Abstract
The diagnosis of therapy-related myeloid leukemia (t-MDS/t-AML) identifies a group of high-risk patients with multiple and varied poor prognostic features. These neoplasms are thought to be the direct consequence of mutational events induced by cytotoxic therapy. Their outcomes have historically been poor compared with those of people who develop acute myeloid leukemia (AML) de novo. The question arises whether a diagnosis of t-AML per se indicates a poor prognosis, or whether their bad outcomes result from other clinical and biologic characteristics. Because of lingering damage from prior cytotoxic therapy and, in some cases, the persistence of their primary disorder, patients with t-AML are often poor candidates for intensive AML therapy. The spectrum of cytogenetic abnormalities in t-AML is similar to de novo AML, but the frequency of unfavorable cytogenetics, such as a complex karyotype or deletion or loss of chromosomes 5 and/or 7, is higher in t-AML. Survival varies according to cytogenetic risk group, with better outcomes observed in patients with t-AML with favorable-risk karyotypes. Treatment recommendations should be based on performance status and karyotype. Patients with t-AML should be enrolled on front-line chemotherapy trials, appropriate for de novo AML patients with similar disease characteristics. Allogeneic hematopoietic cell transplantation can cure some patients with t-AML. Most important , the molecular and genetic differences that appear to determine the phenotype and the outcome of these patients need to be investigated further.
What Further Therapy Would You Recommend for These Patients?
Patient 1: A 50-year-old woman developed carcinoma of the ampulla of Vater and underwent surgical resection, followed by 60 Gy local radiation therapy plus 5-fluorouracil. Three years later, she presented with leukocytosis and peripheral myeloblasts. She was diagnosed with acute myeloid leukemia. Her karyotype was 46,XX,inv(16). She achieved a complete remission (CR) after 1 course of continuous infusion cytarabine plus 3 doses of daunorubicin. She received 1 course of consolidation therapy with high-dose cytarabine. She had no siblings. Several volunteers who were mismatched at one HLA allele were listed in the National Marrow Donor Program (NMDP) registry.
Patient 2: A 30-year-old man presented with superior vena cava syndrome and was found to have a large mediastinal mass. A diagnosis of Hodgkin lymphoma, nodular sclerosis subtype, stage IIB was made. He achieved a CR after 6 cycles of ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) followed by 30 Gy radiation to the residual mass. Two years later, the Hodgkin lymphoma recurred in the mediastinum and lung. He achieved a second remission after 2 courses of gemcitabine, navelbine, and Doxil. Stem cells were mobilized with cyclophosphamide, and he underwent autologous transplantation after BEAM (carmustine, etoposide, cytarabine, melphalan) chemotherapy. Three years later, he became pancytopenic and was diagnosed with therapy-related myeloid leukemia. His karyotype was 45,XY,− 7. A CR was induced with high-dose cytarabine and mitoxantrone. He had no siblings, but several HLA-matched donors were listed in the NMDP registry.
Introduction
Therapy-related myeloid leukemia (t-MDS/t-AML) is a well-recognized clinical syndrome occurring as a late complication following cytotoxic therapy.1–5 The term “therapy-related” leukemia is descriptive and based on a patient’s history of exposure to cytotoxic agents. Although a causal relationship is implied, the mechanism remains to be proven. These neoplasms are thought to be the direct consequence of mutational events induced by cytotoxic therapy, or via the selection of a myeloid clone with a mutator phenotype that has a markedly elevated risk for a mutational event. Several distinct clinical and cytogenetic subtypes of t-AML are recognized that are closely associated with the nature of the preceding treatment. The latency between primary diagnosis and therapy-related disease ranges between a few months to several years, depending in part on the cumulative dose or dose intensity of the preceding cytotoxic therapy, as well as the exposure to specific agents. Most patients have clonal chromosome abnormalities in their bone marrow cells at diagnosis. A spectrum of morphologic abnormalities is observed.4,5 There is a continuum in the percentage of marrow blasts from a myelodysplastic syndrome (t-MDS) to overt acute myeloid leukemia (t-AML), and rapid progression from the former to the latter. Thus, it is reasonable to consider this as a single clinical syndrome. The clinical course is typically progressive and relatively resistant to conventional therapies used for leukemias arising de novo.
