Abstract
Although major progress has been made in the prevention of CMV disease after hematopoietic cell transplantation (HCT), specific problems remain and available antiviral agents are associated with major toxicities. This article reviews current aspects of CMV diagnosis, prevention, and treatment in HCT recipients and defines areas of unmet medical need.
Introduction
CMV continues to be an important complication after allogeneic hematopoietic stem cell transplantation (HCT). This article discusses recent development in CMV in HCT recipients.
Complications
The clinical complications of CMV can be divided into direct and indirect effects.1 The spectrum of disease manifestations has been described extensively.2 The most common disease manifestation today is gastrointestinal disease, which can escape blood-based surveillance by PCR and the pp65 antigenemia assay in approximately 25% of patients. CMV pneumonia is clearly the most serious complication, but has become infrequent with current prevention strategies.3 Rare manifestations include retinitis and encephalitis. The stem cell source and the conditioning regimens can influence both the time to reactivation and disease severity, however, preventative strategies are an important variable.4,5 CMV also exhibits an immunosuppressive effect, which can lead to an increased susceptibility to invasive bacterial and fungal disease as well as GVHD in selected clinical settings.2
CMV seropositivity remains associated with a poorer outcome, mainly in highly immunosuppressed patients such as unrelated donor or cord blood donor recipients.1,5 The survival disadvantage appears to be due to an increase of nonrelapse mortality.1 A recent single-center study suggested a beneficial effect of CMV reactivation on leukemic relapse in patients with acute myelogenous leukemia.6 Validation studies of these findings have not been reported. Earlier studies searching for an association of CMV serostatus and relapse have not been able to reproducibly demonstrate such an effect.7,8
Diagnosis of CMV infection and disease
The most frequently used tests for the diagnosis of CMV infection are detection of antigen (the pp65 antigenemia assay), DNA, or mRNA. The use of quantitative DNA detection techniques has been increasing in recent years9 because they are highly sensitive and provide viral load measurements that can give important prognostic information. Detection of mRNA by nucleic acid sequence-based amplification is not quantitative but appears to work well in preemptive treatment strategies, as documented by randomized trials comparing this technique with pp65 antigenemia or detection of DNA by PCR.10
The diagnosis of CMV gastrointestinal disease and pneumonia, by far the most common manifestations of CMV disease in the current era, has remained unchanged over the past 2 decades.11 Accepted diagnostic methods to document CMV disease include rapid cultures, direct fluorescent antibody tests, DNA hybridization, and cytology. There is presently no consensus on how to use molecular methods to conclusively diagnose CMV pneumonia and gastrointestinal disease because there are presently no data on what level of CMV DNA in bronchoalveolar lavage (BAL) fluid or tissue correlates best with CMV disease. Due to its high sensitivity, the negative predictive value of PCR is high, so it can be used to rule out disease. Whereas high viral loads in BAL or tissue likely point toward a CMV disease in the right clinical context (ie, signs and symptoms compatible with disease), the complicating issue is that CMV silently reactivates in seropositive recipients without disease.12 It is unknown what the range of viral load in tissue or BAL is in these CMV shedders. Therefore, low and moderately high CMV DNA levels in BAL or tissue are presently not interpretable. PCR is therefore not accepted as definitive proof of CMV pneumonia or gastrointestinal disease by current internationally accepted diagnostic criteria.11 For rare complications such as CMV encephalitis, PCR is a useful diagnostic tool.11
Treatment of CMV disease
CMV disease should be treated with antiviral agents such as ganciclovir or foscarnet. Induction doses for at least 2 weeks (preferably 3 weeks, if tolerated) are generally recommended, followed by maintenance dosing for another 3-4 weeks.13 Treatment should be continued until resolution of symptoms and negativation of the viral load. In patients with continued immunosuppression, continued maintenance or close virologic monitoring is recommended and additional treatment courses may be necessary.14
Gastrointestinal disease is generally treated with antiviral agents alone. CMV pneumonia treatment includes the use of intravenous immunoglobulin. Recent analyses have renewed the debate about whether concomitant treatment with immunoglobulin is needed13 ; however, current treatment recommendations continue to recommend its use.
Prevention of CMV disease
Prevention of CMV via blood products and the stem cell product
The practice of using “CMV-safe” blood products for recipients that are CMV seronegative is widely accepted.9 Two options exist for reducing the risk of CMV transmission via blood products: blood products from CMV-seronegative donors and leukocyte-reduced, filtered blood products. Both strategies are widely used.9 Leukocyte filtration should be performed at the blood bank and the established quality standards should be followed.15 No controlled study has investigated whether there is an extra benefit from the use of both seronegative and filtered blood products.
