Abstract
Major, spontaneous bleeding is uncommon in patients with plasma cell dyscrasias despite frequent abnormal screening hemostasis tests. However, acquired von Willebrand deficiency and light-chain (AL) amyloidosis, and amyloidosis complicating multiple myeloma can present with serious hemorrhagic complications that are challenging to manage. While patients with monoclonal gammapathy of undetermined significance and multiple myeloma share an intrinsic increased risk of venous thromboembolic events (VTE), treatment with thalidomide and lenalidomide increases the incidence of VTE in certain multiple myeloma patient subsets. Our understanding of the complex interactions among malignant plasma cells, inflammatory and hemostasis pathways, and treatment modalities that combine to produce thrombotic complications is incomplete. Prospective, randomized trials are clearly needed to assist clinicians in providing optimal VTE prophylaxis to their patients with plasma cell dyscrasias.
Hemorrhagic Complications
While overt bleeding is a relatively uncommon presenting symptom of monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma (MM), Waldenström macroglobulinemia, and other B-cell malignancies, patho-physiologic interactions between paraproteins and coagulation factors, platelets, and vessels can produce hemostasis abnormalities. The consequences are often incidental, abnormal hemostasis test results, but overt bleeding and thrombotic presentations occur as well.1
Incidence
Relying on distant, retrospective surveys, bleeding complications are more likely with IgM and IgA paraproteins than with IgG, and are associated with higher concentrations of serum immunoglobins, higher serum viscosity, and prolonged bleeding time, but not with decreased platelet counts or prolonged prothrombin time (PT), activated partial thromboplastin time (aPTT), and thrombin time.2,3 More contemporary reviews report symptomatic bleeding at presentation in 0% of multiple myeloma,4 and 17% of Waldenström’s macroglobulinemia.5 Bleeding complications are a more common cause of morbidity and death during treatment of plasma cell dyscrasias6 due to progression of disease, renal insufficiency, infections, therapy related toxicity, and invasive procedures rather than a direct consequence of the paraprotein.7
Disorders of Primary Hemostasis and Acquired von Willebrand Syndrome
Impaired platelet function, based on prolonged bleeding time or abnormal platelet aggregation studies, has been associated with clinically significant bleeding and elevated serum paraproteins, particularly IgM.2,8 The proposed patho-physiology is nonspecific coating of platelets by immunoglobin.9 Control of bleeding and shortening of bleeding time following plasmapheresis indirectly supports this mechanism, but improvement may also be due to correction of hyperviscosity. Rarely, in vitro investigations have confirmed that bleeding and abnormal platelet aggregation studies were due to a paraprotein-specific qualitative platelet defect such as binding to GPIIIa.10
Over 450 cases of acquired von Willebrand factor (VWF) deficiency, representing multiple underlying disorders and probable mechanisms, have been published or summarized in an international registry of acquired von Willebrand syndrome (AVWS).11 Although an accurate estimate of the incidence of AVWS is not available, clinically apparent cases are uncommon. AVWS most often occurs in the setting of a lymphoproliferative disease, particularly MGUS (Table 1 ). Presenting in older patients with new onset of mucocutaneous bleeding and no family history of inherited bleeding disorders, the typical laboratory findings of AVWS and coexisting lymphoproliferative, myeloproliferative, and autoimmune disorders are similar to type 2A von Willebrand disease: abnormal bleeding time or PFA-100® (Dade Behring) closure times, markedly reduced VWF activity (ristocetin cofactor or collagen-binding assays), decreased VWF activity-antigen ratio, absence of large multimers, and normal plasma concentrations of VWF propeptide.12,13 Factor VIII (FVIII) activity and VWF antigen concentration may be reduced or normal. VWF inhibitory antibodies, detected by in-vitro mixing studies, are found in a small minority of patients with AVWS and lymphoproliferative diseases while