Alkylating agents are clearly mutagenic and leukemogenic. Topoisomerase-II inhibitors, especially mitoxantrone in studies from Europe and etoposide and doxorubicin in the U.S., can induce t-AML, perhaps augmented by granulocyte colony-stimulating factor (G-CSF). The routine addition of G-CSF to cancer therapy has been controversial. It has been suggested that the ability of G-CSF to promote proliferation of damaged stem cells, which might otherwise undergo apoptosis, may contribute to leukemogenesis. Although life-saving with regard to severe infections, G-CSF has been associated with a significantly increased cumulative hazard of AML over time in patients with severe congenital neutropenia. More recently, t-AML has been seen in patients, particularly solid organ transplantation patients, following treatment with immunosuppressive therapies not previously thought to cause DNA damage directly. A mechanism for the development of t-AML has been proposed for azathioprine, an immunosuppressant widely used in recipients of organ transplantation, through selection of a mutator phenotype to allow the emergence of AML with abnormalities of chromosomes 5 and 7.2
The etiology and specific predisposing features of therapy-related leukemia remain elusive since fortunately only a small fraction of patients exposed to cytotoxic therapy develop the syndrome. It has not yet been possible to determine whether the development of t-MDS/t-AML is a stochastic event, occurring by chance, or whether certain individuals are at higher risk—perhaps due to a DNA-repair deficiency or a heritable predisposition, such as altered drug metabolism. The identification of such an underlying pre-existing condition would help the screening and counseling of patients at the time of treatment for their primary disease.
We have previously reported that the frequency of an inactivating polymorphism in the NQO1 gene (NAD(P)H:quinone oxidoreductase) is increased among individuals with t-AML.6 Both homozygotes as well as heterozygotes, who are at risk for treatment-induced mutation or loss of the remaining wild-type allele in their hematopoietic stem cells, may be particularly vulnerable to leukemogenic changes induced by carcinogens. Other polymorphisms involving detoxifying enzymes have also been reported.7 A large Japanese study of patients with AML de novo and t-AML found that the NQO1 polymorphism was more strongly associated with t-AML than polymorphisms in GST-M1, GST-T1 and CYP3A4.8
In general, two paths of investigation have been explored. The first involves meticulous clinico-pathologic and cytogenetic analyses and, more recently, molecular analyses, of individual patients as they present with therapy-related leukemia.3,4,9 The second involves large scale epidemiologic surveys of patients at risk. There are now many such studies of each type in the literature. After long-term follow-up, hundreds of patients with therapy-related leukemia among cancer survivors have now been reported on and analyzed. Although MDS and AML occasionally occur in cancer survivors treated only with surgery, suggesting a possible predisposition to malignant diseases, the risk of therapy-related leukemia is shared by patients with nonmalignant primary disorders if they have received cytotoxic treatment.1,2,4Table 1 shows the various primary diagnoses and primary cytotoxic therapies received by 306 patients with therapy-related myeloid leukemia studied at the University of Chicago.4
Therapy-Related Leukemia after Treatment of Breast Cancer and the Adjunctive Role of G-CSF
Several large studies have examined the risks for women receiving adjuvant chemoradiotherapy for breast cancer. In six trials completed by the National Surgical Adjuvant Breast and Bowel Project, the incidence of therapy-related leukemia was sharply elevated among patients receiving intensified doses of adriamycin and cyclophosphamide that required G-CSF support; the relative risk (RR) was 6.16 (P = .0001).10 Breast radiotherapy increased the RR to 2.38 (P = .006). In a second study, 5510 women older than 65 years who received chemotherapy for stage I to III breast cancer were analyzed.11 Sixteen (1.77%) of the 906 patients who also received G-CSF or granulocyte-macrophage CSF (GM- CSF) developed therapy-related leukemia compared to 1.04% of those who did not receive myeloid growth factors. The hazard rate was 2.59 (95% confidence interval [CI], 1.30–5.15) for the development of leukemia within 4 years. Finally, a large case-control study from France compared 182 patients who developed therapy-related leukemia after breast cancer treatment and 534 matched controls.12 The risk of leukemia was markedly increased after chemotherapy that included a topoisomerase-II inhibitor (P < .0001), and was higher after mitoxantrone (RR = 15.6) than after anthracyclines. The risk was increased 3.9-fold after breast radiotherapy (P = .003). The risk was also increased among those who received G-CSF (RR = 6.3; P = .0009), even when controlling for chemotherapy doses.
