Abstract
It is now twenty years since the first legal gene transfer studies were approved, and there has been considerable disappointment in the slow rate of progress that followed the initial studies. Gradually, however, as the limitations of available vectors are acknowledged and overcome, and with advances in our understanding of the molecular and cell biology of genetic diseases and of cancer, unequivocal successes are now being reported. In this paper we describe the remaining major roadblocks to successful gene therapy and outline approaches to overcome them. We also illustrate how genetically modified immune system cells are already being used for the effective treatment of hematological and other malignancies, and how these approaches are being modified so that they can be effective in treating a broader range of malignancies.
The promise of gene therapy has never been in doubt. The potential for treating diseases resulting from loss of cell and molecular function, in particular, are clearly greater than for small molecules, and the potential permanence of such benefits makes the approach superior to protein replacement therapies in many common disease settings. Unfortunately, gene therapy has held this “promise” for almost 20 years and, as with any aging ingénue, the audience is tiring of promises and wants to see tangible accomplishments.
Overcoming Obstacles to Success
To understand why gene therapy has been slow to make an impact on human disease and to appreciate why that lag phase is coming to an end, it is necessary to know the major obstacles to success and the means by which they are being overcome.
Biodistribution
Following introduction into the systemic circulation or local tissue, current vectors have a passive initial biodistribution, which is then actively impeded by host innate and adaptive immunity. Local injections will diffuse only a few millimeters from the needle track while systemic intravenous injections may be neutralized by complement components (eg, oncoretroviral vectors) or by pre-existing antibodies (eg, adenoviral vectors) even before they manage to attempt a first pass through the capillary beds of the liver or lung, which themselves represent a potent viral filter. Moreover, many vectors are limited in their target cell range so that even if delivery does occur to a broad array of potential target organs, only a minority may be effectively transduced. Investigators have tried to overcome these problems by manufacturing synthetic vectors in which there is encapsulation of viruses or of plasmid DNA (eg, with lipids or PEG), or deletion or occlusion of pre-existing ligands and addition of new ones (eg, measles vectors), or by preparing conditionally replication-competent vectors that can spread locally under the conditions existing in diseased organs. None of these approaches overcome the limitations of passive biodistribution, and an alternative may be to use cellular carriers to actively transport vectors to the desired sites, even if this requires traversing multiple tissue planes. The vectors may be passively attached to the cell surface1 or encoded internally so that vector production is triggered when the cell reaches its desired target.2
For the moment, the realities of limited biodistribution have compelled investigators to focus on treating diseases in which these restrictions are not critical. Examples include ex vivo gene transfer to modify cells, which are then infused into patients as described in “Gene Transfer and T-cell Therapies for Viral Infection and Cancer” and in the accompanying article by Aiuti and Roncarolo, beginning on page 678, and by local injection of vectors directly into sites with highly circumscribed anatomy, such as the retina.3 Other solutions have been (1) to inject vectors that encode soluble proteins (such as Clotting Factor IX),4 which can be secreted to produce long-term effects; (2) to introduce immunostimulatory transgenes into local tissues to produce a local and then systemic immune response (eg, adenoviral vectors encoding interleukin [IL]2, granulocyte-macrophage colony stimulating factor [GM-CSF] or IL125: or (3) to express transgenes which directly or indirectly produce potent bystander activity on unmodified cells (eg, thymidine kinase gene transfer and ganciclovir to release the toxic phosphorylated metabolite).6 Other strategies try to overcome limited biodistribution by brute force, saturating or blocking physiological clearance mechanisms, or using hydrostatic pressure to force in vivo uptake by cells. These approaches do not yet have a favorable toxicity profile for human application.
Innate and Adaptive Immune Response
The immune system may destroy vectors before they enter cells or may become a major problem following cell transduction. Antibodies and cytotoxic T-cell responses can be directed to transduced cells either because they express low levels of vector-associated antigens (for example, adenovirus or adeno-associated virus [AAV]), or because the proteins the transduced cells produce are abnormally processed and presented, generating new epitopes to which the immune system is not tolerant. These immune responses may cause an inflammatory response that eliminates both transduced cells and their non-transduced neighbors.
