Abstract
Platelet dysfunction is commonly acquired due to medications, procedures, medical conditions, and underlying hematologic disease. These issues are presented, the data reviewed, and recommendations given herein. Many medications and dietary supplements have platelet-inhibitory effects in vitro, although the clinical effects on bleeding risks are unclear for many. Platelet-inhibitory drugs are key in the treatment of vascular disease. Data are available to aid in the management of these medications to prevent hemorrhagic complications. Bleeding in patients with renal failure has decreased with improved dialysis and the use of erythropoietin, but remains a challenge. Platelet dysfunction accompanies cardiac valvular disease and use of cardiopulmonary bypass (CPB) and extracorporeal membrane oxygenation. Hematologic disorders including myeloproliferative disorders (MPDs), myelodysplasia, paraproteinemias, and immune thrombocytopenia (ITP) can also be associated with hemorrhagic complications due to platelet dysfunction. Knowledge of which factors affect bleeding risk and how to treat individuals with acquired platelet dysfunction are important in optimizing patient care.
Platelet dysfunction is commonly acquired due to medications, procedures, medical conditions and underlying hematologic disease, as listed in Table 1. Knowing when this occurs, appropriate diagnostic tools, and the clinical impact of platelet dysfunction is important to avoid hemorrhagic complications in patients.
Drugs and supplements
More than 100 drugs, foods, and supplements have been reported to inhibit platelet function, although the clinical effect of most of these is unclear. Many drugs affect platelet function, some of which are given with therapeutic intent and some with platelet inhibition as a side effect. Drugs that inhibit platelet function are listed in Table 2.
Bleeding complications of drugs used for therapeutic platelet inhibition
The most commonly used drug that inhibits platelet function is aspirin, which inhibits cyclooxygenase I (COX-1) conversion of arachidonic acid to thromboxane A2, inhibiting platelet function by preventing the enhancement and propagation of agonist-induced platelet signaling. Additional drugs that inhibit platelet COX-1 include nonspecific nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen and naproxen. COX-2–specific inhibitors have little platelet-inhibitory activity.1
In most patients on aspirin therapy alone, bleeding symptoms are minimal. However, antiplatelet therapy is often poorly tolerated in patients with underlying or acquired disorders of platelet function, including VWD, and may unmask mild inherited or acquired bleeding disorders that have not been diagnosed previously.
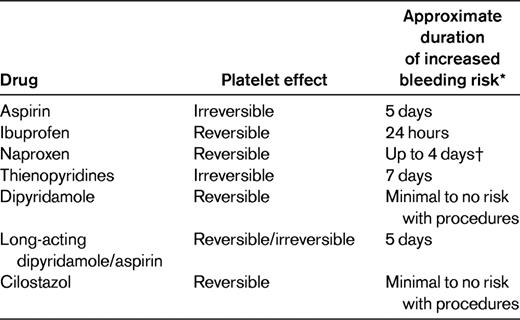
Patients are advised about discontinuing antiplatelet therapy before surgical procedures. Many times, these recommendations are for days far in excess of what is needed for resolution of platelet inhibition. Aspirin inhibits COX-1 irreversibly and persists for the life of the platelet, 7-10 days. However, given new platelet production, overall functional inhibition persists for approximately 5 days.2 In addition, many procedures can be undertaken with noncoagulopathic patients on low-dose aspirin with minimal or no increased risk of bleeding, including cardiopulmonary bypass (CPB)3 and dental extraction.4 Other NSAIDs inhibit platelets while the drug is in the circulation. Ibuprofen has the shortest elimination half-life at ∼ 2 hours. In a study of 11 normal volunteers who had taken ibuprofen 600 mg every 8 hours for 7 days, 7 of 11 had normal platelet function analyzer (PFA-100) closure times with the collagen/epinephrine cartridge at the end of treatment.5 Three of 7 subjects had normal PFAs at 8 hours and all were normal by 24 hours. A smaller study of naproxen found abnormal arachidonic acid–induced platelet aggregation in 2 of 3 patients at 2 hours and in 0 of 3 patients at 4 days after the last dose.6 Recommendations for cessation of antiplatelet therapy, particularly in those with significant cardiovascular risks or need for anti-inflammatory medications, should be made in light of data on the length of platelet inhibition after stopping these medications (Table 3).
