Abstract
A number of lines of evidence now support the hypothesis that vaso-occlusion and several of the sequelae of sickle cell disease (SCD) arise, at least in part, from adhesive interactions of sickle red blood cells, leukocytes, and the endothelium. Both experimental and genetic evidence provide support for the importance of these interactions. It is likely that future therapies for SCD might target one or more of these interactions.
Introduction
Homozygosity for the hemoglobin S (Hb S) mutation, as well as a number of heterozygous states involving one gene encoding Hb S, lead to shortened red blood cell (RBC) survival and hemolysis. Thus, patients with sickle cell disease (SCD) and Hb S heterozygous syndromes are variably anemic. Recently, anemia and hemolysis have also been linked to the development of pulmonary hypertension and other pathophysiologic processes possibly related to nitric oxide bioavailability, including priapism.1 In addition, cardiac remodeling and diastolic dysfunction develop as a consequence of anemia, and both pulmonary hypertension and cardiac sequealae such as diastolic dysfinction have been associated with accelerated mortality.2,3 Dysfunction is also directly related to the development of pigment gallstones and the frequent necessity of cholecystectomy.
Vaso-occlusion, the painful blockage of small vessels, is another hallmark of SCD. This process leads both to typically self-limited episodes of pain as well as a panoply of end-organ damage, including acquired functional asplenia, sickle cell nephropathy, hepatic sequestration, and acute chest syndrome. However, patients with SCD also suffer from a premature and increased rate of strokes, which may involve either large or small vessels.
In the last several decades, our therapeutic armamentarium for the treatment of acute vaso-occlusive episodes has not changed appreciably and continues to include narcotic analgesics, such as morphine, as well as oxygen supplementation and fluids as needed. We do not have therapies targeted against the pathophysiologic mechanisms precipitating or causing vaso-occlusive episodes. This stands in sharp contrast to other events involving acute compromise of circulatory systems, such as acute coronary syndromes. Thirty years ago, acute coronary syndromes were also treated with morphine and oxygen. However, in the intervening years, many targeted therapies have been developed to forestall the pathophysiologic processes that contribute to acute coronary artery occlusion. Among those available in the 1970s and early 1980s but not proven safe and effective for acute coronary syndromes until later were propranolol, aspirin, and heparin. Nitropaste for acute coronary syndromes was used soon after it became available in the 1970s. And, in subsequent years, clinical studies proved the effectiveness of platelet IIb/IIIa inhibitors, thrombolytic therapy, and statins as well as more invasive therapies such as primary angioplasty and coronary artery bypass grafting.
Thus, in order to improve our therapeutic options in SCD, we propose that we need to take a lesson from the cardiologists and apply our understanding of the patho-physiology of vaso-occlusion to the development of targeted therapies. We know that in acute coronary syndromes, occlusion of large epicardial arteries occurs due to a combination of events involving atherosclerosis, inflammation, plaque rupture or erosion, platelet aggregation, and thrombosis. So we must similarly ask ourselves about the mechanisms of vaso-occlusion: Does the event involve occlusion of arteries or veins? Are large or small vessels involved? What is the role of cell adhesion, and what types of cells are involved? Is endothelial dysfunction contributing to the process, and if so, how? What about the contributions of inflammation, thrombosis and platelet aggregation? Finally, what is the acute cause of vaso-occlusion? Individuals with SCD are not constantly experiencing acute painful vaso-occlusion. Therefore, we must understand why people can be living their lives relatively unimpeded at one moment, only to find themselves in acute pain a few hours later. Certainly it is not the hemoglobin content of their red cells that has changed over that period of time.
Sites of Vaso-Occlusion and the Endotheliopathy of SCD
Most in vivo evidence suggests that vaso-occlusion does not occur due to trapping of sickled red cells in small caliber vessels. Rather, ex vivo and in vivo evidence suggests that vaso-occlusion occurs in postcapillary vessels too large to be occluded by single cells, even when they are distorted by HbS polymerization. In animal models, vaso-occlusion occurs primarily when postcapillary venules are blocked by adherent SS RBCs and leukocytes, which can be seen to pile up on one another, leading to eventual compromise of blood flow.4,5 However, stroke occurs due to occlusion of large intracranial arteries, typically in children. Thus, vaso-occlusion in SCD can occur in both large and small vessels, including both arteries and veins. However, the role of “sickled” cells remains uncertain in all these settings.
