Abstract
In recent years, there has been increasing interest in non-invasive iron measurement, especially of the liver and heart, in patients with iron overload. Serum ferritin still remains an essential monitoring parameter in intervals between liver iron measurements; however, confounding factors such as inflammation, chelation treatment changes and the specific disease have to be taken into account. Liver iron measurements can now routinely be performed in clinical applications either by quantitative magnetic resonance imaging (MRI) using the transverse magnetic relaxation rate R2 or R2* (1/T2*) or by biomagnetic liver susceptometry. For iron measurements in the heart, the single-breathhold multi-echo MRI-R2* method has become a standard modality and is now applied in clinical settings beyond research studies. In other tissues like the pancreas, pituitary, and brain, different MRI methods are employed, but their clinical benefit has yet to be proven.
The concentration and total amounts of iron in different tissue are critical parameters that determine clinical outcome in all forms of systemic iron overload, independent of whether the iron overload is caused by blood transfusion (ie, thalassemia major, sickle cell disease, aplastic or refractory anemia or myelodysplastic syndrome) or by up-regulated intestinal iron absorption (ie, hereditary hemochromatosis, thalassemia intermedia, iron loading anemias). The precise assessment of the size of the iron storage pool is essential for the treatment of chronically transfused patients with iron chelators to avoid both toxicity from increased organ iron concentration and side-effects from an excessive chelator dose.1
Iron stores can be assessed by direct and indirect methods. The reference method for direct biochemical measurement of non-heme iron is a liver biopsy. Since the introduction of the serum ferritin test, additional indirect (non-transferrin bound iron, hepcidin) and non-invasive (magnet resonance imaging [MRI], biosusceptometry) methods were developed for the determination of iron stores. Many of these indirect parameters are well suited for the quantitative determination of normal iron stores or of iron deficiency, but fail in the measurement of iron overload.
In recent years, there has been increased interest in non-invasive quantitative iron measurements. These developments were especially influenced by the progress in molecular biology, which led to the discovery of different genes and mutations of hereditary hemochromatosis. Interest was further encouraged by the development of novel oral iron chelators such as deferiprone and deferasirox for the treatment of secondary transfusional iron overload. The recognition that cardiomyopathy and arrhythmia are the most frequent causes of death in thalassemia2 has provided a further stimulus for the development of non-invasive measurements of cardiac iron.
Progress in measurement technology added another stimulus to the development of noninvasive iron assessments. Biomagnetic liver susceptometry (BLS) based on low-temperature (4°K, SQUID) and high (77°K) or room temperature systems is now available or under development as a routine method at five different centers (New York, Hamburg, Turin, Oakland, Genoa).3 Quantitative magnetic resonance imaging (MRI-R2) now allows the measurement of iron distribution and its averaged concentration in an entire slice of the liver.4 The application of fast MRI-GRE-methods (gradient recalled echo, T2*) to the heart in order to detect relatively low cardiac iron concentrations has offered new strategies in the treatment of transfusional siderosis.5 MRI-R2 and –R2* methods have now also been used to assess iron in the spleen, pancreas, bone marrow, pituitary, and in the brain.
Serum Ferritin as Monitoring Parameter
In spite of the limitations of serum or plasma ferritin (SF) for the estimation of iron stores in patients with iron overload, this indirect parameter remains essential in monitoring iron stores. Assays of SF are available worldwide, relatively well standardized, and inexpensive. In the absence of confounding factors, such as inflammation, vitamin C deficiency, oxidative stress, hepatocyte dysfunction, and increased cell death, SF is found in proportion to the size of cellular iron stores. Currently the precise source of serum ferritin is unknown, but SF is seen as an indicator for the iron stores of the reticuloendothelial system (RES). Moreover, the ratio between SF and iron stores is strongly dependent on the underlying disease and is significantly lower in hereditary hemochromatosis, non-transfused thalassemia intermedia, congenital dyserythropoietic anemias, and other iron loading anemias as compared with thalassemia major, myelodysplastic syndrome (MDS), Diamond Blackfan anemia (DBA) or sickle cell disease (SCD).6 –7 Once the SF/LIC-ratio is known for an individual patient, SF is a useful monitoring parameter over a relatively short period (1–3 years) as long as the transfusion and chelation treatment regimen is not changed, the patient’s compliance does not vary, and inflammatory parameters are considered.
