Abstract
A 55-year-old man presented with fever, night sweats, and weight loss of about 20 lbs. in the prior 6 months. Physical examination revealed multiple cervical, axillary, and inguinal lymphadenopathy. The spleen was enlarged. A complete blood count revealed leukocytosis with absolute lymphocytosis: 30,000/μL. Peripheral blood-flow cytometric analysis showed a clonal lymphocyte population with immunophenotypes positive for CD5, CD20(dim), and monotypic kappa light chain. Fluorescence in situ hybridization (FISH) analysis revealed del(11q22.3), but negative for t(11:14). What should be used to treat his chronic lymphocytic leukemia (CLL) disease?
CLL patients with del(11q22–11q23) represent a distinct clinical subset. This genomic aberration occurs in approximately 20% of patients with advanced CLL. These patients are usually younger than the typical CLL patient (median age, 59 years1 ), and often have extensive lymphadenopathies at the time of diagnosis.1–3 The clinical course of patients with the 11q deletion is usually characterized by rapid disease progression with shorter treatment-free survival; a shorter overall survival has been reported in patients younger than 55 years.2,4 Therefore, the choice of the initial treatment is particularly important in these young patients.
In the last decade, therapies including the purine analog have emerged as the main therapies for CLL disease. The superiority of single therapy with fludarabine over chlorambucil has been established for response rate, progression-free survival (PFS), and, most recently, overall survival, despite crossover studies between the two arms.5,6 Three independent studies have demonstrated that fludarabine + cyclophosphamide combination therapies (FC) achieve a better response rate and PFS compared with fludarabine alone, although no significant difference was reported in terms of overall survival.7–9 Subsequently, single-center phase II clinical trials evaluated “chemoimmunotherapy” (CIT) combining a purine analog with or without the alkylating agent cyclophosphamide and the anti-CD20 antibody, rituximab.10,11,16–18 High response rates were obtained with CIT combinations, and in many centers these strategies have become first-line therapies for CLL. Recently, the German CLL study group has reported the results of a study comparing fludarabine + cyclophosphamide + rituximab (FCR) with FC, and demonstrated that FCR is superior to FC in terms of response rate and PFS; there was also a trend toward better survival in the FCR group.12 However, the importance of cyclophosphamide in CIT compared with combination therapies of purine analog and rituximab has not been clearly defined.
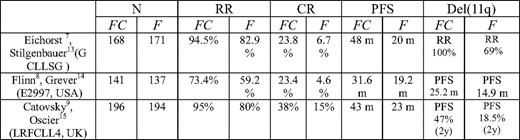
In this review, we focus on the results of purine analog-based front-line therapies of CLL patients with del(11q22–11q23). We therefore performed a literature search combining the National Library of Medicine's Medical Subject Heading (MeSH) terms “leukemia, lymphocytic, chronic, B-cell” and “cyclophosphamide” and limiting the search to human clinical trials in English. This strategy yielded 94 citations. When “purine analog” was used as a key word in the combination search, 58 citations were yielded. Among these, there are five randomized, controlled trials. Three addressed the efficacy of FC compared with fludarabine in front-line therapies for CLL. A search of PubMed and ASH annual meeting abstracts revealed three subsequent analyses including patients with deletion of 11q. Table 1 provides the details of the three randomized, controlled trials and their subsequent analysis.
In these three trials, patients with deletion of 11q had shorter PFS. In the Leukemia Research Foundation CLL4 trial and the German CLL study, del(11q) patients had statistically significant longer PFS or higher response rates when treated with FC compared with their counterparts treated with fludarabine alone. Additionally, although PFS in del(11q) patients treated with FC appeared longer compared with similar patients treated with fludarabine in the E2997 trial, it did not reach statistical significance after approximately 3 years of follow-up.
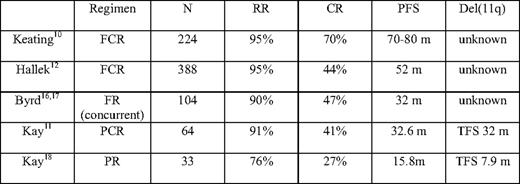
Several studies have addressed the clinical efficacy of CIT; the details of these phase II and III trials are summarized in Table 2. Interestingly, in PCR trials performed by the Mayo Clinic and the Ohio State University, subgroup analysis revealed that patients with the 11q deletion had similar PFS compared with the whole group. While these results were compared with the PR trial conducted at the same institutions, the addition of cyclophosphamide appeared to confer a higher overall and complete remission rate and longer duration of response. This finding was further supported by a recent retrospective analysis performed at M.D. Anderson Cancer Center on a group of 69 CLL patients with the 11q deletion. The del(11q22.3) patients treated with FCR had a 100% response rate and high PFS, suggesting that CIT therapy could overcome the adverse prognosis of the 11q deletion.1 A large randomized intergroup trial comparing FCR with FR therapy is currently ongoing, and the results may further clarify the role played by alkylating agents in CIT combination therapy in this subgroup of patients.
In summary, there is evidence from a subgroup analysis of randomized clinical trials that the addition of alkylating agents, in particular cyclophosphamide, to fludarabine is associated with superior response rate and longer PFS in patients with the 11q deletion (Grade 1A). While data on response duration and overall survival still indicate that this group of patients has an inferior outcome compared with patients with favorable genomic abnormalities (Grade 1A), this suggest that further intensification strategies should be evaluated in this population, and the attainment of a response to the initial therapy is a relevant clinical end point. CIT therapy containing cyclophosphamide achieves longer PFS than the combination therapy with the purine analog and rituximab (Grade 1C). For high-risk CLL patients with del(11q22.3), complete CIT therapy could overcome the adverse prognosis of the 11q deletion (Grade 1C). Therefore, in fit CLL patients, we recommend that cyclophosphamide is included in the CIT therapy of patients with del(11q) (Grade 1C).
Disclosure
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Off-label drug use: None disclosed.
Correspondence
Alessandra Ferrajoli, MD, Department of Leukemia, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center 1515 Holcombe Blvd., Houston, TX 77030; Phone: 713-792-2063; Fax: 713-745-4612; e-mail: aferrajo@mdanderson.org