Therapy-Related Leukemia after Autologous Hematopoietic Cell Transplantation
Several thousand autotransplantations are performed each year in North America for patients with recurring lymphoma and other diseases. Estimates of the incidence of therapy-related leukemia among these lymphoma and Hodgkin disease patients range between 1% and 14% at 3 to 15 years.13 The risk appears lower in patients undergoing autologous hematopoietic cell transplantation (HCT) for breast or germ-cell cancers or myeloma. Important risk factors include age, extent of prior therapy, and exposure to certain agents before and during the transplantation procedure. Genotoxic damage and stresses imposed on hematopoietic stem cells during the priming or mobilizing chemotherapy and engraftment are also likely to play a role. Clonal hematopoiesis has been identified in some patients prior to HCT. Finally, inherited polymorphisms in genes governing drug metabolism and DNA repair likely contribute to leukemogenesis. Studies of the latency periods between first cytotoxic exposure, the autologous HCT itself, and the emergence of therapy-related leukemia suggest that the initial malignant event occurs prior to HCT in most patients.4,13 However, the cytotoxic therapy delivered during the HCT is likely additive to previous genomic damage and contributes to the etiology by cooperating mutations.
Classical Therapy-Related Myeloid Leukemia
In the classic form of therapy-related leukemia that follows treatment with alkylating agents and/or radiation therapy, the blood and bone marrow findings resemble those seen in primary MDS, although the degree of dysgranulopoiesis and dysmegakaryocytopoiesis is typically greater. Anemia and thrombocytopenia are extremely common. Leukopenia may also be present. Marked dysplastic changes are observed in all three cell lines. Mild to marked reticulin fibrosis may be present. Auer rods are rarely seen, and myeloperoxidase and nonspecific esterase reactivity are often only weakly expressed.
Clonal chromosomal abnormalities, often of a complex nature, are identified in most patients with classical therapy-related leukemia.1,3–5 Loss of part or all of chromosomes 5 and/or 7 are the characteristic findings and have been reported in more than 90% of patients in some series.4 The karyotypes are often complex. The most common single abnormality is monosomy 7, followed in frequency by deletion of the long arm of chromosome 5 [del(5q)] and by monosomy 5. These same abnormalities are observed in primary MDS and AML de novo, especially in older patients and those with occupational exposure to potential carcinogens such as benzene.
Therapy-Related Leukemia Following Topoisomerase-II Inhibitors
The leukemias secondary to agents that target topoisomerase-II typically result in translocations involving the MLL gene on chromosome 11, band q23 and, less commonly, the AML1 gene on chromosome 21, band q22.3,4 At first, the association was linked only to the epipodophyllotoxins, etoposide and teniposide. However, subsequent reports have also implicated DNA intercalating agents such as doxorubicin and mitoxantrone. In contrast to classic t-AML, these leukemias have a much shorter latency between initiation of chemotherapy for the primary cancer and the development of leukemia. In addition, a preceding MDS is not common.
Genetic Pathways and Cooperating Mutations in the Etiology of Therapy-Related AML
Particular mechanisms of DNA damage that lead either to chromosomal deletions or to balanced translocations may underlie the differences in latencies between the two forms of therapy-related leukemia.1,14 In the case of chromosomal deletions, one allele of a putative tumor suppressor gene may be inactivated. Before the affected cell would gain a proliferative advantage, however, the second allele might also have to be deleted or mutated. More recent evidence suggests that haploinsufficiency of individual genes such as EGR1 on chromosome 5q may allow for malignant transformation.15 However, even loss of both alleles of an individual tumor suppressor may not be sufficient to confer a malignant phenotype. As described in the model of colorectal tumorigenesis, multiple tumor suppressor genes or oncogenes may need to be mutated to ultimately transform a cell. This series of genetic changes may require an extended period of time, thus explaining the long latency of alkylator-induced t-AML. In contrast, balanced chromosome translocations result in the activation of cellular oncogenes in a dominant fashion. These rearrangements, such as those involving the MLL gene at 11q23, may yield a fusion gene that acts as a dominant oncogene. Whereas this fusion gene alone may not be sufficient to fully transform a hematopoietic progenitor cell, relatively fewer genetic events may be required to progress to the leukemic phenotype.
