abstract
Thrombophilia is an inherited or acquired predisposition to thrombosis. This article reviews the clinical manifestations of thrombophilia and addresses common questions on laboratory assessment and management: what are the potential indications for thrombophilia testing, who should be tested, what tests should be requested, when should testing be performed, and how should the test results affect primary prevention, acute therapy, and secondary prophylaxis of thrombosis.
Introduction
Symptomatic thrombosis is a multifactorial disease that manifests when a person with an underlying predisposition to thrombosis (i.e., thrombophilia) is exposed to clinical risk factors. Thrombophilia is not a disease per se, but may be associated with a disease (e.g., cancer), drug exposure (e.g., oral contraceptives) or condition (e.g. pregnancy or postpartum; “acquired thrombophilia,” Table 1 ), or thrombophilia may be inherited (Table 2 ). This concept is important because susceptibility to a disease does not imply an absolute requirement for primary or secondary prevention, or for treatment. Most persons with a thrombophilia do not develop thrombosis. Thus, thrombophilia must be considered in the context of other risk factors for incident thrombosis, or predictors of recurrent thrombosis, when estimating the need for primary or secondary prophylaxis, respectively. With rare exceptions, the therapy for acute thrombosis is no different for those with than for those without a recognized thrombophilia.
Thrombophilia may present clinically as one or more of several thrombotic manifestations (“phenotypes,” Table 3 ). The predominant clinical manifestation of thrombophilia is venous thromboembolism. Consequently, this section will focus on the role of thrombophilia in venous thromboembolism. Thrombophilia may rarely present as purpura fulminans (e.g., neonatalis or adult) or warfarin-induced skin necrosis. Most clinical studies have failed to show a consistent association between thrombophilia and myocardial infarction or stroke. Thrombophilia may also present as recurrent fetal loss and possibly as stillbirth or other complications of pregnancy.
Currently, there is no single laboratory assay or simple set of assays that will identify all thrombophilias. Consequently, a battery of complex and potentially expensive assays is usually required. Many of these laboratory analyses are affected by other conditions (e.g., warfarin reduces protein C and S levels) such that the correct interpretation of the results can be complicated and always requires clinical correlation. This section addresses several fundamental questions that all clinicians must answer when faced with evaluating a patient for a possible thrombophilia. In addition, test result interpretation and how the results alter primary prophylaxis, acute therapy, or secondary prevention of thrombosis will also be addressed.
Indications for Thrombophilia Testing: Why Should I Test for Thrombophilia?
There are no absolute indications for clinical diagnostic thrombophilia testing. Potential relative indications could include general population screening, selected screening of populations that are potentially “enriched” for thrombophilia (e.g., asymptomatic or symptomatic family members of patients with a known familial thrombophilia, especially first-degree relatives) or populations at increased risk for thrombosis (e.g., prior to pregnancy, oral contraception or estrogen therapy, high-risk surgery, or chemotherapy with angiogenesis inhibitors), and testing symptomatic patients with incident or recurrent thrombosis. With the exception of general population screening (which is not recommended), all of these potential indications are controversial and must be considered in the context of the clinical presentation.
Screening Asymptomatic Family Members
Thrombophilia testing, and especially genetic testing, of asymptomatic family members should be done with caution. Family members (and patients) should receive genetic counseling prior to genetic testing, and such testing should only be performed after obtaining consent. Counseling should include the reasons for testing, such as the potential for avoiding clinical thrombosis by risk factor modification or prophylaxis; and the reasons for not testing, such as stigmatization and mental anguish, the potential effect on obtaining personal health insurance or employment, and the possibility of nonpaternity.
Thrombophilia testing should only be done if the results are likely to change medical management. The risk of idiopathic (“unprovoked”) thrombosis associated with a thrombophilia, while increased, is still insufficient to warrant chronic primary prophylaxis (e.g., warfarin anticoagulation), even for thrombophilias with high penetrance, with the possible exception of paroxysmal nocturnal hemoglobinuria.1
When counseling a family member (or patient) regarding the risk of thrombosis, it’s most useful to provide the “absolute” risk (e.g., incidence) of thrombosis among persons with that particular thrombophilia. For example, the relative risk of venous thromboembolism among women who are factor V Leiden carriers receiving oral contraceptives is increased about 30-fold; however, the incidence is only about 300 per 100,000 women-years, or about 0.3% per woman-year.2 Thus, the absolute risk provides information on both the baseline incidence of venous thromboembolism (e.g., about 10 to 46 per 100,000 women-years for women of reproductive age2,3) as well as the relative risk.