enzyme-linked immunosorbent assay (ELISA)-based assays detect VWF antibodies in most cases.14 Decreased in-vivo survival of VWF following DDAVP (desmopressin acetate), cryoprecipitate, and Humate-P infusions supports increased clearance of VWF-autoantibody immune complexes by reticuloendothelial system as the primary mechanism. It is unclear if the mono-clonal immunoglobin present in lymphoproliferative MGUS–associated AVWS is specific for VWF.15 In a few cases with IgG paraproteins, the IgM immunoglobin fraction has contained vWF binding activity. Cases of AVWS due to antibodies with specific neutralizing affects on VWF have been reported including abnormal platelet adhesion due to paraprotein binding to VWF glycoprotein 1b binding domain,16 and paraprotein interference with vWF binding to collagen.17 Increased VWF clearance has also been linked to absorption onto malignant lymphocytes and plasma cells, and in two cases, aberrant expression of GPIb on the tumor cells was confirmed.13
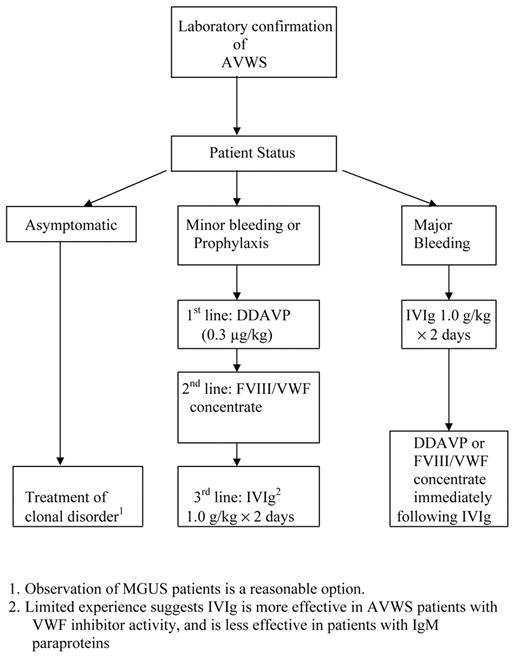
Management of AVWS will vary based on the associated disorder (Table 1 ), and involves interventions to stop acute hemorrhage as well as treatment of the underlying cause. Lacking evidence from large prospective trials, one must rely on small case series and expert opinions for guidance on the management of lymphoproliferative-associated AVWS18 (Figure 1 ). Based on limited experience, attempts to suppress the autoantibody responsible for AVWS in patients with MGUS with immune suppression or chemotherapy are usually unsuccessful, with response rates of 0 of 613 and approximately 36%.18 In patients requiring correction of acquired VWF deficiency prior to invasive procedures, infusion of DDAVP (0.3 μg/kg) and monitoring of VWF activity every 2 hours, based on ristocetin cofactor activity, collagen binding, or PFA-100 closure time, will identify some responders with VWF half-lives of 4 hours or more for whom DDAVP dosing at 12 hour intervals would be sufficient. If DDAVP therapy is unsuccessful, bolus or continuous infusions of a FVIII/VWF-containing factor concentrate (Humate-P, Alphanate), dosing based on monitoring VWF activity, would be second-line therapy. Some patients with IgG MGUS and AVWS will be refractory to both DDAVP and VWF infusions, but will respond to high-dose IVIg infusions (1 g/kg/day for 2 days).12,13,19 Response occurs within several days and may persist for several weeks. In actively bleeding patients, administering DDAVP or FIII/ VWF containing concentrates shortly after infusing IVIg can shorten the time to correction of the hemostasis defect to hours.19 Maintenance infusions of IVIg (1 g/kg) have been effective in a 2 patients, but lower doses (0.5 g/kg) failed.19 Based on fewer than 5 cases in the literature, patients with IgM MGUS and AVWS do not respond to IVIg,11,20 and would be appropriate candidates for aggressive VWF concentrate infusions, plasmapheresis, or extra-corporeal immune absorption in addition to therapy targeted to the underlying plasma cell dyscrasia. Despite case reports of successful treatment with recombinant factor VIIa (rFVIIa) of AVWS associated bleeding refractory to IVIg, DDAVP, and FVIII/VWF concentrate,21,22 it is premature to consider rFVIIa a standard treatment option. Clinicians are encouraged to add their cases of AVWS to an international registry (www.intreavws.com) in order to advance the understanding and management of this syndrome.
Acquired Coagulopathies and Amyloidosis
The most common coagulation abnormalities in patients with plasma cell dyscrasias, prolonged thrombin time and reptilase time, are almost always asymptomatic and are due to monoclonal protein interference with fibrin clot formation.7,23 In rare cases of multiple myeloma complicated by severe bleeding, paraproteins with specificity for thrombin24 and factor VIII7 have been identified. Both solid tumors and multiple myeloma have been associated with rare cases of acquired hyperfibrinolysis due to excess release of tissue plasminogen activator or urokinase-type plasminogen activator,25 and circulating heparin-like anticoagulants.26,27 Although the pathophysiology of both of these hemostasis disorders remains obscure, treatment with protamine infusions and antifibrinolytics respectively has been effective.
