Abstract
Idiopathic (immune) thrombocytopenic purpura (ITP) is a common autoimmune disorder resulting in isolated thrombocytopenia. ITP can present either alone (primary) or in the setting of other conditions (secondary) such as infections or altered immune states. ITP is associated with a loss of tolerance to platelet antigens and a phenotype of accelerated platelet destruction and impaired platelet production. Although the etiology of ITP remains unknown, complex dysregulation of the immune system is observed in ITP patients. Antiplatelet antibodies mediate accelerated clearance from the circulation in large part via the reticuloendothelial (monocytic phagocytic) system. In addition, cellular immunity is perturbed and T-cell and cytokine profiles are significantly shifted toward a type 1 and Th17 proinflammatory immune response. Further clues into immune dysregulation in ITP may be gleaned from studies of secondary ITP. Some infections can induce antiplatelet Abs by molecular mimicry, and there may be common elements involved in breaking tolerance with other autoimmune disorders. There is also evidence for a genetic predisposition to both ITP and responsiveness to therapy, which may in part lie within immune-related genes. Lastly, treatment with immunomodulatory agents remains the mainstay of ITP therapies.
Introduction
Immune thrombocytopenia (ITP) is a disorder characterized by immune-mediated accelerated platelet destruction and suppressed platelet production. The etiology of ITP is not yet known, and the diagnosis continues to be one of exclusion. ITP may present either as primary (isolated) ITP or as secondary ITP in the context of other associated diseases. The epidemiology, clinical presentation, diagnosis, and treatment recommendations for primary and secondary ITP have been reviewed elsewhere.1–3 This chapter highlights aspects of our current understanding of immune dysregulation in ITP, reviews insights gained from studies into the pathogenesis of secondary ITP, considers clues from genetic studies, and addresses immunomodulatory mechanisms of action in ITP therapies.
Immune dysregulation in ITP
The initial pathogenic mechanisms underlying primary ITP have not yet been identified. Approximately 80% of patients present with primary ITP, and 20% can be identified as secondary ITP.3 The categorization of patients as having primary or secondary ITP, although distinct, is in some ways a moving target, because successful identification of new etiologies or associated conditions in ITP results in the reclassification of patients to secondary ITP. Regardless of the nomenclature, there is still a limited set of inciting mechanisms common to subsets of patients currently designated as primary ITP, whereas patients diagnosed with secondary ITP may share pathogenic mechanisms when the underlying ITP-associated disease processes are similar.
Identification of factors that precipitate ITP is extremely difficult due to the likely transient nature of the provoking event, the inherent difficulty in diagnosing ITP early in its course, and the prolonged time period over which monitoring would need to occur to capture the onset of ITP. Even in subjects known to be at high risk for ITP due to a personal history of ITP, a strong family history of ITP (rare), or comorbidity with a condition predisposing to secondary ITP, the onset of ITP often occurs seemingly rapidly and between monitoring time points. Therefore, the study of immune dysregulation in ITP has been necessarily confined to characterization of the disorder after the autoimmune process is well under way and tolerance to platelets has already been broken.