Although immune responses directed to transduced cells may severely impede efforts to correct deficiency states (eg, hemophilia), they may also be beneficial. For example, they may amplify the effects of gene therapies that recruit an immune response to treat cancer, or provide an additional immune based killing mechanism to complement other cancer gene therapies (eg, p53 transgene expression after adenoviral vector mediated gene transfer).7 Investigators are trying to avoid immune responses by switching to allegedly less immunogenic vectors (such as liposomes, AAV and helper-dependent adenoviral vectors) and by co-administering immunosuppressive agents such as cyclosporine and mycophenolate mofetil during and after vector exposure.
Genotoxicity
While treatment of some disorders requires only transient expression of a transgene in episomal form, other diseases can only be cured by integration of the vector in the host cell genome, for example if the target cell is highly mitotic. Under these circumstances, genotoxicity may occur, in which the integrating vector disrupts the function of critical genes, resulting in cell damage and even malignant transformation. Graphic illustration of this possibility was provided by subjects with X-linked severe combined immunodeficiency who received common gamma chain gene transfer into their hemopoietic stem cells. Almost one third of these patients have developed T-cell leukemias due to integration-associated activation of proto-oncogenes.8 Investigators hope to overcome these problems by restricting their choice of transgenes to those that are not themselves potentially oncogenic (see accompanying article by Aiuti and Roncarolo, beginning on page 678),or by introducing regulatory or barrier elements ahead of a potential transgenic oncogene, or by substituting alternative vectors for the Moloney-based retroviruses responsible for the cases of T cell leukemia. These substitutes include human lentiviral vectors (see accompanying article by DA Persons, beginning on page 690), which do not seem be naturally associated with malignant transformation, or synthetic plasmid vectors containing transposons and transposases (eg, sleeping beauty or piggy Bac) that allow integration with adequate frequency. These plasmid vectors may be further engineered to incorporate zinc finger domains with the intent of targeting them to predetermined sequences in host cell DNA. In a similar way, AAV integrates preferentially into apparently safe sites in the human genome, and efforts are being made to accentuate this process and avoid “off target” integration events.
Unwanted Persistence of Genetically Modified Cells
Even without malignant change, transduced cells may be problematic. Unlike a small molecule therapeutic or a monoclonal antibody, transduced cells have the potential to last the lifetime of the host and even to expand in number. Hence, any adverse effect attributable to gene transfer or gene modified cells may actually worsen over time. In recognition of this problem, efforts have been made to develop a variety of “suicide” strategies in which a second transgene accompanies the primary gene of interest. This second gene allows the cell to be destroyed on exposure to a specific signal. The most widely used of these is the herpes simplex viral thymidine kinase (Tk) gene, the product of which will phosphorylate gancyclovir or acyclovir to the active moiety, which interferes with DNA synthesis. The Tk gene has been introduced into allogeneic T lymphocytes used as donor lymphocyte infusions following stem cell transplantation. If the infused cells produce graft-versus-host disease rather than the desired antiviral and antileukemic activity, they can be destroyed by administration of the ganciclovir prodrug. Evaluation of the safety and benefits of this approach have now reached phase III clinical trial.9 Though apparently effective, the Tk gene may itself be immunogenic, leading to undesired elimination of a transduced cell population. Moreover, gancyclovir is a useful drug for immunocompromised patients who develop cytomegalovirus (CMV) infections; in these patients, administration of GCV to treat CMV would produce cell suicide irrespective of need. Finally, Tk/GCV may have limited ability to kill cell populations that are post mitotic. These concerns have led investigators to study alternative agents, for example, inducible Caspase9 (icasp9). Since icasp9 is a naturally occurring component of the caspase pathway it should be non-immunogenic and produce apoptosis even in non-dividing cells. The molecule can be triggered by administration of a small molecule dimeriser that brings together two non-functional icasp9 molecules to form the active enzyme.10
Development and Implementation of Gene Therapies
While some gene therapies can fit into the standard pharmaceutical drug development model, other more complex uses, such as production of gene modified cells for infusion, are less well matched to this scheme. These complex biological therapies (CBTs) may have to be made on an individual patient basis and require expression of several transgenes from one or more vectors followed by ex vivo expansion to large cell numbers. We describe below a specific example of how efforts are being made to overcome the technical problems of CBTs, but the associated lack of a good business model is less amenable to solution. A standard pharmaceutical product has linear development from phase I through phase III and licensure. Cost of goods is low, margins are high, and scalability of manufacturing and administration is readily accomplished. These tenets simply do not hold for CBTs. Hence, many CBTs will have to be developed in an analogous way to other complex medical procedures, such as surgery and transplantation, that are primarily developed, assembled and delivered by major medical institutions. Under this concept, commercialization occurs at the level of component manufacturers, while it is the institution that assembles these components into a therapeutic entity and thereby adds value, whilst using its other resources, such as imaging and diagnostics laboratories, as revenue generators. The funding to develop such an approach is complex, but a combination of longer-term academic and philanthropic grant funding, cost recovery, case rate support, and orphan drug designation may allow implementation and commercialization. Implementation may be favored by likely shifts in health care priorities to use comparative effectiveness data, which will support treatments, irrespective of origin, that health services research shows to have the best pharmaco-economics. Over the next few years, the commercial exploitation of Dendreon’s Provenge,11 a genetically modified autologous dendritic cell vaccine for prostate cancer will be closely watched, since it will provide insights into the feasibility of current strategies for developing individualized complex biological therapies.