The thienopyridines clopidogrel and prasugrel inhibit ADP-induced platelet aggregation. Prasugrel provides a more rapid and consistent response, resulting in higher levels of active metabolites compared with clopidogrel, but carries an increased risk of bleeding.7 Both inhibit the platelet irreversibly, but unlike the case with aspirin, the effect can be detected 7 days after dosing. In the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38), combined TIMI major and minor bleeding in patients requiring emergency coronary artery bypass grafting within 3 days of stopping prasugrel therapy was 26.7% compared with 5% with clopidogrel. Within 4-7 days of stopping therapy, the rate was 11.3% and 3.4%, respectively, for prasugrel and clopidogrel.8 Because the half-lives of the active metabolites of these antiplatelet medications are relatively short (∼ 7 hours), platelet transfusion can be helpful in mitigating the bleeding risks, particularly if given later than 4-6 hours after dosing.
Combinations of antiplatelet and anticoagulant drugs are common in clinical medicine, particularly in the secondary prevention of cardiovascular disease and in patients with atrial fibrillation and coronary artery stents. A meta-analysis of 9 trials evaluating the safety and efficacy of triple therapy (dual antiplatelet plus anticoagulant therapy) compared with less intense therapy after coronary artery stenting showed a decrease in ischemic stroke (odds ratio [OR]: 0.29; 95% confidence interval [95% CI]:0.15-0.58), but with increased major bleeding (OR 3.16; 95% CI:1.81-5.52).9 Dual antiplatelet therapy with a thienopyridine and aspirin is indicated for at least 1 month for bare metal stents and for 12 months for drug-eluting stents, and treatment duration recommendations are increasing. Discontinuing dual antiplatelet therapy in these early critical time points carries a significant risk of graft thrombosis and should be done only when bleeding is life-threatening.
Gastrointestinal (GI) bleeding is the major site of serious bleeding with antiplatelet therapy. Dual antiplatelet therapy increases the risk of GI bleeding 2- to 3-fold compared with aspirin alone, although the absolute risk is relatively low at 0.6%-2%.9 Patients at highest risk are those with a history of GI bleeding. Other risk factors include advanced age, concomitant use of anticoagulation, steroid therapy, or NSAIDs including aspirin, and presence of H pylori infection. Proton pump inhibitors have been shown to decrease the risk by ∼ 50% in patients with a history of GI bleeding or with 3 or more risk factors. Concern has been raised that proton pump inhibitors, particularly those metabolized through the CYP2C19 pathway, interfere with clopidogrel metabolism; however, a prospective trial found no difference in cardiovascular outcomes in patients receiving clopidogrel with or without omeprazole.9 Clopidogrel therapy may carry a risk of thrombotic thrombocytopenic purpura within the first few weeks of therapy that is estimated at 1 in 1 000 000 patients, much lower than that seen with ticlopidine, which is estimated at 1 in 1600 to 1 in 5000 patients.10
Other platelet inhibitors used for therapy include the phosphodiesterase inhibitors dipyridamole and cilostazol and the platelet glycoprotein (gp) IIb/IIIa receptor antagonists. Dipyridamole and cilostazol inhibit phosphodiesterase, resulting in increased platelet cAMP and decreased agonist-induced platelet response; neither alone appears to increase the risk of bleeding with surgery.11,12 Caution with the long-acting dipyridamole/aspirin combination (Aggrenox) should be the same as with aspirin alone. The platelet gpIIb/IIIa inhibitors abciximab, tirofiban, and eptifibatide are used in percutaneous cardiac interventions. In current use, they cause little increased bleeding in this setting (2.4% with vs 1.4% without), and do not increase intracranial bleeding.13 The pathogenesis of abciximab-associated thrombocytopenia is not understood. Its acute onset and its higher incidence in patients exposed to the drug a second time suggests that antibodies may be responsible. Rarely, they result in acute thrombocytopenia and if this occurs, platelet transfusion can be used to treat bleeding symptoms.