CMV-seronegative donors are generally selected for CMV-negative recipients in an HLA-identical sibling situation if multiple donors are available.13 The situation is more complex in the unrelated donor setting. An important question is how to weigh the factor of CMV serological status compared with other relevant donor factors, especially if multiple possible donors are available. Although no study has examined the relative importance of HLA match versus CMV serology, an antigen-matched donor for HLA-A, HLA-B, or HLA-DR would most likely be preferred to a CMV-negative donor.13 However, for a lesser degree of mismatch, such as allele-mismatches or mismatches on HLA-C, HLA-DQ, or HLA-DP, the situation is different and a CMV-negative donor could be considered even if the match was poorer.13 Compared with other donor factors such as age or blood group, a CMV match has preference.13 Whether CMV serostatus should play a role in the donor selection for CMV-seropositive recipients is a subject of debate. Some experts advocate using CM- seropositive donors for CMV-seropositive recipients in the unrelated donor, non–T-cell-depleted setting to optimize the adoptive transfer of donor immunity13 ; however, this strategy has not been universally adopted.
Antiviral strategies
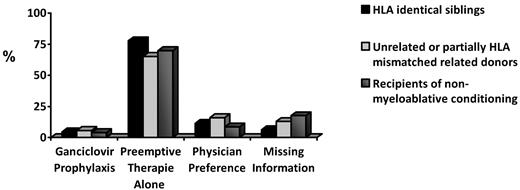
Both prophylaxis and preemptive therapy are used to manage CMV in the HCT setting. A recent international survey documented the current use patterns9 (Figure 1). Preemptive therapy is most commonly used, but prophylaxis is favored by some centers for high-risk patients such as recipients of unrelated, HLA-mismatched, or cord blood stem cell products.
CMV prevention. Ganciclovir prophylaxis versus preemptive therapy by risk group in 2003. (Data are from Pollack et al 2011.9 )
CMV prevention. Ganciclovir prophylaxis versus preemptive therapy by risk group in 2003. (Data are from Pollack et al 2011.9 )
CMV viral load and pre-emptive antiviral therapy.
Preemptive therapy is typically initiated after the detection of CMV pp65 antigenemia, CMV DNA, or mRNA. CMV DNA load is increasingly measured, but there are no validated and standardized thresholds. Details of current approaches have been reviewed recently.13 Ganciclovir is most commonly used for preemptive therapy, with foscarnet serving as second-line agent for situations of neutropenia.9 Valganciclovir provides adequate systemic exposure in HCT recipients, but has not been evaluated in adequately powered clinical trials.16 The current strategies are highly effective in preventing CMV disease, as was recently demonstrated in a large, multicenter clinical trial.3 Today, most cases of breakthrough disease are due to gastrointestinal disease. CMV surveillance and preemptive therapy is continued throughout the first 3 months after transplantation. However, late CMV disease occurs in approximately 4%-15% of seropositive allograft recipients and is associated with poor outcome.17 Most cases occur between months 4 and 12 after HCT.14,17 Risk factors include CMV infection or disease during the first 3 months after HCT, low CD4 T-cell count, undetectable CMV-specific T-cell immunity, GVHD or T-cell depletion in the graft, cord blood transplantation, or use of anti-T-cell agents.17 Continued surveillance and preemptive antiviral therapy is recommended for the management of late CMV complications.18
Prophylaxis.
High-dose acyclovir and (500 mg/m2 3 times daily) valacyclovir prophylaxis (2 g 3-4 times daily) have been shown in earlier studies to reduce the risk for CMV infection, CMV disease, and survival.19 However, the most recent trial in the preemptive therapy era did not demonstrate an effect on CMV disease and survival.20 Ganciclovir prophylaxis has also been studied extensively.21 All studies showed a reduction in the risk of CMV disease compared with placebo, but did not improve overall survival. Prolonged neutropenia is the most important adverse outcome of ganciclovir prophylaxis. Neutropenia can be managed with G-CSF.13 Lower-dose regimens of ganciclovir prophylaxis have been reported in nonrandomized studies; however, the results have varied, with studies showing high rates of CMV disease in high-risk patients.22 No randomized clinical trials on valganciclovir prophylaxis in the HCT setting have been reported. Foscarnet prophylaxis, which is associated with dose-dependent renal toxicity and electrolyte abnormalities, has not been studied in a randomized fashion.
Maribavir has recently been evaluated in a randomized, placebo-controlled phase 3 multicenter trial.3 Based in the initial phase 2 study,23 the dose of 100 mg twice daily was chosen for this trial. The phase 3 trial failed to reduce the incidence of CMV disease and showed only a modest antiviral effect. Overall, the incidence of CMV disease in the control group was < 2.5% during the first 100 days (Table 1). While the reasons for the negative outcome of the trial are being debated, the low-dose regimen and the better than projected performance of the control group are likely reasons for the failure of the drug to meet the primary end point projections.3
Management of pretransplantation CMV infection and disease
Patients with a CMV infection developing close to a planned allogeneic HCT have an increased risk of mortality early after transplantation.24 The use of alemtuzumab and other highly immunosuppressive therapies may lead to an increased risk of CMV infection in HCT candidates25 ; valganciclovir prophylaxis has been shown to be effective in this situation.25 Virologic surveillance and preemptive therapy have not been studied in patients undergoing alemtuzumab treatment. Awareness of CMV in the pretransplantation setting seems prudent. Patients should be screened by PCR during the pretransplantation workup and preemptive therapy administered if CMV is detected.