Gene Transfer and T Cell Therapies for Viral Infection and Cancer
Most current therapies for cancer are poorly targeted, and are therefore toxic and often fail. Although viral infections may be more amenable to conventional therapy, for the immunocompromised host in particular, viral infection or reactivation remains a common cause of morbidity and mortality. Adoptive transfer of T lymphocytes directed to tumor or virus specific/associated antigens has the potential to make a major contribution to the treatment of these disorders. Unlike other targeted therapies, such as monoclonal antibodies, T lymphocytes have the ability to actively traffic through multiple tissue planes and to be self-renewing. These assets, coupled with their ability to destroy tumor- or viral-infected target cells through a range of mechanisms makes them appealing resource for adoptive transfer, and a multiplicity of clinical studies using this approach have now been described. Unfortunately, it has become apparent that their effectiveness is handicapped by immune evasion strategies adopted by malignant or viral-infected target cells, by difficulties in obtaining T cells with the correct antigen specificities, and by problems in manufacturing sufficient numbers of cells in a scalable fashion at feasible levels of cost and complexity. For all these problems, gene transfer may be at least a partial solution.
Adoptive Immunotherapy with Cytotoxic T Lymphocytes
Viral Infections
Viral infections remain one of the commonest causes of morbidity and mortality after stem cell transplant, and indeed are more prevalent as the degree of antigen mismatching between donor and recipient is increased. Cord blood and HLA haploidentical donor transplants in particular are associated with often intractable virus infections, particularly with adenovirus, in addition to the herpes viruses (CMV, Epstein-Barr virus [EBV], human herpesvirus 6 [HHV6], varicella zoster virus [VZV]). Adoptive transfer of virus-specific T cells appears to effectively prevent and treat these infections after transplant. Infusion of even small numbers of specific cells (106 or less) for CMV or EBV12,13 may be sufficient for benefit, since the lymphodepletion of the immediate post-transplant period is associated with the release of homeostatic cytokines such as IL-15, which augments the expansion of virus-specific T cells when they encounter their antigen. Although CMV-, EBV- and adenovirus-specific CTLs have now been safely and successfully given to several hundred patients, preparing these virus-specific cells is expensive and slow, since individual cell lines are required for each patient and for every virus. How gene transfer is overcoming these limitations is described in “Broader Implementation of T cell Therapy.”
Viral-related Malignancy
Adoptive immunotherapy with EBV-specific CTLs in the immunocompromised host was followed by treatment of the EBV associated tumors (lymphoma and nasopharyngeal cancer) that developed in the immunocompetent subject. Unlike EBV-LPD, which expresses the highly immunogenic viral latency antigens EBNA1, EBNA2 and EBNA3, these other EBV tumors express a limited number of poorly processed (EBNA1) or weakly stimulatory (LMP1 and LMP2) EBV-derived antigens. Using gene transfer with adenoviral vectors encoding EBV-LMP2 (and subsequently LMP1 and LMP2) it proved possible to overexpress these weak antigens in antigen-presenting cells (APC) and generate CTLs that were specific for antigens expressed by the tumors from subjects with EBV+ lymphomas (including Hodgkin’s lymphoma). Infusion of these CTLs lead to complete tumor regression in 8 of 12 patients with EBV+ lymphoma,14 and similar, albeit less striking, benefits have been obtained in EBV+ nasopharyngeal carcinoma (NPC), a tumor that originates from the epithelial cells of the nasopharynx.15
Gene Transfer to Enhance and Simplify T-cell Therapy
There is considerable interest in genetically modifying T cells so that they may be used for cancer therapy. Many different types of tumor associated or tumor-specific antigens have been described and are reviewed in Graziano and Finn.16 Most tumor-associated antigens are self-proteins to which the immune system has limited responsiveness, due to the development of tolerance by clonal deletion or anergy.17 Hence, tumor antigen–specific T cells isolated from patients with cancer may have low-affinity T-cell receptors (TCRs), limiting their cytotoxic activity against tumor cells.18 Investigators have overcome this limitation by using gene transfer to express transgenic TCR α and β chains of high affinity, or by expressing a synthetic chimeric antigen receptor.