Medications that inadvertently inhibit platelet function
Many drugs can affect platelet function in vitro, although the clinical impact of increased bleeding with many of these is not well defined. Beta-lactam antibiotics, including penicillins and particularly at high doses, have been shown to inhibit platelet function and increase bleeding. The mechanism is thought to be through the inhibition of epinephrine and ADP-induced platelet aggregation and granule release and interference with platelet-VWF interactions.14 Cephalosporins and other β-lactams given at lower doses can also inhibit platelet function in vitro. However, the clinical significance of this has not been clearly established.
Nitrates inhibit platelet aggregation by increasing cGMP. Propranolol, a nonspecific beta-blocker, affects platelet function in vitro similarly to anesthetics and tricyclic antidepressants, likely by affecting platelet membranes. Calcium channel blockers have many effects on platelets in vitro, with the greatest effect on serotonin-induced platelet aggregation. The clinical impact of these findings, if any, is unclear.
Selective serotonin reuptake inhibitors (SSRIs) decrease serotonin uptake from the blood by platelets. Because platelets do not synthesize serotonin, platelet serotonin content is decreased. There have been several reports of bleeding in patients on SSRIs. In larger studies, the effect was shown to be modest, and those most at risk were the elderly, those with prior GI bleeding, and those on anticoagulation therapy. Using a large Dutch database of approximately 2 million patients on warfarin, Schalekamp et al found that SSRI use was associated with a modest increase in non-GI bleeding (OR 1.7; 95% CI: 1.1-2.5), but no increase in GI bleeding (OR 0.8; 95% CI: 0.4-1.5).15 SSRI therapy may increase the risk of perioperative bleeding. In 14 464 Danish women undergoing breast cancer surgery, the risk of bleeding requiring reoperations within 2 weeks was increased in current SSRI users (adjusted relative risk 2.3; 95% CI: 1.4-3.9).16 Given the importance of SSRIs in the treatment of depression, the hemorrhagic risk of this therapy needs to be balanced with the potential benefit of treatment.
Supplements, foods, and alcohol
Herbal supplements, foods, and alcohol can also inhibit platelet function (Table 4), although the clinical significance for most of these is unclear. There have been several reports of bleeding in individuals taking Ginkgo biloba, although laboratory studies have had inconsistent results.17,18 Patients with underlying bleeding disorders or those on anticoagulants should be advised to avoid this supplement, because it has not been shown to have clinical benefit.19 Because it exerts an antiplatelet effect, gingko is likely to increase symptoms in patients on antiplatelet therapy or with underlying disorders of platelet function or number. The link with other herbal supplements and foods is less clear, but this should be explored in patients with unexplained bleeding. Alcohol decreases platelet function. In the Framingham Offspring Study, moderate alcohol intake was associated with decreased platelet p-selectin exposure in men and with decreased ADP- and epinephrine-induced platelet aggregation in men and women.20
Renal failure
With appropriate dialysis and the use of erythropoietin, bleeding in renal disease has decreased. However, particularly in the setting of acute renal failure and also in preparation for procedures such as renal biopsy, treatment and prevention of bleeding complications remains a common reason for hematologic consultation. Impaired platelet function, reflected by a prolonged bleeding time, has been implicated as a cause of bleeding in renal failure, although the results of platelet aggregation studies are inconsistent. Numerous studies have failed to determine a consistent defect of platelet function that can be causally linked. Correction of anemia with transfusion was shown to decrease uremic bleeding, and this treatment has therefore been supplanted with recombinant erythropoietin therapy.21 Cryoprecipitate decreases bleeding time and bleeding symptoms for up to 24-36 hours,22,23 so its use has generally been replaced with the vasopressin derivative 1-deamino-8-D-arginine vasopressin (DDAVP or desmopressin), which decreases clinical bleeding and improves measures of platelet function.23,24 The effect of this treatment on bleeding time lasts for up to 4-8 hours. However, neither correction of the bleeding time nor PFA closure times has been shown to be correlated with decreased uremic bleeding. The mechanisms of hemostatic response to DDAVP probably include both increases in plasma VWF and factor VIII levels and a direct effect on platelet function.24
Valvular disease, cardiopulmonary bypass, and extracorporeal membrane oxygenation
Pathologic vascular states with high shear can result in bleeding due to acquired VWD. This is best described in patients with aortic stenosis and resolves with valve replacement. Laboratory findings consistent with VWD may be more prevalent than clinical symptoms. Artificial membranes used in CPB and extracorporeal membrane oxygenation can result in both thrombocytopenia and platelet dysfunction.25,26 A detailed study of platelet function throughout CPB and postoperatively found that the defect was not intrinsic to the platelet itself, but rather to an extrinsic defect such as the lack of availability of platelet agonists in vivo.26
MPDs
Myeloproliferative disorders (MPDs) are associated with both bleeding and thrombotic complications.27 Hemorrhagic complications are seen more frequently with the use of high-dose aspirin in patients with essential thrombocythemia or polycythemia vera, but are less common with low-dose aspirin (100 mg/d), and aspirin has more antithrombotic benefit than pro-hemorrhagic risk in that setting.