Liver Iron Quantitation
In patients with secondary iron overload, 70% to 90% of the total body storage iron is stored in the hepatocytes and Kupffer cells, mainly as ferritin and hemosiderin iron. Iron depletion by chelation or phlebotomy can be most accurately monitored in the liver. The assessment of liver iron concentration (LIC) is performed either invasively through liver biopsy or non-invasively using the paramagnetic properties of the storage iron by quantitative magnet resonance imaging (MRI) or biomagnetic liver susceptometry (BLS). Both the non-invasive methods rely on similar physical principles, ie, a change in the magnetic environment of protons (MRI) or iron electrons (BLS) produces a change in the magnetic flux. This change in magnetic flux is measured as a radio wave signal (MRI) or as a voltage change (BLS).8
Quantitative Iron Measurement by Liver Biopsy
The reliability of LIC, as measured in a small biopsy sample, remains a continuously discussed question. A minimum of 4 mg wet weight (fresh tissue) or 1 mg dry weight (dessicated or paraffin block extracted) tissue without significant fibrosis is recommended.9,–10 With a mean dry weight of 0.94 mg, agreement (7.6%) between 2 percutaneous biopsy specimens has been achieved in patients with β-thalassemia major and sickle cell disease.11
Biomagnetic Liver Susceptometry
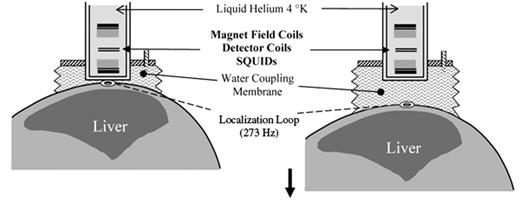
In the case of biomagnetic liver susceptometry (BLS), the change in magnetic flux caused by moving a patient with iron overload through a stable magnetic field generates a voltage in the SQUID sensor. In the practical application of BLS, the difference method is usually employed (Figure 1 ), which measures the difference in magnetic volume susceptibility Dc between the thorax (including the liver with paramagnetic ferritin and hemosiderin) and the reference medium, water.12
This method was validated against the wet-weight iron concentrations of liver biopsies from patients with hereditary hemochromatosis.13 –14 BLS does not require calibration by liver biopsy. Rather, a physical calibration of BLS is accomplished using an object with well known magnetic susceptibility and defined geometry.
Quantitative Magnetic Resonance Imaging of the Liver by R2 and R2*
In spite of its complexity, quantitative MRI of iron offers several advantages. These include 3-dimensional localization (imaging) and the detection of small and/or deep lying organ regions. Since the 1990s, different MRI methods such as spin-echo (SE), signal intensity ratio (SIR) or on gradient recalled echo (GRE) for the detection of liver iron were introduced. A complete survey of the different early MRI techniques was reviewed by Jensen.15
MRI-R2 Method
The single-spin-echo method (SSE) has now become an acknowledged quantitative procedure for the determination of liver iron concentration. Its 2-dimensional liver iron imaging can be captured with a standardized measurement protocol, regular quality control by phantom measurements, and central analysis.16
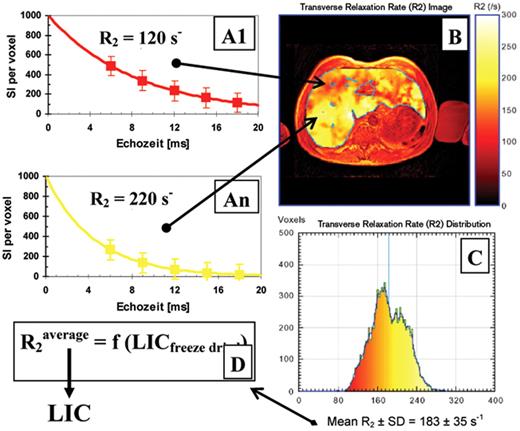
In Figure 2 , the SSE method is illustrated for a transfused patient with HbE/β-thalassemia. (Ferritin: 6600 μg/L, ALT: 200 U/L). Per each volume element (voxel ≈ 10 mm3), the exponential model function is fitted as a function of echo time to the mean signal intensities per voxel (A1 to An). For a representative liver slice, the R2 image is calculated and graphically shown (B). From the resulting R2 histogram (C) and the calibration curve (D), the average LIC is calculated. It is important to note that the nonlinear calibration curve was generated from freeze-dried liver biopsies in 105 patients with β-thalassemia, hereditary hemochromatosis, and patients infected with HCV.16
MRI-R2* Method
Fast gradient recalled echo (GRE) sequences make use of incomplete re-phasing of the proton spins (spin-flip angle < 180°) to measure liver iron. This allows for shorter first echo times, which is important for precise determination of the transverse relaxation rate R2* (1/T2*). Recently, a comparison between R2 and R2* has proven that both methods can accurately measure liver iron in a sufficiently wide LIC range.17 In addition, MRI-R2* can be performed using one breathhold, thus avoiding respiratory artifacts, which have always been a problem with MRI-R2 methods.