In light-chain (AL) amyloidosis, monoclonal plasma cells produce free immunoglobin light chains which form insoluble fibrils that infiltrate and disrupt vascular integrity in multiple tissues and organs. Bleeding is a common presentation of AL amyloidosis, ranging from 15% to 41%, with symptoms ranging from purpura to life-threatening hemorrhage.28–31 Amyloid angiopathy due to vascular fragility and impaired vasoconstriction is the major risk factor for bleeding, but acquired coagulation defects also contribute to hemorrhagic complications. Abnormal screening coagulation tests are more frequently abnormal in AL amyloid patients than in other types of plasma cell dyscrasias (Table 2 ), and are not associated with increased risk of bleeding.32 A unique, but uncommon, feature of AL amyloidosis is acquired coagulation factor deficiencies. Factor X deficiency, isolated or combined with other factor deficiencies, is the most common clinically important coagulopathy in patients with amyloidosis, and the degree of diminished factor X activity does not correlate with the severity of bleeding.33 The generally accepted mechanism is absorption of factor X onto AL fibrils, primarily in the liver and spleen, based on experiments confirming binding of factor X to splenic amyloid fibers34 and recovery of factor X activity following splenectomy.33 However, other causes for an acquired coagulopathy should be considered including vitamin K deficiency, hepatic insufficiency, and consumptive coagulopathy. Rapid clearance of factor X replacement in the form of plasma or prothrombin complex concentrates complicates sustained correction of the acquired deficiency. Additional options include splenectomy if indirect evidence supports the likelihood of factor X deposition, or rFVIIa. Response to chemotherapy is generally poor. High-dose melphalan with autologous stem cell transplantation in 10 patients with AL amyloidosis and acquired factor X deficiency produced 4 complete remissions and 2 partial remissions, but two patients died of hemorrhage in the peritransplantation period.35
Thrombosis and Plasma Cell Dyscrasias
As has been documented for many cancers and hematologic malignancies, patients with plasma cell dyscrasias are at increased risk for venous thromboembolic events (VTE).36 Available data regarding the incidence of VTE and associated risk factors in patients with MGUS and MM are limited. Sallah and colleagues prospectively monitored 310 patients diagnosed with MGUS every 3 months for a median of 44 months and objectively diagnosed VTE in 6.1% (6 VTE/100 patient years).37 A comparison of MGUS patients with and without a VTE identified age older than 65 years, M protein greater than 16 g/L, and subsequent progression to MM, amyloidosis, or other lymphoproliferative disease as risk factors associated with VTE. A retrospective review of 174 patients with MGUS and 404 patients with MM seen at the Cleveland Clinic between 1991 and 2001 confirmed VTEs in 7.5% of patients with MGUS (median 4 months after diagnosis), and 10% of patients with MM (median 8.5 months after diagnosis).38 Risk factors associated with VTE in patients with MGUS included personal and family VTE histories, immobility, low albumin, and elevated WBC. Personal and family history of VTE and positive hypercoaguable testing were associated with an increased risk of VTE in patients with MM.
An unexpectedly high rate of VTEs in patients with MM treated with immune-modulating drugs combined with chemotherapy or glucocorticoids has stimulated investigations into mechanisms of therapy-related VTE in MM. Acquired hypercoagulable risk factors common to patients with solid cancer and MM include chemotherapy effects, central venous catheters, immobility, malignant cells, and inflammation-induced prothrombotic states. Potential hypercoaguable mechanisms unique to MM include cytokine-mediated (predominantly IL-6) interactions between malignant plasma cells, bone marrow stromal cells, and endothelial cells, and paraprotein effects on fibrin polymerization and resistance to fibrinolysis.39 Rarely, paraproteins exhibit specific (anti–protein S40 and anti–protein C41) and nonspecific (lupus anticoagulant) prothrombotic properties.42
Laboratory investigations of acquired prothrombotic states in patients with MM have primarily focused on activated protein C resistance (APCr) and elevated FVIII and VWF (Table 3 ). Acquired APCr (APCr in the absence of FVL mutation) has been associated with an increased risk for VTE in several clinical settings including patients with cancer.24,43,44 In 2002, Zangari and colleagues at the University of Arkansas reported acquired APC resistance in 14 of 62 (23%) newly diagnosed patients with MM, by using an aPTT-based APCr assay in the presence of excess factor V–deficient plasma. Fifty percent (4 of 8) of APCr-positive patients treated with thalidomide and combination chemotherapy experienced a VTE compared with 7 of 22 (32%) VTEs among APCr-negative patients whose treatment included thalidomide.45 Subsequently published acquired APCr rates in patients with MM, also using aPTT-based APCr assays in the presence of excess factor V–deficient plasma, have varied from none46,47 to 14%.48 Recently, Elice reported 9% (109 of 1178) of patients with MM treated at University of Arkansas between 1999–2005 were APC resistant, and 51 of 83 were FVL negative, for an approximate 6% rate of acquired APCr. Independent risk factors for treatment related VTE included light-chain disease, elevated C-reactive protein, APC resistance, recent diagnosis, and thalidomide.49 Most APCr positive patients with MM who responded to treatment were negative when retested.48,49
Elevated FVIII activity is a recognized risk factor for spontaneous VTE, and several groups have reported elevated FVIII and von Willebrand antigen levels in patients with MM at baseline or with relapsed or refractory disease (Table 4 ). Limited laboratory surveys of patients with MM have not identified deficiencies of protein C or antithrombin, but conflicting results have been reported for protein S. Auwerda et al noted low protein S activity in 134 newly diagnosed patients with MM compared with healthy controls, and protein S decreased further with higher stages of MM.50 Neither elevated FVIII/VWF or decreased protein S has been associated with an increased risk of treatment-associated VTE. Due to the limited number of studies and the differences among them regarding APC resistance methods, disease status and treatment history, coagulation panels, and patient follow-up, no definitive conclusions regarding the mechanism of acquired APCr and its role in thalidomide-induced VTE are currently possible.