Hemorrhagic events in MPD unrelated to therapy are sometimes observed. In a study of 494 MPD patients, 5.5% suffered from major bleeding.28 Most were on antiplatelet (63%) or vitamin K antagonist (14.8%) therapy. Bleeding is described in MPD patients with very high platelet counts. In a large observational study of low-risk patients with essential thrombocythemia, an increase in major bleeding was observed with platelet counts > 1 million (incidence rate ratio: 5.4; 95% CI: 1.7-17.2).29 Loss of high-molecular-weight VWF multimers due to spontaneous platelet binding and clearance appears to be a mechanism that results in bleeding30 ; this is apparently caused by the high platelet count because patients with very elevated platelet counts after splenectomy have the same laboratory findings.30 Abnormalities of platelet aggregation assays have been described in samples from patients with MPD, but are nonspecific and are not correlated with either bleeding or thrombotic complications. The most common finding is the loss of the secondary wave of aggregation with epinephrine and ADP.27 Neither the bleeding time nor PFA-100 closure times have been shown to predict bleeding in MPD.
Because of the increased risk of bleeding with high platelet counts, the platelet count should be lowered to less than 1 million before procedures and in treating bleeding complications.
Myelodysplasia
Bleeding in patients with myelodysplasia is usually due to thrombocytopenia. However, abnormal thrombopoiesis can result in dysfunctional platelets. Platelet electron microscopy in 8 patients with myelodysplasia demonstrated significantly fewer alpha granules and abnormalities in the dense tubular system.31
In the setting of myelodysplasia, bleeding symptoms can occur at platelet counts above that which would be suspected. As in other disorders of platelet function, symptoms usually consist of increased bruising, epistaxis, GI bleeding and other bleeding, particularly from mucosal sites. This bleeding tendency may be enhanced in the setting of antiplatelet therapy, and the diagnosis of myelodysplasia should be considered in elderly individuals who begin to have new bleeding symptoms. These patients will usually have anemia and/or thrombocytopenia, but occasionally bleeding can be the first manifestation of myelodysplasia.
Paraproteinemia
Paraproteins, particularly in the setting of multiple myeloma and Waldenstrom macroglobulinemia, can result in multiple hemostatic defects32 such as prolonged coagulation assays, including the thrombin time, the prothrombin time, and occasionally the activated partial thromboplastin time (aPTT). In addition, associated amyloid can result in acquired deficiencies of vitamin K–dependent factors, particularly factor X, through adsorption to the amyloid fibrils. Nonspecific immunoglobulin adherence to the platelet surface results in platelet dysfunction. In this setting, platelet transfusion has limited effectiveness because the transfused platelets quickly become dysfunctional. Removal of the paraprotein is generally effective in improving platelet function.
In patients with lymphoproliferative disorders with new onset bleeding, one should also consider acquired VWD (aVWD), which is seen most commonly in this setting.33 Given the key role of VWF in platelet adhesion, mucosal bleeding or “platelet-like” symptoms predominate. In patients with aVWD, the prothrombin time and thrombin time are normal and the aPTT is either prolonged or normal. VWD in this setting most commonly resembles a “type 2” subtype with loss of the highest-molecular-weight multimers. Factor VIII activity and VWF antigen can be normal or only slightly decreased, whereas a measure of VWF function (ristocetin cofactor or collagen binding) is lower. If the aPTT is prolonged, it will correct with mixing, because the decrease in FVIII is caused by an acquired deficiency due to lack of its cofactor and not an inhibitor. The decrease in VWF is usually due to increased clearance.