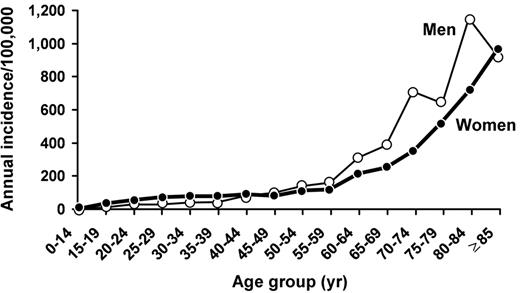
When estimating the absolute risk of thrombosis, it is especially important to include the effect of age on the baseline incidence. For example, among women of perimenopausal age (ages 50 to 54 years), the incidence of venous thromboembolism is 123 per 100,000 woman-years,4 and the incidence increases exponentially thereafter (Figure 1 ). Among factor V Leiden carriers of perimenopausal age, the relative risk of venous thromboembolism associated with hormone therapy may be increased 7- to15-fold.5,6 However, while the relative risk for venous thromboembolism is less for hormone therapy compared with oral contraceptives, the absolute risk is substantially higher (approximately 900–1800 per 100,000 woman-years, or approximately 1% to 2% per woman-year). Given recent studies questioning the benefit of postmenopausal hormone therapy, most women likely would choose to avoid this risk if they were known to be carriers. Thus, it may be relatively cost effective to screen asymptomatic peri- or postmenopausal women with a known family history of familial thrombophilia who are considering hormone therapy.5
Primary prevention of incident venous thromboembolism
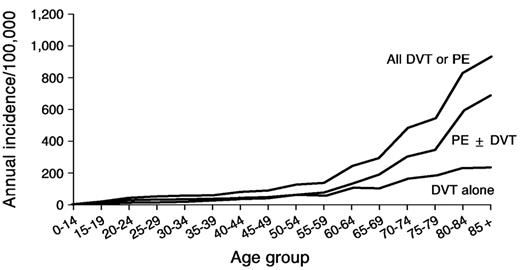
Venous thromboembolism is a major health problem, with at least 300,000 first-lifetime cases per year in the United States.4 Observed survival after venous thromboembolism is significantly less than expected, especially after pulmonary embolism. For about one-quarter of patients with acute pulmonary embolism, the initial clinical presentation is sudden death. Pulmonary embolism accounts for an increasing proportion of venous thromboembolism with increasing age for both sexes (Figure 2 ).4 Hence, as the average U.S. population age increases, the total number of incident venous thromboembolism events per year will increase, and an increasing number of these events will be pulmonary embolism. Because of the markedly reduced survival after pulmonary embolism, the number of U.S. deaths due to venous thromboembolism per year also will increase. Primary prevention of venous thromboembolism, either by risk-factor modification or by appropriate prophylaxis of patients at risk, is essential in order to improve survival and prevent complications. However, despite improved prophylaxis regimens and more widespread use of prophylaxis, the overall incidence of venous thromboembolism has been relatively constant at about 1 per 1,000 since 1979.4,7,8
To avoid or modify risk, or appropriately target prophylaxis, patients at risk for venous thromboembolism must first be identified. Independent risk factors for venous thromboembolism, as well as the magnitude of risk associated with each, are shown in Table 4 .9 Among women, additional risk factors for venous thromboembolism include oral contraceptive use and hormone therapy,10,11 selective estrogen receptor modulators,12 and pregnancy and the postpartum period.3
Family-based studies indicate that venous thromboembolism is highly heritable and follows a complex mode of inheritance involving environmental interaction.13 Inherited reductions in plasma natural anticoagulants (e.g., anti-thrombin, protein C, or protein S) have long been recognized as uncommon but potent risk factors for venous thromboembolism.14 More recent discoveries of additional reduced natural anticoagulants or anticoagulant cofactors, impaired downregulation of the procoagulant system (e.g., activated protein C resistance, factor V Leiden), increased plasma concentrations of procoagulant factors (e.g., factors I [fibrinogen], II [prothrombin], VIII, IX, and XI) and increased basal procoagulant activity,15 impaired fibrinolysis,16 and increased basal innate immunity activity and reactivity17 have added new paradigms to the list of inherited or acquired disorders predisposing to thrombosis (thrombophilia). Inherited thrombophilias interact with such clinical risk factors (i.e., environmental exposures) as oral contraceptives,2,18 pregnancy, hormone therapy, surgery19 and cancer20 to compound the risk of incident venous thromboembolism. Similarly, genetic interaction increases the risk of incident venous thromboembolism.21 Thus, it may be reasonable to consider thrombophilia testing for asymptomatic men and women family members with a known family history of familial thrombophilia. The results of such testing may aid in further stratifying venous thromboembolism risk after exposure to a known risk factor (e.g., hip or knee replacement surgery19).