Despite these limitations, data from numerous studies in recent years are beginning to come together to form a picture of an unbalanced immune response. Not surprisingly, the immune changes observed during ITP are complex (an overview is provided in Table 1). The long-held dogma of platelet-bound Abs leading to Fcγ receptor (FcγR)–mediated clearance of platelets by phagocytes residing in the spleen (and liver) continues to be a central theme in our current understanding of ITP. In addition to this, the evidence supports a wide array of immune shifts involving all components of the immune system, resulting in both shortened platelet survival and inhibition of the production of platelets.3
B cells and antiplatelet antibodies
Although the initial inciting event resulting in provocation of antiplatelet Abs remains unknown, platelet autoantibodies are often present by the time of diagnosis.3 Macrophages and dendritic cells of the reticuloendothelial system (ie, the monocytic phagocytic system) function to phagocytose circulating Ab-bound antigens, including Ab-targeted platelets. Opsonization of Ab-platelet complexes by these APCs facilitates intracellular processing of platelets and can lead to presentation by T cells via MHC II as an array of “foreign” platelet peptides. Presentation of platelet peptides by MHC II in a stimulatory context activates T cells, leading to enhancement of the antiplatelet immune response and the possibility of epitope spread to additional platelet antigens.3
In patients with ITP, autoantibodies frequently appear to be directed against GpIb/IX and GPIIb/IIIa,4 although specificity for other platelet antigens can occur. Although antiplatelet autoantibodies appear to play a central role in the pathogenesis of ITP, some patients have no detectable Abs at the time of diagnosis (for a recent example, see Najaoui et al5 ). This may be explained by limitations inherent to laboratory testing methods and the biology of ITP: brisk clearance of some types of Ab-platelet complexes may reduce circulating antiplatelet Ab titers to below the threshold of detection; tightly bound antiplatelet Abs may be difficult to dissociate for study; Abs with specificity to minor or cryptic antigens on platelets or antigens that reside primarily on megakaryocytes may be missed; and there may simply be a subset of patients in which antiplatelet Abs are not present. Therefore, although the majority of ITP patients present with features consistent with Ab-mediated autoimmunity as a central feature of their disease, there exists considerable heterogeneity in the types, titers, and likely biology, of antiplatelet Abs in ITP.
FcγR and the reticuloendothelial system
In ITP, platelets bound by antiplatelet Abs directed against platelet surface membrane glycoproteins are thought to be cleared by FcγR–bearing macrophages in the reticuloendothelial system (ie, the monocytic phagocytic system). The FcγRs are receptors present on monocytic phagocytic cells, where they bind the constant Fc region of Abs and mediate opsonization. The FcγR system is composed of “activating” (eg, FcγR1, FcγRII, and FcγRIII) and “inhibitory” (eg, FcγRIIb) FcγRs. The activating receptors FcγRII and FcγRIIIa are both indirectly implicated in the phagocytosis of Ab-coated platelets,3 whereas there are some data suggesting a role for decreased FcγRIIb in ITP.6–8 Therefore, there is evidence that the balance of FcγRs present on the monocytic phagocytic cells is shifted in ITP toward a propensity for FcγR-mediated clearance of Ab-antigen complexes.
Complement system
In general, Abs specifically bound to cell-surface antigens not only mediate clearance from circulation by FcγRs, but also can serve to fix complement on cells. Recently, plasma from ITP patients was shown by 2 groups to be capable of fixing complement to platelets in vitro.5,9 Furthermore, platelets from ITP patients also exhibit detectable complement,5 and the ability to fix complement was correlated with the presence of detectable antiplatelet Abs.5 Therefore, complement-mediated immunity, either by enhanced clearance or direct cell destruction, represents another mechanism by which platelet autoantibodies may lead to immune-mediated platelet destruction.
T-cell phenotypes in ITP
From studies of the immune system in animals, immune homeostasis is thought to be maintained via a balance of type 1 (IFNγ, IL-2, TNFα, and TNFβ1 related) and type 2 (IL-4, IL-5, IL-6, IL-10, and IL-13 related) responses. Although the distinctions between type 1 and type 2 T cells and cytokine profiles in humans is less clear, these categories are helpful in broadly placing common immune patterns into context. Type 1 reactions are classically thought to be involved in response to intracellular pathogens, and a type 1 response is one which generally promotes pro-inflammatory, cell-mediated, complement fixing phenotypes. On the other hand, type 2 immunity is thought to function in the fight against extracellular pathogens, and a type 2 response typically elicits an immediate-type hypersensitivity response. During an inflammatory event, dominance of either a type 1 or 2 profile is characteristic, because the prevailing dominant type both expands and engages in negative feedback loops to suppress the “other” T-cell types. Resolution of an immune inflammatory event is then characterized by suppression of the dominant type phenotype and restoration of the type 1/type 2 balance. Therefore, the shifting of the balance of type 1 and type 2 permits tailoring of the immune response to the perceived threat.