Pedersen-Bjergaard and his colleagues have proposed 8 different genetic pathways for the multistep development of t-MDS/t-AML (see Color Figures, Pedersen-Bjergaard et al Figure 1, page 510).16 There is growing evidence that mutations in a limited number of molecular pathways may cooperate in the genesis of leukemia. Gilliland and colleagues have described an emerging paradigm in AML, namely, the cooperation between constitutively activated tyrosine kinase molecules, such as FLT3, and transcription factor fusion proteins.17 In this model, the activated tyrosine kinase confers dysregulated proliferative and/or antiapoptotic activity, whereas the fusion protein impairs normal differentiation pathways but has a limited effect on cellular proliferation. Gene expression array experiments with CD34+ t-AML cells have provided evidence to support this hypothesis.9 Loss of TAL1, GATA1, and EKLF expression has been observed, which might result in impaired differentiation of hematopoietic cells, whereas overexpression of FLT3, PIK3C2B, and BCL2 result in a proliferative and survival advantage.
Factors that Influence Outcome in t-AML
Therapy-related leukemia is generally a fatal disease. The life-threatening complications of this disorder are the result of persistent and profound cytopenias due to the failure of normal hematopoiesis regardless of the fraction of myeloblasts accumulating in the bone marrow or blood. There has been general agreement that patients with t-AML have shorter survivals than patients with de novo AML. Supportive care is still considered by many to be the standard management.
A number of potential factors explain the poor outcome of patients with therapy-related leukemia. The persistence of the primary malignant disease, particularly meta-static cancer or lymphoma, causes morbidity and mortality independent of the bone marrow failure caused by leukemia. Injury to organs and their vascular supply from prior treatment may compromise the ability of these patients to receive intensive remission induction chemotherapy or bone marrow transplantation. There may be depletion of normal hematopoietic stem cells as a consequence of previous therapy, so that these patients suffer prolonged cytopenias after induction chemotherapy. The bone marrow stroma may have been damaged, especially by therapeutic radiation to fields that include the pelvis or lumbosacral spine, so that it will not support regeneration of normal hematopoiesis. Patients with t-AML are often chronically immunosuppressed from prior disease or ongoing therapy or may have dysfunctional phagocytes, and thus are often colonized with pathogenic or antibiotic-resistant bacteria and fungi. Following prior supportive care, patients may be refractory to additional transfusion support and are therefore not ideal candidates for intensive myelosuppressive chemotherapy. Finally, the high frequency of unfavorable cytogenetic aberrations arising during or after chemoradiotherapy appears to result in the rapid emergence of chemotherapy resistance in t-AML stem cells.
Treatment of Therapy-Related Myeloid Leukemia
The survival of patients with therapy-related leukemia is often poor despite prompt diagnosis and treatment. There is a paucity of prospective treatment data since these patients are most often excluded from frontline clinical trials. There are no randomized studies comparing standard AML therapy to other forms of treatment. In a nationwide Japanese study of 256 patients with t-MDS (41%) or t-AML (59%), a poor prognosis was associated with abnormalities of chromosome 5, hypoproteinemia, high C-reactive protein, thrombocytopenia, and persistence of the primary malignancy.18 The median age was 61 years. The median survival was only 9.7 months. Most of the Japanese patients (72%) received antileukemia chemotherapy, either a standard combination using an anthracycline plus cytarabine, or low-dose cytarabine, or tretinoin (ATRA) in the case of 7 patients with therapy-related acute promyelocytic leukemia (t-APL). A CR was seen in 85 patients (46%). The median remission duration was 8.2 months.
Poor hematopoietic reserves make the administration of standard AML therapy difficult. Many patients have poor tolerance for the acute toxicity of treatment. Because therapy-related leukemia evolves in the milieu of chemotherapy, the malignant cells are relatively drug resistant. Expression of the multidrug resistance phenotype is common. In a review of 644 patients with t-AML treated with a variety of standard AML chemotherapy regimens, only 182 (28%) achieved a CR.19 Individual small series report CR rates of 40% to 50%. This is considerably lower than the 65–80% CR rate observed in patients with de novo AML. In addition, remissions are often short even when confirmed cytogenetically and consolidated intensively.20
HCT for t-AML
The treatment most likely to cure t-AML is allogeneic HCT. Several small case series have described the outcomes of these patients, and the survival appears to be about 20% to 30%.1,21 However, chronic and cumulative toxicities from prior chemoradiotherapy affect the ability to perform HCT and adversely affect survival. Early deaths from regimen-related toxicity are more common after HCT for therapy-related leukemia than for primary AML.