Secondary prevention of recurrent venous thromboembolism
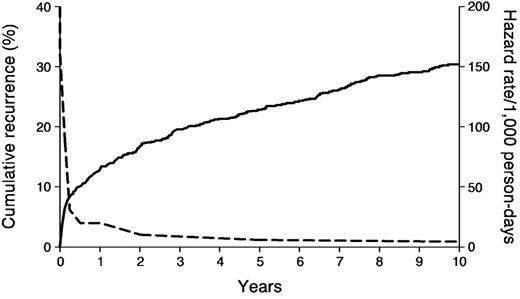
Venous thromboembolism recurs frequently; about 30% of patients develop recurrence within the next ten years (Figure 3 ).22 The hazard of recurrence varies with the time since the incident event and is highest within the first 6 to 12 months (Table 5 ).22,23 Independent predictors of recurrence and the hazard of recurrence associated with each are shown in Table 6.22 Other predictors of recurrence include “idiopathic” venous thromboembolism,23,24 a persistent lupus anticoagulant and/or persistent high-titer antiphospholipid antibody, antithrombin, protein C or protein S deficiency,14 combined heterozygous carriers for more than one familial thrombophilia (e.g., heterozygous for the factor V Leiden and prothrombin G20210A mutations) or homozygous carriers,25–27 and possibly increased procoagulant factor VIII and factor IX levels,23,25,28 decreased tissue-factor pathway inhibitor levels26 and persistent residual deep vein thrombosis. Recent meta-analyses suggest a modest 1.4- and 1.7-fold increased risk of recurrence among factor V Leiden and prothrombin G20210A heterozygous carriers, respectively.29 The estimated population-attributable risk of recurrence was 9.0% and 6.7% for the factor V Leiden and the prothrombin G20210A mutations, respectively. An increased D-dimer measured at least 1 month after stopping warfarin therapy may be a predictor of recurrence independent of residual venous obstruction.30,31
Secondary prophylaxis with anticoagulation therapy should be considered for patients with the above-described characteristics. While the incident event type (deep vein thrombosis alone vs pulmonary embolism) is not a predictor of recurrence, patients with recurrence are significantly more likely to recur with the same event type as the incident event type.32 Because the 7-day patient fatality rate is significantly higher for recurrent pulmonary embolism (34%) compared with recurrent deep vein thrombosis alone (4%), secondary prophylaxis should be considered for incident pulmonary embolism, especially for patients with chronic heart or lung disease and reduced cardiopulmonary functional reserve.
Diagnostic Thrombophilia Testing: Who Should Be Tested?
Currently recommended indications for thrombophilia testing include idiopathic or recurrent venous thromboembolism; a first episode of venous thromboembolism at a “young” age (e.g., < 40 years); a family history of venous thromboembolism (in particular, a first-degree relative with thrombosis at a young age); venous thrombosis in an unusual vascular territory (e.g., cerebral, hepatic, mesenteric, or renal vein thrombosis); and neonatal purpura fulminans or warfarin-induced skin necrosis. When two or more of these thrombosis characteristics are present, the prevalence of antithrombin, protein C or protein S deficiency as well as the factor V Leiden and prothrombin G20210A mutations are increased. Consequently, a “complete” laboratory investigation (vide infra) is recommended for patients who meet these criteria, while more selective (e.g., activated protein C resistance/factor V Leiden, prothrombin G20210A mutation) is recommended for other patients.
Diagnostic Thrombophilia Testing: What Should I Test For?
A complete history and physical examination is mandatory when evaluating individuals with a recent or remote history of thrombosis, with special attention given to patient age at onset, location of prior thromboses, and results of objective diagnostic studies documenting thrombotic episodes. An inquiry regarding interval imaging to establish a new baseline is particularly important when diagnosing recurrent thrombosis in the same vascular territory as a previous thrombosis. Patients should be questioned carefully about diseases, exposures, and conditions or drugs that are associated with thrombosis (Tables 1 and 4 ). Thrombosis can be the initial manifestation of cancer, so a complete review of systems directed at symptoms of (occult) malignancy is important, including whether indicated screening tests for normal health maintenance (e.g., mammography, colon imaging) are current. However, routine screening for occult cancer in patients presenting with idiopathic venous thromboembolism has not been shown to improve cancer-related survival and is not warranted in the absence of clinical features and abnormal basic laboratory findings suggestive of underlying malignancy.33,34 The laboratory evaluation for individuals with thrombosis should be selective and based on the history and physical examination. Potential assays for general diagnostic testing and recommended assays for initial and selected additional special coagulation testing for a familial or acquired thrombophilia are provided in Table 7 . Testing for common methylene-tetrahydrofolate reductase (MTHFR) polymorphisms (e.g., C677T) is not recommended.35 It is essential that all abnormal test results be confirmed by repeat testing after correction of any acquired causes for an abnormal result.
