Abstract
A clear understanding of the molecular basis of VWD can guide the choice and interpretation of appropriate diagnostic tests. This review briefly describes the lifecycle and molecular interactions of VWF and how they lead to the current clinical classification. It also includes a brief discussion of the differential diagnosis and general workup of mucocutaneous bleeding, a review of the various VWD subtypes, and pertinent laboratory assays for each, including genetic tests. Finally, common testing pitfalls and diagnostic dilemmas are covered, including the challenge created by the overlap of borderline low VWF levels and mild bleeding.
Introduction
VWD is the most common inherited bleeding disorder and is characterized by abnormally low VWF levels and excessive mucocutaneous bleeding. Over the last decades, many aspects of the genetics and pathogenesis of the disease have been elucidated. These recent discoveries allow for a clearer understanding of the mechanisms of the disease, but they have not necessarily translated yet into better diagnosis and clinical practice. Current tools for the accurate diagnosis of VWD and recent developments that may improve its diagnosis in the future are discussed in this review.
Approach to patients with mucocutaneous bleeding
The evaluation of patients with mucocutaneous bleeding should begin with a careful personal and family history. Important topics to discuss include family history of bleeding and type/chronicity of bleeding symptoms that triggered the referral. Specific consideration should also be given to questions about timing of bleeding and any necessary interventions (eg, blood transfusions) after challenges such as surgery, dental extractions, and trauma (as well as menstrual periods/childbirth for women).1 In addition, a thorough medication review (including herbal supplements and over-the-counter drugs) is essential, because many of them can cause platelet dysfunction (eg, aspirin or other nonsteroidal anti-inflammatory drugs).
The International Society of Thrombosis and Hemostasis (ISTH) Scientific Subcommittee (SSC) on VWF developed provisional consensus criteria on the definition of significant mucocutaneous bleeding history. According to this statement, it requires at least 2 symptoms (Table 1) in the absence of a blood transfusion history or 1 symptom requiring treatment with blood transfusion or 1 symptom recurring on at least 3 distinct occasions in order for the bleeding to be considered significant.2 The VWF SSC also defines a positive family history compatible with type 1 VWD as: (1) at least 1 first-degree relative or 2 second-degree relatives with a personal history of significant mucocutaneous bleeding and (2) laboratory tests compatible with type 1 VWD.2 These definitions attempt to standardize the quantification of bleeding; however, accurate characterization of bleeding symptoms still presents a significant challenge to the diagnosis of VWD, especially type 1 VWD. Recently, several bleeding assessment tools have been developed for the determination of a more accurate bleeding phenotype in healthy subjects and those with bleeding disorders.3 The development of these new assessment tools will significantly change the way we evaluate bleeding in the future. However, for the time being, it is clear that, in most cases, the personal history of bleeding triggers the laboratory testing and a positive family history acts as supportive evidence for a hereditary bleeding disorder.
VWF production, secretion, and clearance
The biosynthesis and lifecycle of VWF provides a framework for understanding how mutations affect plasma levels, and therefore assist in the classification of subtypes and the selection of appropriate laboratory tests.4 VWF is a large, multimeric glycoprotein that promotes platelet adhesion to the exposed subendothelium, contributes to platelet aggregation, and chaperones clotting factor VIII (FVIII) in plasma, protecting it from proteolytic degradation.5 Therefore, quantitative and qualitative defects in VWF can cause bleeding by impairing platelet adhesion or by reducing the concentration of FVIII.6 VWF is synthesized in the vascular endothelium (stored in Weibel-Palade bodies and released in response to stress or vasopressin) or in BM megakaryocytes (stored in alpha-granules and released in response to platelet activation signals).4–7 The complex cellular processing consists of dimerization in the endoplasmic reticulum (ER), glycosylation in the ER and Golgi complex, multimerization in the Golgi complex, and packaging into storage granules after the VWF propeptide (VWFpp) is cleaved off. The VWF propeptide is released in equimolar concentrations to the mature VWF molecule.6 After multimers are secreted into the blood, they are subject to proteolysis, including cleavage under shear stress conditions by the metalloprotease ADAMTS13 and clearance by macrophages in the liver and spleen.4
VWD
VWD is often categorized by quantitative or qualitative defects of VWF. Whereas type 1 VWD is a partial quantitative defect, type 3 VWD is represented by the complete absence of VWF. Type 2 VWD describes a qualitative defect of VWF that can be further subdivided: type 2A (loss of intermediate- and high-molecular-weight multimers due to decreased secretion or increased susceptibility to ADAMTS 13); type 2B (gain-of-function mutation resulting in spontaneous VWF-platelet binding under physiologic shear conditions, which leads to clearance of the highest-molecular-weight multimers and mild thrombocytopenia); type 2M (loss-of-function mutations that decrease the interaction of VWF with its platelet receptor and decrease ristocetin cofactor activity); and type 2N (mutations in VWF causing reduced binding to FVIII, allowing for increased clearance).5 The transmission of VWD is often autosomal dominant (ie, types 1, 2A, 2B, and 2M), but can also be rarely inherited in a recessive manner (ie, types 2N and 3). The most common form (type 1) is characterized by low penetrance (not all who inherit the mutation show signs of clinical disease) and variable expressivity (those who inherit the same mutation show variable clinical signs).5 This phenomenon usually leads to a great deal of difficulty in consistently diagnosing type 1 VWD correctly.