Relationship of Liver Biopsy to MRI and BLS Measurements
MRI and BLS are both in vivo methods, and therefore measure LIC in the native wet condition. The calibration or validation of MRI and BLS, respectively, by LIC (“gold standard”) from dry weight biopsies is affected by variations in the wet-to-dry weight conversion factor. The long-standing conversion factor of 3.33 and the resulting recommended LIC safety thresholds18 for the treatment of thalassemia patients should now be reassessed using new correction factors based on recent investigations.19 In Table 1 , the updated conversion factors and the resulting safety thresholds are shown for the most widely used liver biopsy processing procedures.20
Clinical Application of Liver Iron Measurements
Thus far, we have shown that the liver is the primary site for iron storage, that liver iron concentration can be accurately measured by several techniques, and that LIC can be used to estimate total body iron. Monitoring iron overload in patients is usually performed annually or biennially over decades. A practical use of LIC is to monitor transfusion-dependent patients with the aim of achieving equilibrium or a negative iron balance by chelation dose adjustment.
However, with the simultaneous influx of iron from blood transfusions and different degrees of patient adherence this may be difficult. The concept of the Therapeutic Index (TI), which relates the deferoxamine dose to the ferritin level,21 can be adopted for LIC measurements in order to avoid chelation toxicity. Following that concept, the chelator index (CI) is calculated as the ratio between the molar dose rate D and the total body iron store. A CI of 1.2 mmol/d/g of Fe corresponds with the DFO toxicity risk level of TI equal to 0.025 (mg/kg/d)/(μg/L).20 In patients with high ferritin levels related to inflammation, the CI may be superior to the TI. Patients above this threshold have increased risk of growth delay (age <18 years) and sensorineural hearing defects. A chelation index has not been established for other chelators (deferiprone, deferasirox).
Cardiac Iron Quantitation
Although the majority of iron is stored in the liver in iron overload states, pathologic iron can occur in other organs leading to specific organ dysfunction. Cardiomyopathy, congestive heart failure, arrhythmia, and cardiac death are related to iron overload of the heart,22 and is the most frequent cause of death (60%) in thalassemia.2 In recent years, the paradigm of a direct relationship between an increased risk of cardiac morbidity in patients with thalassemia and elevated iron stores was challenged by the finding that heart problems also occur in patients with low LIC and ferritin. With the introduction of modern MRI-R2* methods, this paradox was directly confirmed by the demonstration of short cardiac T2* transverse relaxation times (equals increased heart iron) in well chelated patients with low LIC.5
Evidence for Heart Iron by Cardiac Biopsy or Autopsy
In contrast to the liver, the direct determination of the cardiac iron concentration (CIC) by endocardial biopsy is rarely performed. In addition, the CIC by endocardial biopsy does not represent total heart iron because of the small sample size and the inhomogeneity of the iron distribution in the myocardial tissue. In post-mortem examinations of hearts with significant histological iron staining, Buja and Roberts23 reported CIC values between 160 and 1470 μg/gwet weight. All patients with CIC > 600 μg-Fe/gwet weight and more than 23 g of transfused iron had developed clinical heart failure.