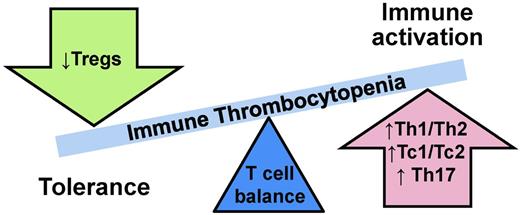
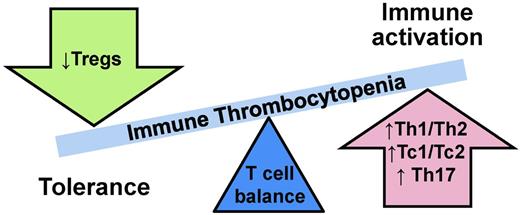
In ITP, type 1/type 2 ratios are unbalanced (Figure 1).3 In primary ITP, adult chronic primary ITP patients have high Th1/Th2 (“helper” CD4+ cells) ratios and high Tc1/Tc2 (“cytotoxic” CD8+ cells) ratios.10 Furthermore, the Th1/Th2 ratio imbalance is inversely correlated with disease severity,11 meaning the higher the Th1/Th2 ratio, the lower the platelet count. ITP patients also exhibit decreased numbers of CD4+CD25+ T-regulatory cells (Tregs), which function to down-regulate T-cell responses.12 Not surprisingly, the degree of decrease in numbers of Tregs is associated with more severe disease in ITP.12 In addition to these type 1/2–specific changes, the total CD4:CD8 ratio is also observed to be diminished in ITP10,13 and improves with disease remission.10
More recently, other subsets of T cells distinct from type 1 and type 2 have also been in implicated in autoimmune diseases, including ITP. Similar to type 1 and type 2 T cells, these T-cell subsets are defined by their cytokine secretion profiles. T cells, which secrete IL-17, are pro-inflammatory and of interest in ITP in part due to a large body of evidence implicating IL-17 in autoimmunity. Within IL-17–secreting T-cell subsets, Th17 (CD4+) cells are increased in ITP,14 as are Tc17 (CD8+) cells.13 Moreover, the increase in Tc17 cells is correlated with skewing of the CD4:CD8 ratio in ITP.13 An additional discrete T-cell subset, Th22 (CD4+IFNγ−IL-17−IL-22+), has recently been identified and found to be up-regulated in several autoimmune diseases. Th22 cells are also significantly increased in ITP patients, and this increase is correlated with the observed increased numbers of Th1 and Th17 cells.15 Another cytokine, IL-21, is produced by some CD4+ T cells and natural killer T cells and is capable of up-regulating both Th17 cells and B cells. In ITP, IL-21 expression on T cells is elevated in untreated newly diagnosed ITP patients, although circulating IL-21 is unchanged.16
Simplistically, the evidence supports a type 1 T-cell response with an up-regulated Th17 response in ITP. Interestingly, similar patterns of immune dysregulation can also be seen in other autoimmune disorders (reviewed in Sakakura et al,12 Hu et al,13 Hu et al,15 and Zhu et al16 ). These types of responses, stereotypically proinflammatory, cell-mediated, complement-fixing phenotypes, would be expected to propagate and enhance the ongoing autoantibody-mediated platelet immune process.
Platelets as immune cells
Platelets themselves are active immune participants17 and may contribute to immune responses in ITP.3 Specifically, expression of the cytokines CXCL5, CCL5, EGF, and CD40L have been found to be significantly decreased in both ITP and aplastic anemia patients, and the levels of these cytokines were strongly associated with the degree of thrombocytopenia.18 The investigators of that study postulated that decreases in these platelet-derived cytokines collectively work to both tip the Th1/Th2 balance and affect hematopoiesis.
Gene-expression networks in ITP
New and emerging molecular technologies have also been applied to investigating ITP. Recently, one such effort profiled whole blood gene expression (the ITP blood “transcriptome”) in adult and pediatric ITP patients.19 This work identified both unique differentially expressed genes and altered gene-expression networks in ITP, although, not unexpectedly, there was heterogeneity between patients. Interestingly, ITP-specific differential gene transcript profiles, specifically from IFN-regulated genes, appeared similar to those reported in other autoimmune diseases, and these signatures were abrogated with therapy, suggesting that these profiles reflected active disease processes. These data demonstrate that the application of system-wide “omics” technologies (ie, RNA, DNA, and protein) offers the potential for new insights into the pathogenic mechanisms of and prospects for identification of novel therapeutic targets in ITP.