Screening tests
Laboratory evaluation of the patient with mucocutaneous bleeding usually begins with specific screening tests. A complete blood count will identify thrombocytopenia and/or other blood cell abnormalities.1 Prothrombin time, activated partial thromboplastin time, and fibrinogen tests may help to exclude liver disease–related coagulation abnormalities and inherited or acquired clotting factor deficiencies (covered in greater depth in Mannucci8 ) but may be difficult to interpret in the setting of suspected VWD. The activated partial thromboplastin time in particular can be problematic as a screening test, especially for types 1 and 2 VWD, because the value is often normal in these cases (except in type 2N VWD, in which it is increased).9 The test yields abnormal results if FVIII is sufficiently reduced, which is typically only noticeably abnormal in types 2N and 3 VWD.
Laboratory screening methods have been used to identify persons with a high likelihood of VWD or a platelet disorder. For many years, the bleeding time represented the gold standard of screening tests, but its clinical use is questionable due to difficulties with standardization, reproducibility, and lack of sensitivity and specificity.10
The closure time obtained in the PFA-100 (platelet function analyzer) test has also been proposed to have a role in the screening of persons with suspected VWD. Because the sensitivity and specificity of the PFA-100 approach 90% for patients with VWD (when their VWF levels are less than 25%) or severe platelet disorders, it has been suggested that the PFA-100 could have utility as an initial screening and diagnostic tool in the evaluation of patients with suspected VWD or a platelet-related bleeding disorder.11–14 Unfortunately, the initial enthusiasm for the efficacy of the PFA-100 as screening tool has diminished due to low sensitivity (24%-41%) of the device reported in persons with VWD and VWF levels greater than 25%, mild platelet secretion defects, and storage pool disorders, especially in the preoperative setting.15–18
In summary, the existing screening laboratory methods available to hematologists have very limited value. Therefore, in clinical practice, if the personal history of mucocutaneous bleeding is significant, specific laboratory assays for VWD should be ordered.
Diagnosis of VWD
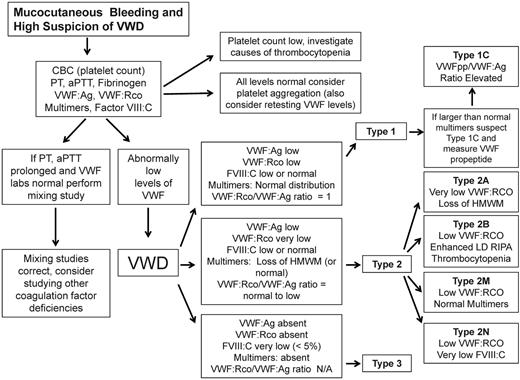
Based on the nature of VWD categorization, it is important to determine whether the defect is quantitative or qualitative in nature. Once the diagnosis of VWD is being considered, the clinician can follow a simplified VWD diagnostic algorithm (Figure 1) beginning with screening tests and assessment of the VWF:Ag level, VWF ristocetin cofactor activity (VWF:RCo), coagulation FVIII (FVIII:C), and VWF multimers.