Timing of Diagnostic Thrombophilia Testing: When Should I Test?
As acute phase reactants, plasma levels of antithrombin and occasionally proteins C and S may transiently decrease, and fibrinogen and factor VIII levels may increase, with acute thrombosis. Although protein C and S levels may be accurately assayed during acute thrombosis,36 in general, thrombophilia testing should be delayed for at least 6 weeks to allow acute-phase reactant proteins to return to baseline. Heparin therapy can lower antithrombin levels and impair interpretation of clot-based assays for a lupus anticoagulant. Warfarin therapy reduces vitamin K–dependent factors, including proteins C and S. Many authorities recommend delaying testing until the effects of heparin and warfarin therapy have resolved. In those for whom temporary discontinuation of anticoagulation is not practical, heparin can be substituted for warfarin when testing protein C and S levels. However, the effect of warfarin on protein S levels may not resolve for 4 to 6 weeks. Any abnormal result should be confirmed with repeat testing and/or by testing symptomatic relatives. DNA testing for the factor V Leiden and prothrombin G20210A mutations is unaffected by anticoagulation therapy.
Diagnostic Thrombophilia Testing: How Do I Manage Patients with Thrombophilia?
Primary prophylaxis
All patients should receive appropriate antithrombotic prophylaxis when exposed to risk factors (Table 4 ). Despite accumulating evidence that an underlying thrombophilia increases the risk of symptomatic thrombosis among individuals exposed to a clinical risk factor, thrombophilia screening for such persons in the absence of a known family history of familial thrombophilia is not recommended at this time.37 Current recommendations regarding venous thromboembolism prophylaxis for surgery or hospitalization for medical illness are based solely on clinical characteristics;38 in general, prophylaxis regimens are not altered based on a known inherited or acquired thrombophilia. However, given the increased risk of symptomatic venous thromboembolism after high-risk surgery,19 patients with a known thrombophilia should be considered for a longer duration (e.g., “out-of-hospital”) of prophylaxis.
Acute therapy
In general, patients with a familial or acquired thrombophilia and acute venous thromboembolism should be managed in standard fashion with intravenous unfractionated heparin, low-molecular-weight heparin or fondaparinux. Special attention may be required for patients with deficiencies of antithrombin or protein C. Some patients with antithrombin deficiency may be relatively heparin resistant. Antithrombin concentrate can be used in special circumstances such as recurrent thrombosis despite adequate anticoagulation, unusually severe thrombosis or difficulty achieving adequate anticoagulation, before major surgery or in obstetric situations when the risks of bleeding from anticoagulation are unacceptable. Antithrombin concentrate appears to have a low risk of transmitting bloodborne infections. Hereditary protein C deficiency can be associated with warfarin-induced skin necrosis. Consequently, warfarin should be started only after therapeutic heparinization, and the initial warfarin dose should be low (e.g., 2 mg) and increased slowly. Individuals with a history of warfarin-induced skin necrosis can be anticoagulated after receiving a source of exogenous protein C (e.g., fresh frozen plasma, investigational protein C concentrate).