Clues to mechanisms from secondary ITP
Observations in secondary ITP, in which an ITP-causative or -associated condition has been identified, may provide clues into common mechanisms underlying ITP in general. As discussed in more detail in a recent review,20 the presentation of secondary ITP is often more complex than in primary ITP. This complexity is due to both heterogeneity of the associated disorders and concomitant risk of thrombocytopenia due to nonimmune causes. This section reviews our current understanding of the pathophysiology of some types of secondary ITP to provide examples of characterized ITP-causative events and ITP-associated immune dysregulation.
Chronic infections: Helicobacter pylori, HIV, and HCV
ITP is associated with several chronic infections, notably Helicobacter pylori, HIV, and hepatitis C virus (HCV) infections (ITP-associated immune modulation in these diseases is broadly summarized in Table 1). Other chronic infections can also be associated with ITP (reviewed in Cines et al20 and Stasi et al21 ).
H pylori is a gastrointestinal bacterium that is often pathogenic in humans, causing chronic inflammation and ulcers and posing a risk of malignancy. Therapies to eradicate H pylori have demonstrated success in treating H pylori–associated ITP, but the rates of success vary in different geographic populations.20 The pathogenesis of H pylori–associated ITP likely includes elements inherent to both the bacterium and the human host. From the side of the H pylori bacteria, there is evidence that H pylori may provoke an autoimmune platelet response via molecular mimicry to Helicobacter antigens such as CagA.20,21 Moreover, some strains of H pylori can induce platelet aggregation and platelet expression of p-selectin and phosphatidylserine.22 From the perspective of the human host, genetic factors such as Lewis type (a carbohydrate blood group antigen system) or HLA type20,21 are associated with H pylori–associated ITP. Therefore, the risk of H pylori–associated ITP appears to be due to a combination of intrinsic host factors, including susceptibility to the infection itself (ie, variation in Lewis antigens at the host mucosal surface), variation in the ability of the host to present of bacterial peptides to the immune system (ie, variation in the individual patient's HLA), and the capacity for molecular mimicry. These factors may in part explain the observed geographic differences in prevalence and treatment successes of H pylori–associated ITP between different populations. In addition to these factors, the immune balance is shifted in the setting of H pylori–associated ITP. For example, monocytes demonstrate increased phagocytic activity and decreased FcγRIIb (an inhibitory FcγR), consistent with a phenotype of accelerated opsonization that returns to normal with successful treatment.6
Similar to the antiplatelet Abs provoked during H pylori infection, HIV can provoke anti-HIV Abs that cross-react with platelet glycoproteins and form immune complexes, as can HCV.4,21 Additional mechanisms of platelet destruction also become apparent from studies in virus-associated ITP. For example, HIV can provoke anti-GpIIIa Abs, which lead to complement-independent platelet fragmentation,23 whereas in HCV, the virus itself can bind platelets directly, leading to circulating anti-HCV Ab-antigen-platelet complexes.20 In both HIV and HCV, suppression of viral replication can result in improvement in thrombocytopenia.20,21 Interestingly, HIV-associated ITP tends to occur early in HIV infection, whereas nonimmune thrombocytopenia tends to predominate in more advanced HIV, when the immune system has suffered greater effects from the infection. One possible explanation is that the immune systems of HIV patients are more capable of developing autoimmunity in the earlier phases of the disease.
Acute infections
An acute infectious event has long been suspected to be a trigger in the initiation of primary ITP. In newly diagnosed ITP, there is often a history of antecedent symptoms that may be attributed to infection in the days or weeks before diagnosis of ITP. In a very minor subset of cases, a pathogen is detected (eg, EBV, influenza viruses, Varicella-zoster virus),3 which then qualifies these cases as secondary ITP. However, in most acute ITP cases, a pathogen is not identified, and the vague constitutional symptoms predating or accompanying the diagnosis of ITP are difficult to distinguish from those which that be expected with inflammation from an ongoing autoimmune process. Therefore, unidentified acute infection remains a plausible candidate to induce ITP either by providing an opportunity for molecular mimicry or similar targeting of the immune system to platelets (as discussed in the “Chronic Infections” section above) or by the mere presence of an acute inflammatory response tipping the balance in a predisposed patient to break tolerance.