Simplified algorithm for the evaluation of the patient with mucocutaneous bleeding and high index of suspicion of VWD. Due to space limitations, not all potential diagnostic laboratories were included.
Simplified algorithm for the evaluation of the patient with mucocutaneous bleeding and high index of suspicion of VWD. Due to space limitations, not all potential diagnostic laboratories were included.
Available tests for VWF
Ag levels are usually measured by quantitative immunoassays and are available in most hemostasis laboratories. VWF:RCo reveals the plasma's ability to agglutinate platelets in the presence of the antibiotic ristocetin. This assay can be challenging to interpret, in part because of the associated high coefficients of variation, which can be as high as 30% or even greater when the VWF:RCo is lower than 12-15 IU/d,16 potentially affecting the ability to diagnosis VWD or differentiate between type 1 and type 2 VWD. Despite these limitations, however, VWF:RCo is widely used and is accepted as the gold standard for VWF activity. Other versions of this assay that are being evaluated for clinical utility include latex immunoassay and ristocetin ELISA, which have the potential to alleviate some of the VWF:RCo limitations. Low-dose ristocetin-induced platelet aggregation (LD-RIPA) is used to identify abnormally increased binding between VWF and platelets. VWF multimers are usually run on an agarose gel to appreciate the full range of molecular weights within the mature VWF molecule. This can help to differentiate between various subtypes of type 2 VWD and can easily identify type 3 VWD. FVIII level and the FVIII-binding assay allow for a more accurate diagnosis of type 2N VWF. The collagen-binding assay measures the binding of large VWF multimers to collagen and represents another way to measure the functional activity of the molecule without using ristocetin. The test may be useful to differentiate type 2 VWD from type 1 VWD. Two recent studies have revealed that higher values are obtained with type I collagen compared with types III and VI, and the different types differ significantly in relative sensitivity to the loss of high-molecular-weight multimers.19,20 Therefore, results from one collagen type may not be generalizable to all collagen assays.
In addition, many preanalytic variables must be considered to make the most accurate interpretation of the specific laboratory results. Platelet contamination in frozen plasma can lead to protease-induced alterations in VWF structure, causing increased VWF:Ag but decreased activity.5 In addition, refrigeration of whole blood samples before separation can cause reduced plasma VWF levels. Other clinical conditions must also be considered due to VWF's changes as an acute-phase reactant (eg, increased levels or altered multimeric structure) in situations of surgery, collagen vascular disease, general inflammatory diseases or infection, pregnancy, and increased endothelial stimulation (eg, diffuse intravascular coagulation, liver disease, thrombotic thrombocytopenic purpura, and hemolytic uremic syndrome).5
Type 1 VWD is defined by partial quantitative deficiency of VWF and bleeding symptoms in a patient who usually has family members with the same features.2 Patients with very low VWF levels (less than 20 IU/dL) typically have mutations in the VWF gene (VWF) and significant bleeding symptoms.4 Approximately 75% of type 1 VWD mutations are missense, leading to single amino acid substitutions with a dominant-negative effect that impair the intracellular transport of VWF subunits and cause a subsequent reduction in VWF secretion.21 Recently, investigators have identified other mutations that lead to rapid clearance of VWF from the circulation.22,23 In the case of these mutations, VWF is made and secreted normally, but is cleared from the circulation more rapidly than expected. The impaired secretion and increased clearance are likely the most common molecular mechanisms that lead to type 1 VWD (Figure 2).
Mechanism of disease for quantitative variants (types 1 and 3 VWD) and the effect on diagnostic laboratories.
Mechanism of disease for quantitative variants (types 1 and 3 VWD) and the effect on diagnostic laboratories.