Artificial αβ T Cell Receptors
The cDNAs for the α and β chains of the TCR are cloned from class I HLA-restricted TCRs of tumor-reactive cytotoxic T cells and transferred to fresh T cells by an integrating vector, potentially giving the recipient cells the same antigen specificity as the donor T cells.19 This approach allows rapid production of large numbers of tumor antigen–specific T cells. Preclinical studies have shown that infusion of αβ TCR transgenic T cells can eradicate tumors in vivo.20,21 Recently, Morgan and colleagues treated melanoma patients with T cells genetically modified with MART-1–specific TCRs and reported regression of meta-static lesions in two patients together with prolonged persistence of CTLs.22 The same procedure has also been applied to generate T cells specific for the minor histocompatibility antigens HA-1- or HA-2- to treat leukemic relapse after HLA-mismatched HSCT23,24 and to common oncoproteins such as MDM2 and WT-1.21,25 To date, however, success has been less than desired, and a major constraint is the development of hybrid TCR, which contain a mixture of native and transgenic receptors. These are usually functionless, but in preclinical models can produce autoreactivity with a graft-versus-host-disease–like syndrome.
Chimeric Antigen Receptors (CARs)
Instead of transducing T cells with additional αβ TCR, it is possible to transfer chimeric TCRs, which are usually generated by joining the light and heavy chain variable regions of a monoclonal antibody expressed as a single-chain Fv (scFv) molecule with the transmembrane and cytoplasmic signaling domains derived from CD3 δ chain or Fc receptor γ chain through a flexible spacer.26,27 Thus they combine the antigen specificity of an antibody and the cytotoxic properties of a T cell in a single fusion molecule. Since CARs bind to target antigens in an HLA-unrestricted manner, they are resistant to many tumor-immune evasion mechanisms, such as down-regulation of HLA class I molecules or failure to process or present proteins. First generation CARs incorporated the cytoplasmic region (endodomains) from the CD3 δ or the Fc receptor γ chains as their signaling domain. Although these receptors successfully redirected T-cell cytotoxicity, they failed to stimulate T-cell proliferation and survival in vivo, likely because of the lack of appropriate costimulatory signals to T cells following engagement of the CAR. Efficacy was therefore modest in subjects with lymphoma, ovarian or renal cancer.28–30 Second-generation CARs were constructed by incorporating signaling domains from costimulatory molecules such as CD28, OX40, and 4-1BB within the endodomain, and improved antigen-specific T cell activation and expansion.31,32 An alternative approach is to express CARs in antigen-specific T cells, which will then also be activated and expanded through engagement of their native αβ TCR by antigen on professional antigen-presenting cells, with attendant co-stimulation. For example, subjects receiving EBV-specific CTLs engineered with a CAR (CAR-CTLs) specific for the disialoganglioside antigen GD2a on neuroblastoma cells show longer in vivo persistence of CAR-CTLs compared with unselected T cells engineered with the identical CAR, since the CAR-CTLs encounter (persistent) Epstein-Barr virus antigens. Longer persistence is associated with tumor responses including complete remission.33
Overcoming Immune Evasion Strategies