There are a number of lines of evidence suggesting that the endothelium is abnormal in SCD as well. Pathologic studies show a broad range of endothelial and vascular abnormalities, of which perhaps the most remarkable is the formation of Moya Moya type intracranial blood vessels. Studies have also shown that SCD is accompanied by abnormal vascular tone, perhaps caused by the presence of free Hb in the plasma, leading to lack of bioavailable nitric oxide (NO).6 In addition, it is known that endothelin-1 levels are elevated in SCD. Finally, there is an increased number of circulating endothelial cells in patients with SCD, and these cells have an activated phenotype. Experimental work in vitro has shown that contact with flowing SS RBCs activates endothelial cells in culture, leading to an increase in expression of adhesion receptors as well as procoagulant proteins.7–10 In addition, the demonstrated increase in a variety of cytokines also leads to activation of endothelial cells.
Contribution of Inflammation and Hypercoagulability to Vaso-Occlusion in SCD
Numerous inflammatory markers are known to be elevated in SCD, including TNFα, C-reactive protein, and interleukins-1 and -8. In addition, mediators of endothelial activation are elevated, including vascular cell adhesion molecule-1 (VCAM-1), endothelin-1, and sCD40 ligand. Clinical studies have also shown that baseline leukocyte counts are elevated in SCD and, more importantly, are known to correlate with many manifestations of SCD, including stroke, acute chest syndrome, and mortality.11–17 Markers of neutrophil activation are also increased in SCD, concordant with increased levels of circulating cytokines and increased leukocyte adhesion.18 In animal models, vaso-occlusion causes hypoxia/reperfusion injury,19,20 which is also mediated by an inflammatory response, and some anti-inflammatory agents can reduce damage due to experimentally induced vaso-occlusion.21
Typical thrombotic events, including strokes, avascular necrosis, and pulmonary emboli, are common in SCD. In fact, almost every aspect of hemostasis is abnormal in SCD. Platelets are activated and abnormally numerous. RBCs are procoagulant due to exposed phosphatidylserine. Levels of von Willebrand factor (VWF) and factor VIII (FVIII) are elevated. And finally, there is chronic thrombin generation, with elevated d-dimers, thrombin-antithrombin (TAT) complexes, and other markers.
So, we must finally ask ourselves: Why isn’t vaso-occlusion happening all the time? Why can a person with SCD be asymptomatic at noon and in severe pain at dinner time? And our conclusion must be that something, and perhaps something preventable or reversible, must precipitate vaso-occlusive events. About 25% of vaso-occlusive episodes are associated with identifiable physiologic stress, especially infection. For that reason, all patients with acute pain should have a thorough evaluation for signs and symptoms of infection, including pulmonary, urinary, biliary, skin, and other systemic infections (e.g., port infection, osteomyelitis). Even afebrile patients with “typical” pain should have a chest x-ray, urinalysis, and pulse oximetry, as well as a complete blood count with differential. But what about the other 75% of episodes? Patients often report emotional stress, physical stress, or environmental factors, such as change in ambient temperature, as associated with onset of pain. But we thus far lack solid, carefully gathered evidence that there is a causal connection between physiologic stress and vaso-occlusion, although such a connection remains an attractive hypothesis for explaining how a person may transition from steady state to vaso-occlusion.
Thus, after considering all these factors, we must conclude that possible therapeutic targets in SCD include RBC adhesion; endothelial cell activation and vascular tone; leukocyte activation, inflammation and hypoxia/re-perfusion injury; hypercoagulability, including platelet activation; inciting events; and Hb S polymerization (i.e., prevention of sickling).