Vaccination
For decades, ITP has been known to be a rare complication of the measles-mumps-rubella vaccination. This is likely due to provocation of the immune system by the vaccine antigens in a manner similar to actual infection with these childhood diseases, each of which is also associated with ITP. In a recent study in a large network of managed care organizations, the association between measles-mumps-rubella and increased risk of ITP in young children was confirmed, although overall such events were still rare.24 The investigators also reported a possible (even more rare) increased risk of ITP after hepatitis A, Varicella, or dTap vaccination in older children. These observations are also consistent with a scenario of molecular mimicry and/or immune provocation by specific antigen exposures that tip the immune response to break tolerance to platelets in susceptible persons.
Autoimmune disorders
Patients with systemic autoimmune diseases, such as systemic lupus erythematosus, antiphospholipid antibody syndrome, and rheumatoid arthritis, are prone to developing ITP.25 A diagnosis of secondary ITP in these patients is complex because nonimmune thrombocytopenia due to underlying disease or related therapies is also common. These observations are consistent with the notion that a patient with one autoimmune disease is at high risk to develop a second. The mechanisms underlying the development of many autoimmune disorders, including ITP, is unknown. It may also be that during the immune dysregulation leading to autoimmunity to one self-antigen, there is a risk of immune presentation of (and breaking of tolerance to) other self-antigens. Interestingly, many of the features of immune dysregulation described in ITP, such as the shift in Th1/Th2 balance, increased Th17, and altered Treg profiles described above, are also common to other autoimmune diseases.
Other ITP-associated immune altered conditions
Other conditions commonly associated with ITP are pregnancy26 and malignancies, particularly some lymphoid malignancies.27 Although the mechanisms underlying secondary ITP in each of these cases are unknown and quite distinct, they warrant mention here in that they share a common feature: the immune system is perturbed by the patient's underlying condition before the onset of ITP.
Inherited risks for ITP
A genetic component in ITP has long been suspected to predispose some persons to develop ITP when exposed to a provocative event.
Familial ITP
The hypothesis of underlying genetic risk for ITP is supported by the rare anecdotal and case reports of familial ITP. Affected members of these families present with ITP that meets the clinical criteria for primary ITP, but demonstrates a convincing pattern of inheritance. A 2006 review of the Pediatric and Adult Registry of Chronic ITP (PARC-ITP) found that 10 of 445 (2.2%) of pediatric patients reported a positive family history of ITP.28 However, application of unbiased genomic approaches to the identification of susceptibility genes in ITP, such as family linkage studies or genome wide association studies, have not yet been forthcoming, likely due to the rarity of familial ITP families available for study and the heterogeneity of ITP sporadic cases (reviewed in Bergmann et al29 ). It may be that in the future, characterization of genes by studying familial ITP will lead to important clues into the pathogenesis of more common forms of ITP, similar to the application of the knowledge gained in the study of other rare familial hematologic disorders to their more common sporadic counterparts.30
Candidate genes
Many studies have sought to assess genetic risk for ITP through the study of candidate genes already suspected to participate in the disease process. These efforts have yielded a variety of positive, conflicted, and negative findings. These mixed results may in part be due to the inherent limitations in candidate gene approaches for ITP: these studies were limited to a small number of genes, only detected known DNA variants, and were heterogeneous with respect to the phenotypes of ITP. In addition, many of these studies were also confounded by population demography. The selection of candidate genes for study in ITP has largely focused on immune-related genes for which there is evidence of perturbation in ITP or other autoimmune diseases. The results of several studies of candidate DNA polymorphisms in or near candidate genes was recently summarized.29 These efforts both demonstrate the scientific interest in looking for genetic risks in ITP and, by the nature of the genes selected, highlight the pathways thought to contribute to the pathogenesis of ITP.