Because VWF is synthesized on a 1:1 ratio with VWFpp, an alteration of the ratio in favor of the propeptide suggests increased VWF clearance (type 1C).22 The propeptide assay may become more widely used in the future, because the diagnosis of accelerated clearance has therapeutic implications. A recent study described the utility of measuring VWFpp to identify patients with a reduced VWF survival phenotype, because these patients can have a robust initial desmopressin response, but levels decrease precipitously within 3 hours.22
Low VWF levels
More than half of patients with mildly low levels of VWF (30-50 IU/dL) are asymptomatic or have minimal bleeding symptoms that are most of the time indistinguishable from those in the general population. Therefore, the presence of plasma VWF levels between 30 and 50 IU/dL does not automatically define type 1 VWD. Moreover, patients whose blood group is O have 25%-30% lower levels of VWF than those with group A; therefore, 14% of those with blood type O in the United States are expected to have VWF levels equal to or lower than 50 IU/dL. Based on the fact that mild bleeding is likely to be commonly reported in the healthy population, it is possible that many of those diagnosed with mild type 1 VWD may not have the genetically inherited VWD, but an association of mildly low (perhaps normal for blood group O) VWF levels and mild bleeding without a direct causative effect.24 A modestly low VWF level does not predict significant bleeding in the future in the absence of bleeding symptoms and, conversely, severe bleeding in the face of modestly low VWF may suggest other causes for bleeding, leading some to consider moderately low VWF as a risk factor, not as a disease unto itself.4
Types of VWD
Laboratory test results are compatible with type 1 VWD if the levels of both VWF:RCo and VWF:Ag are greater than 2 SDs below the population mean and the plasma VWF multimer distribution is normal.2 In addition, the VWF:RCo/VWF:Ag ratio is close to 1. Finally if abnormally large VWF high-molecular-weight multimers are detected in the presence of a low VWF:Ag and VWF:RCo, a VWFpp level allows for the discrimination of type 1C VWD by determining the VWFpp/VWF:Ag ratio, which will be elevated (Figure 1).
Type 3 VWD is usually inherited in an autosomal-recessive mode and is characterized by the complete lack of VWF protein with undetectable VWF:Ag and VWF:RCo levels and very low FVIII:C levels (< 5%) that represent the steady state of FVIII without being chaperoned by VWF. Multimers are absent and the bleeding patterns in patients with type 3 VWD are usually very severe.
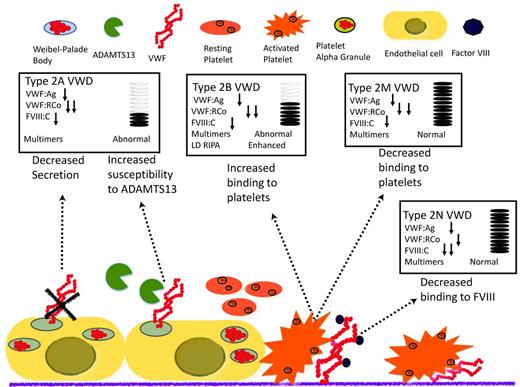
Type 2 VWD is a qualitative defect caused by mutations in VWF that result in abnormal interactions with several of its ligands (Figure 3). Type 2A VWD, which is associated with a selective loss of intermediate- and high-molecular-weight multimers, is caused by either retention in the ER or increased susceptibility to proteolytic cleavage by ADAMTS13 in the plasma. The diagnosis is made in the presence of a low VWF:Ag and a very low VWF:RCo, with a pronounced loss of high-molecular-weight multimers. Type 2M VWD is caused by mutations of the platelet glycoprotein 1bα (GP1balpha)–binding site, resulting in decreased binding of VWF to GP1bA and subsequent impairment of platelet-dependent function. One of the keys to differentiating this laboratory profile from other type 2 VWD profiles is the presence of a normal multimeric structure. Type 2B VWD is characterized by gain-of-function mutations of the binding site for GP1bA, leading to rapid clearance of the platelet/VWF complex. To confirm the diagnosis of type 2B VWD, LD-RIPA is used to enhance platelet agglutination in the presence of gain-of-function mutations. This phenomenon is seen in patients with type 2B VWD or in patients with platelet-type or pseudo-VWD, a rare disorder caused by mutations in platelet GP1bA.25 It is important to differentiate these 2 phenotypically similar entities, because the therapeutic approach is very different: type 2B VWD is treated with VWF concentrates (because the molecular defect is in VWF) and pseudo-VWD is treated with platelet transfusions (because it is caused by mutations in platelet GP1bA). If pseudo-VWD is suspected, the patient's platelets with a normal source of VWF can be the substrates for a LD-RIPA–based mixing study. Enhanced binding will confirm the diagnosis. Finally, type 2N VWD results from mutations in the FVIII-binding site of the VWF that disrupts the interaction between these 2 proteins.9 It is characterized by normal or low VWF:Ag and VWF:RCo and disproportionately lower FVIII:C. Specific FVIII-binding assays can be used to confirm the diagnosis. Type 2N VWD should be suspected in patients diagnosed with mild hemophilia who either do not respond well to FVIII infusions or belong to families in which males and females are equally affected. Symptomatic patients are either homozygous or compound heterozygous.