Thalidomide, and its related analog, lenalidomide, inhibit angiogenesis, produce complex immune-modulating effects, and block the antiapoptotic effects of IL-6 on malignant plasma cells. Encouraging response rates in relapsed and refractory patients with MM to thalidomide monotherapy and low VTE rates51 prompted adding thalidomide to existing glucocorticoid and chemotherapy combinations and applying them to newly diagnosed as well as relapsed patients with MM. However, thalidomide-containing regimens were accompanied by higher VTE rates when combined with dexamethasone52 and with doxorubicin and dexamethosone.53 Similarly, an initial clinical trial establishing lenalidomide as an effective agent against relapsed and refractory MM reported lower VTE rates.54 Subsequently, increased venous thrombotic complications were reported when lenalidomide was combined with high-dose dexamethasone, ranging from 5%51 to 18%,55,56 and dexamethasone plus erythropoietic agents.56Table 4 summarizes thrombotic complication rates for thalidomide- and lenalidomide-treated patients.57 Thrombotic complications during treatment of a malignancy with an angiogenesis inhibitor are not unique to thalidomide or multiple myeloma. Treatment of metastatic melanoma, renal cell carcinoma, prostate cancer, and glioblastoma with combination therapies including thalidomide were complicated by unexpectedly high VTE rates. Other inhibitors of vascular endothelial growth factor (VEGF)–activated pathways, such as bevacizumab, have been linked to arterial and venous thromboses.58
Several deep venous thrombosis (DVT) prophylaxis approaches have been evaluated, but no prospective, randomized study comparing different prophylaxis strategies in patients with similar disease states and receiving similar thalidomide- or lenalidomide-containing treatment regimens has been completed. Low-dose warfarin (1 to 2 mg/ day) appears to be safe, but reported efficacy for preventing thalidomide-related VTE varies widely.59–61 Anticoagulation with warfarin (international normalized ratio [INR] target of 2–3) appears to be effective for both primary62 and secondary prevention of thalidomide-associated VTE.61 VTE incidences among patients with MM receiving thalidomide and low-molecular-weight heparin (LMWH) pro-phylaxis were similar to VTE rates in patients receiving the same combination therapy without thalidomide and no pro-phylaxis, and LMWH did not cause bleeding complications.61,63 A high VTE rate among patients with MM treated with pegylated liposomal doxorubicin, vincristine, dexamethasone and thalidomide, and in vitro evidence of increased platelet aggregation and VWF antigen after chemotherapy prompted Baz and colleagues to add aspirin (81 mg) for VTE prophylaxis for subsequently enrolled patients. Patients who never took aspirin had a VTE rate of 58% (11 of 19) compared with 19% for patients who took aspirin (11 of 58).64 Additional before/after and retrospective studies have reported reductions in VTE rates using aspirin doses of 81 to 325 mg/day, mostly with lenalidomide combination therapies. Presently, there is no consensus regarding the optimal prophylaxis strategy,52,65,66 and prospective, randomized comparisons of VTE prophylaxis options for patients with MM at high risk for VTE are advocated by hemostasis67 and oncology52,65,66 experts.
Management of acquired von Willebrand syndrome (AVWS) associated with lymphoproliferative disorders.
Management of acquired von Willebrand syndrome (AVWS) associated with lymphoproliferative disorders.
Washington University School of Medicine, St. Louis, MO