Acute therapy aims to prevent extension or embolism of an acute thrombosis, and needs to continue for a sufficient duration of time and intensity to insure that the acute thrombus has either lysed or become organized, and the “activated” acute inflammatory/innate immunity system has returned to baseline. The total duration of anticoagulation for acute therapy should be individualized based on the circumstances of the thrombotic event. In general, a duration of 6 weeks is adequate for isolated calf vein thrombosis related to transient risk factors,39 while patients with persistent risk factors require 3 months of therapy.39,40
Secondary prophylaxis
It is important to make a distinction between acute therapy and secondary prophylaxis. Beyond 3 months, the aim of continued anticoagulation is to prevent recurrent thrombosis (i.e., secondary prophylaxis). Venous thromboembolism is now viewed as a chronic disease (likely because all such patients have an underlying, if not recognized, thrombophilia) with episodic recurrence.8 The decision regarding secondary prophylaxis is complex and depends on estimates of the risk and consequences of unprovoked venous thromboembolism recurrence while not receiving secondary prophylaxis, the risk of anticoagulant-related bleeding, and patient preference. Clinical predictors of venous thromboembolism recurrence, and the hazard of recurrence associated with each, are presented in Table 6.22 In addition, a meta-analysis of the relative risks of recurrence associated with a familial or acquired thrombophilia is presented in Table 8 .41
In general, secondary prophylaxis is not recommended after a first-lifetime venous thrombosis, especially if the event was associated with a transient clinical risk factor. Secondary prophylaxis may be considered for idiopathic, recurrent, or life-threatening venous thromboembolism (e.g., pulmonary embolism, especially in association with persistently reduced cardiopulmonary functional reserve; phlegmasia with threatened venous gangrene; or purpura fulminans), persistent clinical risk factors (e.g., active cancer, chronic neurologic disease with extremity paresis, or other persistent secondary causes of thrombophilia [Table 1 ]),22,23 a persistent lupus anticoagulant and/or high-titer anticardiolipin or anti-β2 glycoprotein-1 antibody, anti-thrombin, protein C or protein S deficiency,14,42 increased basal Factor VIII activity,43 substantial hyperhomocysteinemia, combined heterozygous carriers for more than one familial thrombophilia (e.g., heterozygous for the factor V Leiden and Prothrombin G20210A mutations, etc.) or homozygous carriers,26,27 and possibly a persistently increased plasma fibrin D-dimer30 or residual venous obstruction.44 The risk of recurrence among isolated heterozygous carriers for either the factor V Leiden or Prothrombin G20201A mutations is relatively low and insufficient to warrant secondary prophylaxis after a first-lifetime thrombotic event in the absence of other independent predictors of recurrence.29 A family history of venous thromboembolism is not a predictor of an increased risk for venous thromboembolism recurrence45 and should not influence the decision regarding secondary prophylaxis. Because of the high risk of recurrence among patients with active cancer due to warfarin failure, low-molecular-weight heparin is recommended as long as the cancer remains active.
The risks of recurrent venous thromboembolism must be weighed against the risks of anticoagulant-related bleeding. The relative risk of major bleeding is increased about 1.5-fold for every 10-year increase in age,41 and about 2-fold for patients with active cancer.41 Additional risk factors for bleeding include prior gastrointestinal bleeding or stroke, or one or more comorbid conditions, including recent myocardial infarction, anemia (hematocrit < 30%), impaired renal function (serum creatinine > 1.5 mg/dL), impaired liver function, and thrombocytopenia. Moreover, the ability to perform activities of daily living should be considered because of the increased risk of bleeding associated with falls. The patient’s prior anticoagulation experience during acute therapy should also be considered; patients with unexplained wide variation in the international normalized ratio (INR) or noncompliant patients likely should not receive secondary prophylaxis. Finally, the mechanism(s) by which the anticoagulation effect of secondary prophylaxis with warfarin will be monitored and the warfarin dose adjusted should be considered; the efficacy and safety of such care when rendered through an “anticoagulation clinic” or when “self-managed” at home is superior to usual medical care. With appropriate patient selection and management, the risk of major bleeding can be reduced to about 1% per year.
Because the risk of venous thromboembolism recurrence decreases with time since the incident event,22 and because the risk of anticoagulant-related bleeding also may vary over time, the need for secondary prophylaxis must be continually re-evaluated. It is inappropriate to recommend “lifelong” or “indefinite” anticoagulation therapy.
Annual incidence of all venous thromboembolism, deep vein thrombosis (DVT) alone, and pulmonary embolism with or without DVT (PE ± DVT).4,22
Cumulative incidence of first venous thromboembolism recurrence (—), and the hazard of first recurrence per 1000 person-days (- - -).22
Cumulative incidence of first venous thromboembolism recurrence (—), and the hazard of first recurrence per 1000 person-days (- - -).22
Director, Mayo Clinic Coagulation Laboratories and Clinic; Professor of Medicine, Mayo Clinic College of Medicine From the Divisions of Cardiovascular Disease (Vascular Section) and Hematology (Section of Hematology Research), Department of Internal Medicine; and the Divisions of Hematopathology and Laboratory Genetics, Department of Laboratory Medicine and Pathology; Mayo Clinic and Foundation, Rochester, MN
Acknowledgments
Funded, in part, by grants from the National Institutes of Health (HL66216, HL83141, HL83797 & RR19457) and the Centers for Disease Control and Prevention (TS1255), U.S. Public Health Service; and by Mayo Foundation.