One of the main challenges to effective adoptive T-cell therapy is the lack of in vivo expansion and maintenance of ex-vivo manipulated, adoptively transferred T cells because of tumor-induced immune evasion mechanisms. Gene transfer technologies allow us to modify T cells and restore their functionality in a hostile environment (Table 1 ). For example, many tumor cells or their associated stroma produce TGF-β, which favors the development of immune tolerance and T-cell anergy,34 inducing T effector cell growth arrest with induction of Tregs. Bollard and co-workers showed that human and murine antigen-specific T cells could express a dominant negative (dn) TGF-β receptor following retroviral transduction and become resistant to the anti-proliferative effects of TGF-β retaining their effector function in vivo.35 Subsequently, Zhang and colleagues showed that T cells expressing a dnTGF-β RII had improved persistence and superior tumor elimination in a murine prostate cancer model.36 T cells may also be modified to express cytokine or cytokine receptor genes that mimic the milieu found during lymphoid regeneration and restoration of homeostasis, such as IL-2, IL-7, or IL-15.37–39 As yet, we do not know how safe or effective these transgenic cytokines and their receptors will be, but the opportunity to provide T cells with effective transgenic countermeasures to tumor-immune evasion mechanisms is likely to greatly increase their therapeutic potency
Broader Implementation of T-cell Therapy
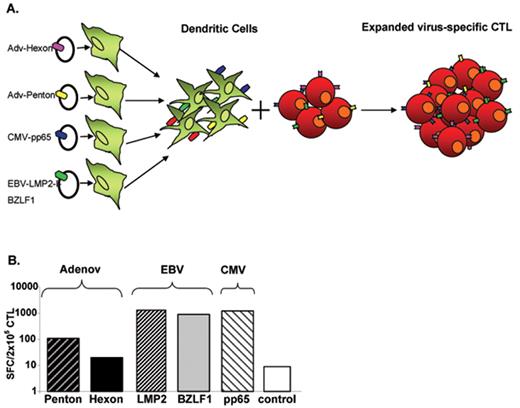
As described in “Development and Implementation of Gene Therapies” above, complex biological therapies are indeed intricate to implement. Fortunately, the successes described in the preceding paragraphs have spurred methodological changes to simplify and accelerate manufacture of engineered viral- or tumor-specific CTLs. For example, investigators have developed methods to generate CTL lines that have anti-viral activity for EBV, CMV and Adv in a single culture,40 and mini-bioreactors in which to prepare these cells in a closed system. Plasmid mediated gene transfer into antigen-presenting cells can force simultaneous expression of CMV-, adenovirus- and EBV-associated antigens (Figure 1 ) without having to manufacture EBV-transformed lymphoblastoid cell lines. In combination, these techniques allow sufficient CTLs for patient treatment to be made in less than 10 days instead of longer than 10 weeks. Moreover, these lines can be banked and given to partially HLA matched patients as “off the shelf” reagents,41 and a multicenter study is evaluating whether trivirus-specific CTL lines may have similar activity against EBV, CMV and adenovirus in partially HLA-matched allogeneic patients. If successful, extension to the treatment of malignant disease would follow.
Conclusion
As we begin to acknowledge and overcome the limitations of gene therapies, our achievements increase. Cancer therapy with gene-modified T cells is one area of established clinical success, but as for all complex biological therapies, commercial implementation remains a challenge.
Virus-specific cytotoxic T cells. (A) Generation of virus-specific cytotoxic T lymphocyte (CTL) by using plasmid system. (B) Specificity of plasmid-activated CTL. The frequency of Adv (Hexon and Penton), Epstein-Barr virus (EBV; LMP2 and BZLF1) and cytomegalovirus (CMV; pp65) reactive T cells in multivirus CTL population was analyzed by interferon (IFN)-γ Elispot assay. The control was IFN-γ release in response to stimulation with an irrelevant peptide mixture.
Virus-specific cytotoxic T cells. (A) Generation of virus-specific cytotoxic T lymphocyte (CTL) by using plasmid system. (B) Specificity of plasmid-activated CTL. The frequency of Adv (Hexon and Penton), Epstein-Barr virus (EBV; LMP2 and BZLF1) and cytomegalovirus (CMV; pp65) reactive T cells in multivirus CTL population was analyzed by interferon (IFN)-γ Elispot assay. The control was IFN-γ release in response to stimulation with an irrelevant peptide mixture.
Disclosures Conflict-of-interest disclosures: The authors declare no competing financial interests. Off-label drug use: None disclosed
Acknowledgments
Some of the work described in this review was supported by: The Leukemia and Lymphoma Society (SCOR 7018); NIH-NCI PO1 CA094237;NIH-NHLBI U54 HL081007; and NIH-NCI SPORE P50 CA126752
References
Author notes
Center for Cell and Gene Therapy, Baylor College of Medicine, The Methodist Hospital and Texas Children’s Hospital, Houston, TX