Management of antiviral drug resistance
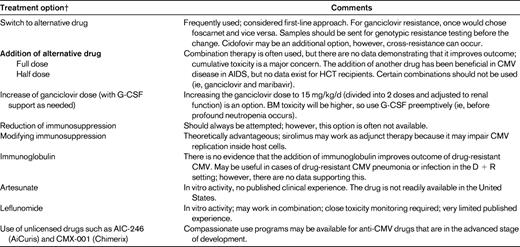
Drug resistance remains rare after HCT, but there are certain situations in which it should be suspected. Most drug resistance has been reported with ganciclovir or valganciclovir because they are the most commonly used antiviral agents. Genotypic assays are available for diagnostic analysis in reference laboratories.26 Drug resistance should be suspected in patients who are on antiviral drugs and who have had viral load increases for more than 2 weeks.13 After starting preemptive therapy, the viral load increases occur in approximately one-third of patients due to the underlying immunosuppression.27 Therefore, in a drug-naive individual (which is the case in most patients during the first 3 months after transplantation), it is unlikely that these early increases are due to true drug resistance in adult patients (although a few cases of early-onset resistance in highly immunosuppressed pediatric patients have been reported28 ). In contrast, viral load increases for more than 2 weeks, especially with significant prior exposure to antiviral drugs, are suspicious for drug resistance. If drug resistance is suspected, genotypic testing and switching to an alternative drug (eg, foscarnet in the case of ganciclovir resistance) is recommended as a first-line approach.13 Viral load can be used to monitor the response to treatment. In patients who do not respond or those who are critically ill, few options exist and none is supported by high-quality data (Table 2).
Immune augmentation
CMV-specific T cells play a pivotal role in controlling CMV after HCT.29 CMV-specific CD8 and/or CD4 CMV-specific T cells can be monitored using different techniques including detection by tetramers or measurement of peptide-specific lymphocyte responses using intracellular cytokine assays and assessment of polyfunctionality.30–32 However, none of these techniques is standardized for routine use. Adoptive transfer of donor-derived CMV-specific T cells has been reported without significant toxicity and antiviral effects in some small series of patients with refractory CMV viremia or disease.33 Active immunization with DNA vaccines are also being studied in HCT recipients.34
Conclusions and unmet medical needs
Early treatment of CMV has been optimized over the past decade. The recent phase 3 maribavir trial documented how effective preemptive therapy is today, with CMV disease rates of approximately 2.5% during the first 3 months after HCT. This raises issues of study design for the future assessment of CMV therapeutics, because superiority studies that require the traditional CMV disease end point will require sample sizes that are unrealistic to obtain. Therefore, virologic biomarkers of CMV disease such as the viral load in blood or the need for preemptive antiviral therapy should be used in future efficacy trials. Although preemptive therapy works well in most settings, there are notable exceptions for which intensified strategies are needed. The most important one is the cord blood transplantation setting, in which the risk of CMV reactivation and disease is increased.
New treatment options for CMV are urgently needed because the currently available drugs have major limitations. Novel drugs such as lipid cidofovir (CMX-001)36 and a novel nonnucleoside inhibitor, AIC-246,37 are currently undergoing phase 2 evaluation in HCT recipients. Maribavir failed to prevent CMV disease at a relatively low dose,3 but higher doses might be effective in the treatment of refractory and drug-resistant CMV disease.38 In addition, the development of immune augmentation strategies, including vaccination and adoptive T-cell transfer, as well as nonspecific strategies involving keratinocyte growth factor and IL-7, should be pursued because recent studies showed very promising results.33,39 The quantitative assessment of CMV-specific T-cell immunity is now feasible, and initial data on predictive thresholds for protective immunity have been reported.32,40 A strategy that uses laboratory evidence of protective T cell immunity to withhold therapy is now feasible; however, a systematic evaluation in a randomized trial is needed to determine whether such strategy can be safely used.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Chimerix, Genentech/Roche, Vical, and Viropharma; has consulted for Astellas, Boehringer Ingelheim, Chimerix, Novartis, Genentech/Roche, Vical, and Viropharma; and is supported in part by National Institutes of Health grants CA18029, HL93294, and 102547. Off-label drug use: Off-label use of antiviral drugs for CMV management in transplantation recipients.
Correspondence
Michael Boeckh, Vaccine and Infectious Disease and Clinical Research Divisions, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109-4417; Phone: (206) 667-6702; Fax: (206) 667-4411; e-mail mboeckh@fhcrc.org.