Targeting Cell Adhesion as a Therapeutic Strategy
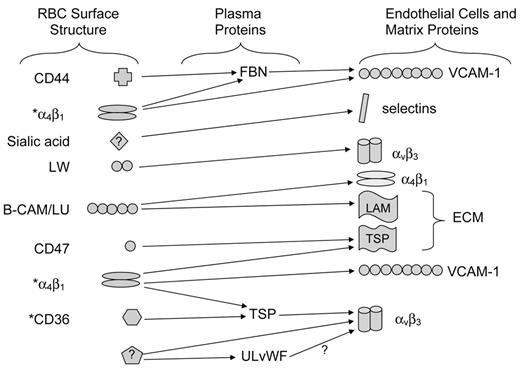
SS RBCs adhere abnormally to extracellular matrix components as well as to endothelial cells. In the 1980s, increased adherence was shown to correlate with severity of vaso-occlusive symptoms. We now know that both normal and SS red cells have multiple adhesion receptors (Figure 1 ). Reticulocytes, but not mature RBCs, express both CD36 and α4β1 integrin, which are capable of binding to thrombospondin and fibronectin, respectively. Both reticulocytes and mature RBCs express a number of additional adhesion receptors, including B-CAM/LU, a high-affinity laminin receptor; LW (ICAM-4), a receptor for a variety of integrins; CD44, a hyaluronan receptor; and an as-yet unidentified molecule that binds to selectins. However, for many years, it was not clear how much each of these potential types of interactions actually contributed to vaso-occlusion in vivo. The first breakthrough was in 2000, when Kaul and colleagues showed that a monoclonal antibody that binds both αIIbβ3 (platelet glycoprotein IIb/IIIa) and αvβ3 markedly inhibited adhesion of human SS RBCs to rat mesocecal vessels in an ex vivo model.4 Zennadi and her colleagues then showed in 2004 that RBC LW (ICAM-4) was the RBC receptor for endothelial αvβ3.22 Since then, at least two groups have shown that the LW-αvβ3 interaction appears to be the major contributor to SS RBC adhesion in animal models.5,23
The LW-αvβ3 interaction is therefore a rational target for anti-adhesive therapy, although not without challenges. Abciximab, a product already approved by the FDA as an antiplatelet agent, crossreacts with both platelet αIIbβ3 and endothelial αvβ3; it is essentially the same antibody shown to prevent SS RBC adhesion in a rodent ex vivo model.4 However, that model looked at SS RBC adhesion in a highly artificial system, in which endothelial cells were activated with platelet-activating factor (PAF), and RBCs were suspended in buffer without other normal blood elements, including plasma, platelets and leukocytes. Nevertheless, two more recent studies are also encouraging regarding the possibility that the LW-αvβ3 interaction can be targeted in vivo.5,23 Kaul and colleagues showed in an ex vivo microvascular model system that two peptides derived from LW markedly decreased SS RBC adhesion, improved hemodynamics, and prevented vessel blockage in vessels pretreated with PAF. Zennadi et al have shown that a soluble form of LW can also be used to prevent epinephrine-stimulated SS RBC adhesion and vaso-occlusion in an animal model in which RBC adhesion takes place in uninstrumented vessels and in the context of whole blood. Thus, while much work remains to be done, I believe we can reasonably expect that anti-adhesive therapy will continue to be developed.
Nor is the LW-αvβ3 interaction the only target. Several other interactions between SS RBC and endothelium or extracellular matrix proteins may also be amenable to blockade. Polaxamer 188 (Flocor), a nonspecific surfactant–like molecule, showed some modest efficacy in treating sickle cell vaso-occlusive crisis and has been shown to reduce RBC adhesion;24,25 however, this product is no longer under development for treatment of SCD. Another possible inhibitor of SS RBC adhesion is heparin, which inhibited SS RBC adhesion to P-selectin as well as to cultured endothelial cells in vitro.26 The use of heparin could thus theoretically ameliorate two pathophysiologic processes involved in vaso-occlusion, RBC adhesion and activation of coagulation. In addition, both dextran and chondroitin sulfate have been shown to block SS RBC adhesion to thrombospondin in vitro. A study of the effect of infusion of dextran reported in 1956 reported both an increase in hemoglobin level as well as improvement in symptoms of vaso-occlusion in 6 of 9 patients, and the authors postulated that such infusions resulted in “mobilizing erythrocytes which have become trapped or stagnated in organ depots and/or small peripheral vessels.”27
Studies have also provided support for the role of α4β1 on SS reticulocytes, which frequently express the α4β1 integrin. In 1993, Swerlick et al showed that SS RBCs can bind to VCAM-1 expressed by TNFα-activated endothelial cells.28 Additional work suggested that α4β1 also mediated adhesion to fibronectin after SS RBCs were stimulated by phorbol ester.29 Perhaps most interestingly, a study of human SS RBC circulation in rat retinal vessels showed retention of human SS RBCs in TNFα-treated rat retinal vessels that could be abrogated by a peptide that blocks the binding of α4β1 or a monoclonal antibody against α4β1.30 Thus, α4β1 may also be an appropriate target for anti-adhesive therapy.