Quantitative Magnetic Resonance Imaging of the Heart (MRI-R2*)
Of all non-invasive methods for the measurement of heart iron, MRI does appear to be the most promising.24 The MRI-R2* method seems to be more sensitive and better suited to measure the relatively low heart iron concentration than the more classic MRI methods (T2, SIR). The transverse magnetic relaxation R2* = 1/T2* characterizes the decay of the proton resonance in the vicinity of local magnetic fields (susceptibility effects). In order to measure the relatively small magnetic susceptibility effects of the neighboring atoms, this method needs very homogeneous magnetic fields of ≥ 1.5 Tesla, short echo times (TE < 3 ms), and the localization of an optimal cardiac slice. The single-breathhold multi-echo sequence has emerged as the standard for measuring cardiac iron.25
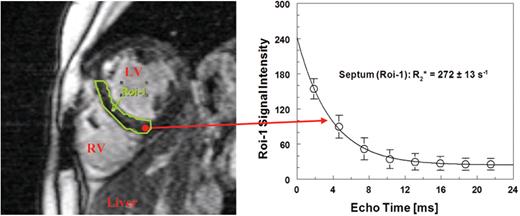
An example of this approach is shown in Figure 3 in a transfused patient with β-thalassemia major. In the last 10 years, her LIC by BLS stabilized at an optimal value of about 1000 μg/gliver by adapting the chelator dose (DFO) to her transfusion rate. However, in the last two years the patient occasionally showed extra-systoles in the ECG and decreased LVEF between 50% and 58 % by MRI.
Calibration of MRI-R2* by iron quantitation in human heart tissue samples is still in progress. In animals, this has been performed for T2 and T2*.26 In patients with cardiac iron overload, a comparison with T2 was semi-quantitatively performed with histological sections from endocardial biopsies.27 A direct determination of the heart iron concentration without the necessity of a calibration by physicochemical iron measurement in tissue samples could take place in the future by the measurement of the magnetic susceptibility in the myocardium by means of MRI.28
Clinical Application of Heart Iron Measurements
Currently, an elevated cardiac iron level of R2* > 50 s−1 (T2* < 20 ms) seems to be the earliest indicator of an increased risk of cardiomyopathy.5 Based on a study in transfused pediatric patients with thalassemia major between 2.5 and 18 years of age,29 a first cardiac MRI-R2* measurement should be considered at an age of 9 or 10 years. For other iron overload diseases, the incidence of elevated cardiac iron levels seems to be lower, but has still to be verified in larger studies.
Patients with cardiac iron concentrations proportional to transverse relaxation rates R2* = 50 – 100 s−1 (T2* = 20 – 10ms) deserve special attention with respect to their actual chelation dose and compliance. Patients with R2* > 100 s−1 (T2* < 10 ms) have an increased risk of cardiac decompensation and require intensified chelation treatment regimen.30 Although there is an association between liver and cardiac iron, short-term monitoring of LIC is of limited use during intensive chelation because of the relatively slower clearance of iron from the heart.31
Magnetic Resonance Imaging of Other Organs and Tissue
Anterior Pituitary Gland (Hypophysis)
The anterior pituitary gland appears very sensitive to early toxic effects from iron overload. This is supported by the high frequency (55%) of patients reported from Italian thalassemia centers to have hypogonadism.2 The quantitative measurement of iron by means of MRI in the relatively small pituitary gland (200 ± 100 mm3) is difficult. Using R2 relaxivity, Argyropoulou et al34 studied 37 patients with β-thalassemia major. Pituitary T2 showed a significant correlation between pituitary gland height and serum ferritin. Pituitary size decreased with progressive siderosis. The relationship between gland siderosis and function was investigated by Lam et al32 in 50 thalassemia major patients using a T2-weighted signal intensity sequence. There was significant correlation of the pituitary signal with IGF-1 and IGFBP-3 and the history of hypogonadism (P = .001).
Pancreas
In iron overload, glucose intolerance and/or a diabetes mellitus type 2 is a frequently found complication, both in hereditary hemochromatosis and in β-thalassemia major.2,35 The pathogenesis of diabetes due to siderosis is not well understood,36 although iron overload in the pancreas is thought to produce oxidative stress in the beta cells followed by cell death, insulin deficiency and glucose intolerance. Au et al37 provided fundamental information on iron measurement in the pancreas and relationship to diabetes in thalassemia. The investigators completed pancreatic MRI-R2* in 72 patients with thalassemia major (21 diabetic, 51 normoglycemic). The two groups were comparable for ferritin and MRI-T2* heart, liver and pancreas although diabetic patients were significantly older (P < .0001) and had smaller pancreas volume (P < .0001). In normoglycemic patients, log-pancreatic T2* values correlated with homeostatic model assessments HOMA-B (beta cell reserve), HOMA-IR (insulin resistance) and fasting insulin/C-peptide levels. The length of exposure to an elevated iron level had an important influence on the clinical status, not the pancreatic iron alone.