Mechanism of disease for qualitative variants (type 2 VWD) and the effect on diagnostic laboratories.
Mechanism of disease for qualitative variants (type 2 VWD) and the effect on diagnostic laboratories.
The results of a recent study suggest that the VWF:RCo assay can be abnormally low in a subset of otherwise-healthy African Americans due to the presence of a common single nucleotide polymorphism (SNP) in exon 28 of VWF.26 This SNP appears to affect ristocetin binding without conferring any hemorrhagic risk, but potentially leading to “false diagnosis” of type 2 VWD. Newly developed functional assays using the platelet ligand for VWF, GP1bA, may allow for the assessment of VWF activity without the need for ristocetin, thus helping to discriminate type 2 variants without the pitfalls discussed earlier in this section.27
Genetic testing
Genetic testing for several human diseases has been debated intensely in recent years, and VWD is no exception. Cost and ethical considerations are always considered in the discussions. The VWF gene is very large, spanning 178 kb and containing 52 exons. In addition, the presence of a highly homologous partial pseudogene in chromosome 22 and the highly polymorphic nature of VWF make the sequencing and its interpretation particularly difficult. The presence of numerous SNPs without apparent clinical implications (more frequently observed in the African-American population) has been described recently.28 Genetic testing is further complicated by the fact that bleeding symptoms and VWF level often are inherited independently and the diagnosis of type 1 VWD does not exhibit consistent linkage to the VWF locus.29
Therefore, the utility of genetic testing will be justified only in cases in which the results would make a difference in the patient's management. One such example is the identification of persons with type 3 VWD, who that exhibit large deletions that place them at higher risk of developing Abs, and therefore anaphylactic reactions, upon treatment. Genetic testing may be also justified in cases in which therapeutic options would be significantly different upon accurate diagnosis, such as type 2N and 2B VWD. However, with the exception of these cases, genetic testing for VWD remains controversial.
Acquired VWD
Another cause of low VWF is acquired von Willebrand syndrome (AWS), a rare disorder in which VWF is synthesized normally, but removed from the circulation more rapidly than expected until the associated underlying condition is resolved. Three pathogenic mechanisms appear to govern this increased clearance: VWF binding to malignant cells (eg, Wilms tumor or lymphoproliferative disorders), autoantibodies against VWF and immune complex formation (eg, hypothyroidism), and increased proteolytic activity of high-molecular-weight multimers under high shear-stress conditions (eg, congenital heart disease, aortic stenosis, and angiodysplasia). The laboratory diagnosis of AWS does not usually differ significantly from the diagnosis of the congenital forms. However, a recent retrospective single-center cohort study described some patients with AWS with normal VWF:Ag and VWF:RCo but abnormal multimers, emphasizing the importance of multimer analysis in all patients with suspected AWS as a sensitive tool for detecting structural abnormalities of VWF and typical patterns that can help to distinguish AWS from congenital VWD.29,30
Conclusions
The diagnosis of VWD could have significant impact on the lifestyle and medical care of affected patients and their families. Therefore, it is essential to understand those aspects of the disease that have a significant effect on diagnosis and treatment. The current approach has limitations, mainly due to the inability to accurately quantify bleeding and to categorize individuals with low VWF and mild bleeding. Although the diagnoses of types 2 and 3 VWD are relatively straightforward, the diagnosis of type 1 VWD disease is still plagued with inaccuracies. Newly developed bleeding assessment tools and laboratory assays will likely advance the field significantly in the years to come.
Disclosures
Conflict-of-interest disclosure: J.D.P. has consulted for and received honoraria from CSL Behring and Pfizer. B.R.B. declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Jorge Di Paola, MD, University of Colorado School of Medicine and Children's Hospital Colorado, Department of Pediatrics, Section of Hematology/Oncology/BMT, 13123 E 16th Ave, B115, Aurora, CO 80045; Phone: 303-724-4000; Fax: 303-724-4015; e-mail: jorge.dipaola@ucdenver.edu.