The case of FcγR polymorphisms illustrates both the success and difficulties in such studies. Significant associations between ITP and known functional FcγR polymorphisms (FcγRIIa-131H and FcγRIIIa-158V) have been reported (represented in Bergmann et al29 ), and variation in FcγR makes sense in the context of our understanding of the pathophysiology of ITP. However, demonstrating the ITP risk conferred by FcγR gene variants is complicated by highly variable FcγR gene family DNA sequences and DNA structural variation at the FcγR locus.31,32 Furthermore, each of these studies are also likely confounded to some degree by demography.29 These caveats extend to other candidate genes that have been found to have an association with ITP, many of which could largely be classified T-cell related but include other genetic loci of interest, such as HLA.29
Genetic syndromes
Clues to the pathogenesis of autoimmunity may be gleaned by the study of genetic disorders associated with autoimmune disease. Studies of a spectrum of primary immunodeficiency syndromes have implicated defective central and peripheral B-cell tolerance resulting from defects in signaling and apoptosis (central) and interaction with Tregs and circulating factors (peripheral) in autoimmunity.33 ITP is associated with several such genetic syndromes, such as common variable immunodeficiency (CVID), autoimmune lymphoproliferative syndrome (ALPS), and hyper IgM syndrome.34 ITP is common in CVID (10%) and is frequently the first manifestation of the disorder.34 Patients with CVID exhibit immune defects late in B-cell maturation characterized by hypogammaglobulinemia, a normal B-cell count, and variable T-cell phenotype. Interestingly, in one study, the incidence of ITP in CVID was reported to be enriched in patients with mutations in the CVID-associated gene TNFRSF13B (which encodes TACI, a B-cell receptor).35 ALPS is characterized by defective lymphocyte apoptosis (a central defect) and lymphadenopathy, splenomegaly, and hyperlymphocytosis with circulating CD3+/CD4−/CD8− T cells; 23%-34% of ALPS patients also develop ITP, in addition to other autoimmune disorders.34 Inherited syndromes that primarily affect T cells are also associated with autoimmune disease and ITP. Although rare, 80% of partial DiGeorge syndrome (del22q11.2) patients present with a quantitative T-cell deficiency, which is proposed to be due in part to diminished CD4+CD25+ T cells (Tregs) due to altered thymic processing early in life. These patients exhibit a 200-fold risk of developing ITP over the general population, in addition to being at high risk for other autoimmune disorders.36 Wiskott-Aldrich syndrome is another genetic syndrome associated with autoimmunity and ITP, in this case due to mutations in a protein (WASP) known to participate in Treg function and TCR-mediated apoptosis.37 Therefore, patients with a variety of inherited immune deficiencies are at increased risk for autoreactivity and ITP, likely attributable to the inherent imbalances resulting from the immune deficiencies themselves. In addition, these patients are often susceptible to frequent infections, which may serve to provoke ITP in the setting of preexisting immune defects.
In summary, the presence of families with inherited ITP, increased frequency of ITP in some genetic syndromes, and evidence of an association between ITP and several candidate immune genes collectively point to the presence of individual genetic risks for ITP.
ITP therapy