In patients with paraprotein-associated aVWD, removal of the paraprotein is most effective in correcting the bleeding diathesis. This is particularly challenging in the setting of monoclonal gammopathies of undetermined significance. With acute bleeding or when the paraprotein cannot be eliminated, intravenous gamma globulin infusions can prolong VWF survival and increase VWF levels. This may need to be combined with DDAVP or VWF-containing factor concentrate depending on the severity of bleeding and baseline levels. These treatments will be short-lived, making management of this disorder challenging.
ITP with platelet dysfunction
Bleeding in patients with immune thrombocytopenia (ITP) usually occurs at very low platelet counts.34 Occasionally, patients will have bleeding symptoms with only mild to moderate thrombocytopenia. Antibodies in ITP are usually directed at the gpIIb/IIIa or gpIb-IX receptor. In some cases, these antibodies result in platelet dysfunction. Testing for the presence of antiplatelet antibodies will not predict who will have bleeding symptoms. Laboratory measures of platelet function are generally not helpful because thrombocytopenia alone results in abnormal values. In mild thrombocytopenia, platelet aggregation studies may be helpful in defining a functional disorder, although this approach is not standardized. If platelet aggregometry is performed on mildly thrombocytopenic patients, control platelet-rich plasma should be adjusted to the same level and interpretation of the patients' studies should be done in consideration of the control values.
Approach to patients with suspected platelet dysfunction
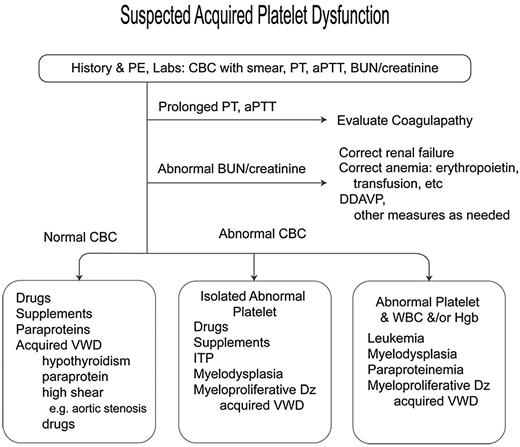
An approach to the diagnosis of patients with suspected platelet dysfunction is presented in Figure 1. The patient's clinical, medication, and supplement history should be carefully reviewed to detect platelet-inhibitory products and symptoms of systemic disease. A basic laboratory evaluation should include a complete blood count, review of the peripheral blood smear, prothrombin time, aPTT, and renal and thyroid functions. In patients with a normal platelet count and no clear etiology for the dysfunction, further evaluation for a paraprotein and acquired VWD should be performed. Bleeding time, PFA-100 closure time, and platelet aggregation studies are of limited utility in diagnosis or assessment of treatment.34,35
Algorithm for suspected platelet dysfunction. PE indicates physical examination; PT, prothrombin time; and Dz, disease.
Algorithm for suspected platelet dysfunction. PE indicates physical examination; PT, prothrombin time; and Dz, disease.
Treatment of patients with suspected platelet dysfunction is generally specific to the underlying disorder and can include DDAVP and platelet transfusion, as noted in the previous sections. Antifibrinolytic therapy (epsilon aminocaproic acid or tranexamic acid) is useful, particularly in mucosal bleeding, but in general should not be used in patients with hematuria or disseminated intravascular coagulation. rFVIIa has been used to treat bleeding in patients with acquired and inherited platelet disorders, albeit with an increased thrombotic risk.
Summary
Many drugs and supplements, clinical circumstances, and underlying hematologic disorders result in platelet dysfunction. However, laboratory findings may not be correlated with clinical risk. Knowledge of which factors affect bleeding risk is important in optimizing patient care. Treatment approaches vary according to the cause of platelet dysfunction and the associated disease.
Disclosures
Conflict-of-interest disclosure: The author has equity ownership in Glaxo-SmithKline. Off-label drug use: None disclosed.
Correspondence
Barbara A. Konkle, Director, Translational Research, Puget Sound Blood Center, Professor of Medicine, Division of Hematology, University of Washington, 921 Terry Ave, Box 359190, Seattle, WA 98104; Phone: (206) 233-3349; Fax: (866) 291-0025; e-mail: barbarak@psbc.org.