Other Glands and Tissues
Iron deposition in other organs and tissue such as the spleen,38,–39 kidney,40 gonads, thyroid, lung, bone marrow,41 adrenal gland42 and brain43 has not been examined extensively. Measurement of low, but elevated levels of brain iron has gained recent interest because of its relation to neurological disorders including (Parkinson, Alzheimer). In a recent trial with thirteen Friedreich ataxia patients receiving low dose deferiprone (20–30 mg/kg/d), slightly elevated R2* rates as compared with controls were measured in the dentate nuclei and in the palladium.43 During the 6-month trial, R2* in the dentate nuclei decreased from 18.3 s−1 to 15.7 s−1 (P < .002, controls: 16.6 ± 1.2 s−1). Susceptibility Weighted Imaging (SWI), which analyzes phase images as well as the usual signal amplitude images44 may become a promising MRI method for studying brain iron deposition and toxicity in iron overload diseases.
Difference method for the measurement of the liver iron concentration by biomagnetic liver susceptometry: From the start position (left), the patient is lowered in an inhomogeneous magnetic field of 20–30 milliTesla by about 5 to 10 cm (right) to a field of 1 milliTesla. During this vertical scan, the water coupling bag follows the patient and the change of the magnetic flux as a function of the distance is registered in the detector coils and transformed by the SQUID electronics.
Difference method for the measurement of the liver iron concentration by biomagnetic liver susceptometry: From the start position (left), the patient is lowered in an inhomogeneous magnetic field of 20–30 milliTesla by about 5 to 10 cm (right) to a field of 1 milliTesla. During this vertical scan, the water coupling bag follows the patient and the change of the magnetic flux as a function of the distance is registered in the detector coils and transformed by the SQUID electronics.
Iron distribution in a 5 mm liver slice of a patient with HbE/β-thalassemia (29 y, HCV+) measured in a 1.5 T imager (Philips Intera Gyroscan®, CHRCO Oakland, USA) with an average LIC of 18.6 mg/gdry wgt(Ferriscan®). The transverse relaxation R2per volume element (voxel) and therefore the iron distribution ranges from 6.0 mg/gdry wgt(red: R2≥ 92 s−1) to 30.5 mg/gdry wgt (yellow: R2 ≤ 280 s−1).
Iron distribution in a 5 mm liver slice of a patient with HbE/β-thalassemia (29 y, HCV+) measured in a 1.5 T imager (Philips Intera Gyroscan®, CHRCO Oakland, USA) with an average LIC of 18.6 mg/gdry wgt(Ferriscan®). The transverse relaxation R2per volume element (voxel) and therefore the iron distribution ranges from 6.0 mg/gdry wgt(red: R2≥ 92 s−1) to 30.5 mg/gdry wgt (yellow: R2 ≤ 280 s−1).
Mid-papillary short axis slice at an echo time of 7.5 ms in a patient with b-thalassemia major (30 y) for assessing the signal intensities in the septum (Roi-1) (single-breathhold multi-echo method TE= 1.9 - 22 ms: 1.5 T Siemens Magnetom Symphony®, UKE Hamburg). The subsequent exponential fit yields a short relaxation time T2* = 1000/R2* = 3.7 ms, while the liver iron concentration in this patient is relatively low LIC (BLS) = 1090 ± 114 μg/gliver.
Mid-papillary short axis slice at an echo time of 7.5 ms in a patient with b-thalassemia major (30 y) for assessing the signal intensities in the septum (Roi-1) (single-breathhold multi-echo method TE= 1.9 - 22 ms: 1.5 T Siemens Magnetom Symphony®, UKE Hamburg). The subsequent exponential fit yields a short relaxation time T2* = 1000/R2* = 3.7 ms, while the liver iron concentration in this patient is relatively low LIC (BLS) = 1090 ± 114 μg/gliver.
Disclosures Conflict-of-interest disclosure: PRH is on advisory committess for Shire Pharmaceuticals, BioMarin Pharmaceutical, Apotex, and Ferrokin Biosciences; he receives research funding from BioMarin Pharmaceutical and honoraria from BioMarin and Shire Pharmaceuticals; he is a consultant for BioMarin Pharmaceutical. RF receives honoraria and research funding from Novartis Pharma GmbH and Novartis Pharmaceuticals Inc. Off-label drug use: None disclosed.
References
Author notes
University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Children’s Hospital & Research Center Oakland, Oakland, CA