Another approach to blockade of cell adhesion may be developed by understanding why SS RBCs but not normal RBCs adhere. Although SS RBCs have slightly higher expression of several adhesion receptors, such as B-CAM/ LU and LW, the degree to which these molecules are more strongly expressed is modest.31,32 More interestingly, and potentially addressable as a therapeutic target, is the fact that the adhesive functions of both B-CAM/LU and LW have been shown to be upregulated in response to adrenergic signaling.5,22,33,34 Simply put, exposure of SS RBCs, but not normal RBCs, to physiologic “stress” levels of epinephrine results in markedly increased adhesion of SS RBCs to these receptors’ ligands, laminin and endothelial αvβ3 integrin, respectively. Not surprisingly, therefore, propranolol has been shown to inhibit SS RBC adhesion to endothelial cells both in vitro and in a rodent model of vaso-occlusion.5 In that model, infusion of human SS RBCs not previously exposed to epinephrine adhered only minimally to microvascular walls, while SS RBCs previously exposed to epinephrine adhered avidly and caused vaso-occlusion. However, pretreatment of either the SS RBCs or the animals with propranolol prevented most of the adhesion and vaso-occlusion.5 De Castro and colleagues presented very promising preliminary results of the first study of the effects of oral propranolol on human SS RBC adhesion measured in vitro at the 2006 meeting of the American Society of Hematology.35 Therefore, although we need to await further studies of both safety and efficacy, the possibility certainly exists that anti-adhesive therapy may address signaling pathways responsible for activation of adhesion rather than, or in addition to, the adhesion receptors themselves.
Is There a Role for Anti-Inflammatory Agents?
Leukocytosis has been shown to be a factor in predicting a poor outcome in SCD.36 Furthermore, during vaso-occlusive crises, leukocytes from patients with SCD exhibit greater adhesion to endothelial cells, as well as increased expression of activation markers such as CD64.37 There are a number of ways in which inflammation may contribute to vaso-occlusion. Inflammatory mediators upregulate the expression of adhesion molecules by endothelial cells. In addition, SS RBCs may also adhere to leukocytes that are themselves adherent to vessel walls.38
Dexamethasone has been shown to improve sickle RBC circulation acutely in animals, but it then causes leukocytosis and rebound increase in adhesion molecule expression.39 Nevertheless, dexamethasone has been studied in the setting of acute chest syndrome, where preliminary studies suggest it might be beneficial.40 Sulfasalazine has also been tested in both mice and a small number of human subjects, due to its ability to inhibit NFκB. Sickle mice received sulfasalazine twice daily for 10 days. After treatment, the mice were examined under normoxic conditions and after 3 hours of hypoxia and 1.5 hours of reoxygenation, conditions that had been previously shown to induce sickling and hypoxia/reperfusion injury. This work showed that sulfasalazine caused a marked decrease in leukocyte adhesion in normoxic conditions, as well as after hypoxia/ reoxygenation, consistent with anti-inflammatory efficacy.41 In another study, 3 patients were given sulfasalazine every 8 hours, and the activation state of their circulating endothelial cells was assessed; sulfasalazine was found to have significantly reduced circulating endothelial cell expression of VCAM, ICAM, and E-selectin but not tissue factor.21 However, larger trials of sulfasalazine in human patients with SCD have not been reported.
Thus, anti-inflammatory therapy in SCD is another area that requires further study but seems promising. In fact, it is already recognized that clinical response to hydroxyurea (HU) correlates with both increases in Hb F as well as the degree of WBC reduction achieved42 and therefore may be viewed as dependent on the anti-inflammatory effects of HU.