In an analysis of 70 patients (31 with t-MDS and 39 with t-AML) who underwent allogeneic HCT between 1980 and 1998 in France, poor outcomes were associated with age older than 37 years, male sex, positive cytomegalovirus serology in the recipient, absence of CR at the time of HCT, and the use of intensive conditioning chemotherapy.22 The treatments given were heterogeneous, and the donors were varied. The estimated 2-year survival rate was 30%, event-free survival rate was 28%, relapse rate was 42%, and transplantation-related mortality was 49%. Thus, for patients who have chemotherapy-responsive t-AML, allogeneic HCT can be curative, but it is unfortunately not often successful. Nonmyeloablative, reduced-intensity allogeneic HCT is under investigation for those who are not eligible for standard HCT.
Similar results have been seen in children who have undergone allogeneic HCT for t-AML developing after therapy for acute lymphoblastic leukemia (ALL). Hale et al reported the outcomes of 21 children who had received epipodophyllotoxin-containing regimens for ALL and subsequently developed t-AML.23 Thirteen received induction chemotherapy prior to HCT, whereas 7 underwent HCT immediately after diagnosis. One patient received an autologous HCT in first CR from t-AML, but later relapsed and was subsequently treated at second relapse with an allogeneic HCT. Eleven patients received bone marrow cells from HLA-matched siblings, while 8 received bone marrow cells from matched unrelated donors, and 2 received haploidentical marrow from family members. Three years after HCT, only 4 patients (19%) were alive. Seven patients died from transplantation-related causes, and 10 patients died from recurring t-AML after a median of 5 months.
The European Bone Marrow Transplant registry has reported on 65 patients with t-AML who underwent autologous HCT.24 The median age was 39 years (range, 3–69 years). Estimates of overall and disease-free survival at 3 years were 35% and 32%, respectively. The relapse rate was lower for patients who received transplants in first CR (48% vs 89%, P = .05). Age over 40 years resulted in higher transplantation-related mortality (47% vs 7%, P = .01)
Cytogenetics Impact on Outcome of Therapy-Related Myeloid Leukemia
The most informative data on the prognostic impact of karyotype on outcome in t-AML were reported by the German AML Cooperative Group (AMLCG).25 This group compared karyotype analysis and survival between 93 patients with t-AML and 1091 patients with de novo AML; all received intensive treatment. Favorable, intermediate, and unfavorable karyotypes were observed in 26%, 28%, and 46% of patients with t-AML, and in 22%, 57%, and 20% of patients with de novo AML. Overall, the median survival was 10 months for patients with t-AML compared with 15 months for patients with de novo AML (P = .0007).
At the University of Chicago, 306 consecutive patients with t-AML were analyzed for clinical outcome according to cytogenetic subset as well as other clinical features, including disease latency.4 In contrast to the German series, not all of our patients underwent intensive remission induction chemotherapy. Many received only supportive care. Survival times are shown in Table 2 . Only 24 patients (8%) were alive 3 years after diagnosis. Even patients with a normal karyotype or with a balanced chromosomal rearrangement did poorly overall. Patients with t-AML who responded to remission induction therapy but subsequently died from their primary malignancy were included in the survival analysis. The incidence of unfavorable karyotypes was greater than 70%. The group with the worst overall survival compared with all other cytogenetic groups were those patients with abnormalities of both chromosomes 5 and 7 (P = .005).
In an updated analysis of the German AMLCG study, the survival of 121 patients with t-AML was compared to 1511 patients with de novo AML according to karyotype.26 All received intensive AML therapy. The median survival for the patients with t-AML ranged from 27 months for those with a favorable karyotype to 6 months for those with an unfavorable karyotype (Table 3 ). Importantly, about half of the patients with t-AML (58 of 121) had an unfavorable karyotype, whereas only about 20% (302 of 1511) of the de novo AML patients had an unfavorable karyotype. For those with a favorable karyotype, the median survival was not yet reached after 5 years for the 306 patients with de novo AML compared with 27 months for the 29 patients with t-AML (P = .02). Within the large intermediate-risk cytogenetic group, no significant difference in survival was observed between patients with t-AML and patients with de novo AML. An unfavorable karyotype predicated a very short survival in both groups of patients with AML.