In addition, it is interesting to speculate that statins may be of value in SCD. An initial study of the effect of lovastatin in severe phenotype sickle mice showed that it effectively reduced the expression of tissue factor in the lungs of such mice; moreover, it also blocked the up-regulation of expression of tissue factor in less severe mice after exposure to hypoxia.43 In an editorial regarding that work, Hillery commented that tissue factor expression might both “cause and result from vascular inflammation in sickle cell disease” and that statins might be effective in breaking this vicious cycle.44 A trial of atorvastatin therapy to improve endothelial function in sickle cell disease is currently being conducted at the National Institutes of Health Clinical Center. The goals of this study are to determine the effects of oral atorvastatin on endothelial-dependent relaxation in patients with SCD, examine the effect of such therapy on peripheral blood markers of inflammation and vascular function, and investigate the role of xanthine oxidase in limiting NO bioavailability in patients with SCD.
Anti-Sickling Therapy
Despite a detailed understanding of the biochemical and physical characteristics of Hb S, the role of red cell sickling in vaso-occlusion remains uncertain. We do know that sickling causes membrane damage, decreased RBC deformability, and increased hemolysis. We also know that drugs that increase Hb F decrease Hb S polymerization, and drugs that prevent RBC dehydration should also decrease Hb S polymerization. Thus, antisickling therapy has received a great deal of attention from clinical investigators, in part because a diminution of hemoglobin polymerization would certainly be apt to lessen hemolysis and improve anemia (and possibly thereby overall well-being) as well as in the hope that reduced sickling would lessen the frequency of vaso-occlusive events.
Induction of Hb F
HU is a deoxyribonucleotide reductase inhibitor that may affect Hb F production, primarily due to its myelotoxicity. The Multicenter Study of Hydroxyurea In Sickle Cell Anemia (MSH) study showed that it can lead to increased Hb F levels in about 50% of patients with SCD.45 HU also lowers leukocyte, platelet, and reticulocyte counts in a manner highly associated with Hb F response.42 The MSH study looked at 299 adults with SCD and found that, during the 2-year study period, the frequency of hospitalization, vaso-occlusive episodes, acute chest syndrome, and blood transfusion were each reduced by almost half.45 Responsiveness correlated with higher baseline reticulocyte and leukocyte counts, possibly due to greater “bone marrow reserve.” Furthermore, long-term follow-up shows a 40% reduction in long-term mortality, although, as mentioned above, clinical improvement may not correlate only with Hb F response. A phase 2 study in children also showed that children treated with HU also experience increased Hb F levels, as well as increased total hemoglobin and a lower rate of SCD-related complications.46 In addition, that study successfully demonstrated that HU did not have adverse effects on growth and development between the ages of 5 and 15 years.
Decitabine appears to work by hypomethylation of the γ-globin gene promoter, with associated changes in histone acetylation and chromatin structure. In pilot studies, responses were even seen in HU nonresponders.47 Treatment with decitabine has been associated with increased Hb F and a decrease in bilirubin and reticulocyte count, as well as decreased markers of activation of coagulation, such as d-dimer, VWF, and sVCAM-1.48 Larger scale studies of decitabine are on the horizon.
Butyrate is another compound that can induce expression of Hb F. It is a short-chain fatty acid that inhibits histone deacetylation and changes chromatin structure. Intermittent infusions give sustained Hb F response in patients whose Hb F baseline is above a certain level.49
Prevention of RBC dehydration
Another type of antisickling therapy involves reagents that do not affect hemoglobin expression but rather promote cell hydration, thus inhibiting Hb S polymerization by keeping the Hb concentration lower. One major class of such compounds are Gardos channel inhibitors.
Clotrimazole was one of the first such compounds to be tried. In sickle mice, it successfully improved RBC hydration as well as Hb level.50 Clotrimazole was also shown in pilot studies in humans to reduced erythrocyte dehydration, increase RBC potassium content, and slightly improve total Hb levels.51
A more effective Gardos channel inhibitor is ICA-17403, now called senicapoc. This compound inhibits the Gardos channel 10 times more efficiently than does clotrimazole. Senicapoc specifically blocks the Ca++-dependent K+ efflux, limiting the loss of K+, Cl−, and water. In a phase 2 study, senicapoc was shown to decrease RBC hemolysis and improve anemia. In this 12-week phase 2 study, 90 patients were randomized to placebo, low-dose, or higher-dose drug. Eighty patients completed the study, and only 3 of 10 dropped out because of adverse events. Hb increased significantly for patients taking the higher dose (P < .001). Dense RBCs and reticulocytes also decreased significantly. Lactose dehydrogenase (LDH) and indirect bilirubin, markers of hemolysis, likewise showed significant drops. However, a multicenter phase 3 trial was stopped due to the Data Safety Monitoring Board’s determination that there was a low probability of achieving a reduction in crisis rate, the primary study endpoint. Nevertheless, preliminary analysis of the data have apparently shown the expected increases in total Hb as well as decreases in reticulocytes, LDH, and bilirubin. In addition, analysis to date indicates no statistically significant differences in safety measurements between the senicapoc and placebo treatment groups. Thus, while this drug will probably not be useful to prevent SCD-related events such as vaso-occlusive crises, it may warrant further investigation for its ability to lower the hemolytic rate and raise hemoglobin levels. The results of the phase 3 study are also particularly interesting in that it appears that a significant decrease in hemolytic rate did not affect the frequency of vaso-occlusive events. Whether or not such a decrease in hemolysis would affect other disease outcomes, such as progression of renal dysfunction or pulmonary hypertension, remains unknown and as of yet untested.
The K:Cl cotransporter is another potential therapeutic target in SCD. Sickle erythrocytes are exposed to relatively acidic conditions in organs such as the kidney, and as a result, their K:Cl cotransporters are activated, leading to loss of both potassium and water and consequent cell shrinkage. This transporter can be inhibited by increasing the abnormally low cytoplasmic magnesium (Mg) content of sickle erythrocytes.
Oral Mg supplementation has been shown to reduce sickle cell dehydration in vivo in transgenic sickle mice and in patients.52 A small uncontrolled trial of Mg pidolate showed that this therapy resulted in increased RBC Mg and K, decreased cotransporter activity, fewer dense RBCs, and decreased numbers of painful episodes. Oral Mg pidolate is now being studied in Hb SC disease, both as a single agent and in combination with HU. This trial will examine several surrogate endpoints, although it will not be large enough to examine the effect of Mg pidolate on clinical endpoints such as frequency of crisis.52
Cation flux is also associated with deoxygenation and sickle RBC sickling. Dipyridamole has been shown capable of inhibiting sickling-induced cation fluxes in vitro.53 However, although there has been limited investigation of dipyridamole in SCD for its antiplatelet effects, it is only now being investigated clinically for its ability to prevent RBC dehydration and sickling.
Therapy for Vaso-Occlusive Episodes—The Future
As discussed above, we are gradually learning how to target the complex processes that appear responsible for vaso-occlusive events in SCD. As is the case of atherosclerotic heart disease, there is no single pathophysiologic process to target (Table 1 ). Rather, it seems we will have to aim for disrupting many contributing events in the vaso-occlusive pathway. If we succeed, then perhaps we, like the cardiologists, will rarely find need for morphine in the treatment of vaso-occlusive pain.
Hopefully, our understanding of the vicious circle of adhesive and inflammatory processes involved in vaso-occlusion, as well as the signaling and transport events that occur within the RBCs, will also help us take aim at chronic prevention of clinical sequelae. Our prophylactic armamentarium will likely include HU and other drugs that act at the level of transcription, as well as drugs that take aim at other pathophysiologic processes. These drugs could include Gardos and other transport channel inhibitors to prevent RBC dehydration, sickling and hemolysis; statins and other anti-inflammatory agents; inhibitors of adrenergic receptors; and antiplatelet agents and anticoagulants, which are discussed by Dr. Ataga, below.
Adhesive interactions between SS red blood cells, plasma proteins, and components of the endothelium. A large body of evidence supports the ability of multiple red cell surface structures to interact with plasma proteins, endothelial cell surface proteins, or components of the subendothelial extracellular matrix.
* Structures present on SS reticulocytes but not mature SS red blood cells.
Abbreviations: FBN, fibronectin; TSP, thrombospondin; ULvWF, ultra-large von Willebrand factor; ECM, extracellular matrix; LAM, laminin
Adhesive interactions between SS red blood cells, plasma proteins, and components of the endothelium. A large body of evidence supports the ability of multiple red cell surface structures to interact with plasma proteins, endothelial cell surface proteins, or components of the subendothelial extracellular matrix.
* Structures present on SS reticulocytes but not mature SS red blood cells.
Abbreviations: FBN, fibronectin; TSP, thrombospondin; ULvWF, ultra-large von Willebrand factor; ECM, extracellular matrix; LAM, laminin
Duke University Medical Center, Durham, NC