Treatment of t-AML with Balanced Chromosomal Rearrangements
In marked contrast to the poor outcome overall for t-AML, those patients who develop t-APL with t(15;17) or those with t(8;21) or inv(16) have treatment outcomes that are similar to patients with de novo AML with the same chromosomal rearrangements. However, non-leukemia comorbidities or persistent primary malignancy may affect ultimate survival. In a report on 106 patients with t-APL identified between 1982 and 2001 in France, Spain, and Belgium, the characteristics of the patients with t-APL were similar to those of de novo APL.27 In addition, more than 80% of those treated with anthracycline-based chemotherapy and/or ATRA achieved a CR. Ten of the complete responders relapsed, and 7 others died from persistent primary tumor. The actuarial survival was 58% at 8 years, and did not differ between patient groups analyzed by primary treatment (chemotherapy, radiotherapy, or both) or prior exposure to particular drugs (alklyating agents, topoisomerase-II inhibitors, or both).
Among patients analyzed at the International Workshop in Chicago in 2000, 33 of 39 intensively treated patients (85%) with t-AML and inv(16), and 24 of 35 (69%) with t(15;17) achieved a CR.28 Both subgroups were associated with prior exposure to topoisomerase-II inhibitors, but importantly, 21% of the patients with inv(16) and 29% of the patients with t(15;17) had received only radiotherapy previously. The median overall survival for patients with t-AML with either inv(16) or t(15;17) was 29 months after receiving intensive AML therapy.
Only 12 of the 33 patients with inv(16) who achieved CR relapsed. Five underwent HCT in first CR (4 allogeneic, 1 autologous), and all 5 were alive and leukemia-free at last follow up. The responding patients were significantly younger than the 6 who did not achieve CR (median, 44 years vs 62 years; P = .012). In the inv(16) subgroup, patients younger than 55 years had improved survival when compared with older patients. The median survival in the young patient group (n = 26) was not reached and was longer than 3 years, but was only 12 months for the 13 older patients (P = .006). A similar tendency was observed in the t(15;17) subgroup, with median survival times of 29 and 20 months in the 21 younger and 14 older patients, respectively (P = .7).
A total of 72 patients with t-AML and t(21q22) were also studied at the International Workshop.29 Their median survival was 14 months, and 18% were alive after 5 years. Patients with t(8;21) had a more favorable outcome than those with other 21q22 rearrangements (P = .014). The median survivals were 17 months for the 11 t-AML patients with t(8;21) only and 31 months for the 33 patients with t(8;21) plus other abnormalities (P = .6). Fifty-three patients with t(21q22) received intensive AML therapy; the median survival for the 7 who underwent HCT was 31 months compared with 17 months for those who did not. Mutations in cKIT were not studied.
Recommendations for Treatment of t-AML
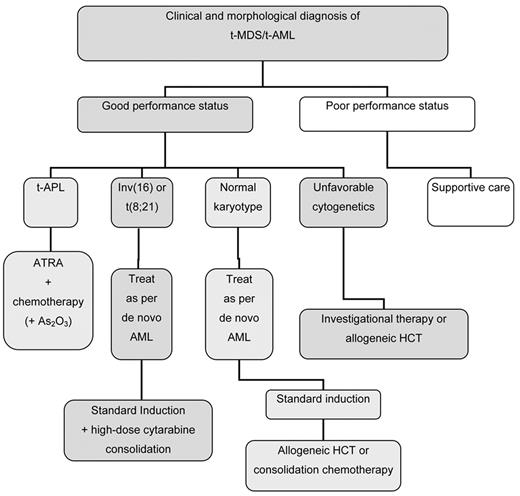
Figure 1 shows a treatment algorithm for the management of patients who develop t-AML. Primary considerations are the patient’s performance status, which likely reflects age, comorbidities, the status of the primary disease, and the presence of complications from primary therapy, as well as the clonal abnormalities detected in the t-AML cells (Table 4 ). In general, these patients should be encouraged to participate in prospective clinical trials that are appropriately designed for other patients with AML with similar cytogenetic abnormalities. Patients who have an HLA-matched donor should be considered for allogeneic HCT, although patients with favorable karyotypes may do well with conventional chemotherapy.
Decision tree for the management of therapy-related myeloid leukemia.
Department of Medicine and Cancer Research Center, University of Chicago, Chicago, IL
Acknowledgments
Supported in part by grants CA40046 and CA14599 from the National Cancer Institute, USA.