ITP therapies, with the possible exception of thrombopoietin (TPO) receptor mimetics, are thought to be immunomodulatory in mechanism (broadly summarized in Table 1). First-line ITP therapy is generally corticosteroids, which are thought to globally influence the immune system by functionally suppressing T- and B-cell reactivity while inducing tolerogenic patterns in T cells, dendritic cells, and circulating cytokines.38 Even after 4 days of high-dose dexamethasone therapy, IFNγ was decreased, IL-4 was increased, and FcγRIIb on monocytes was increased.7 Frontline ITP therapy can also include Ig therapy (reviewed in Cooper39 ), such as IVIg or anti–D globulin. The mechanism of IVIg is uncertain, but is thought to tip the immune balance back toward tolerance by inducing inhibitory phenotypes in the reticuloendothelial system (FcγRIIb) and possibly by inhibiting complement-mediated cell damage, suppressing B and T cells, and exerting a direct anti-idiotype effect on circulating functional antiplatelet Abs.8 Consistent with this concept, FcγR DNA polymorphisms are associated with IVIg responsiveness,40 which may account for some heterogeneity in responses seen after IVIg. Similar to IVIg, infusion of anti–D globulin into RhD-positive patients (who have not been splenectomized) also likely modulates FcγRs (although likely via FcγRs distinct from IVIg) by blockade of phagocytic cells via anti-Rh Abs bound to RBCs. In addition, anti–D globulin may also modulate immunity by mechanisms similar to those postulated for IVIg: anti-idiotype activity, Fc receptor modulation, cytokine shifts, and down-regulation of phagocytosis.41 For both IVIg and anti–D globulin, recent evidence supports that therapy significantly diminishes the accelerated clearance of platelets in ITP with little effect on platelet production.42
Other second-line ITP therapies are also thought to function largely through an immunomodulatory effect. Splenectomy not only removes a large component of the reticuloendothelial (monocytic phagocytic) “filter,” it also serves to remove a lymphoid organ important for immune function, particularly B-cell development, and is associated with restoration in the diversity of T-cell repertoires.3 Other immunomodulatory therapies may also be used in ITP cases that have failed first- or second-line therapies. For example, rituximab (anti-CD20) in conjunction with corticosteroids predictably decreases B-cell populations but also durably increases Tregs43 and diminishes detectable oligoclonality in T-cell populations.3 Other drug agents, such as Cytoxan, azathioprine, and vincristine, also have known immunomodulatory effects. Conversely, TPO mimetic therapies are thought to interact directly with the TPO receptor on megakaryocytes to stimulate platelet production, and therefore would seem less likely to exert an immune effect per se, although an increase in Tregs has been found with thrombopoietic agents.44 Therefore, most ITP treatments suppress active B cells, T cells, and/or the reticuloendothelial (monocytic phagocytic) system, which likely leads to down-regulation of inflammation and tipping of the immune balance back toward tolerance.
Summary
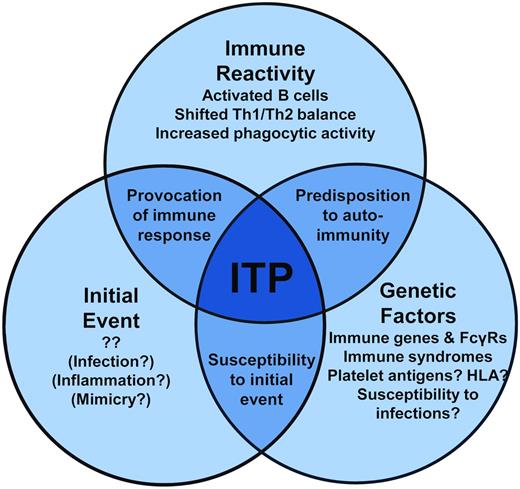
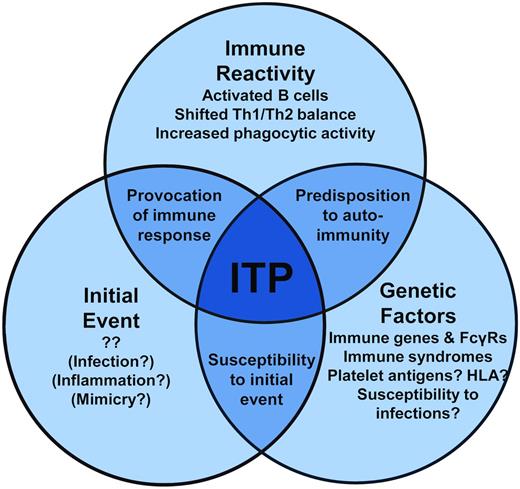
The initial causative event(s) of primary ITP remains unknown, although clues from secondary ITP suggest that specific immune stimuli, such as those that occur during some infections, may instigate a break in platelet tolerance. The immune dysregulation that follows in ITP is becoming clearer. In addition to antiplatelet Ab–mediated clearance of platelets, Abs also may fix complement to platelets, T-cell and cytokine profiles are tipped to type 1 immune responses, and the monocytic phagocytic system appears to be more active. Therefore, the autoimmune attack on platelets (and megakaryocytes) is propagated on multiple fronts. Further, a genetic predisposition to ITP likely plays a role in susceptibility to developing ITP in specific contexts (a model of these events is proposed in Figure 2). Therefore, our current understanding of ITP is one of a complex state of immune dysregulation that, in conjunction with genetic risk factors for ITP, warrants further investigation to better understand the causes of ITP. Although many of these themes may be more reflective of an ongoing autoimmune process than a specific autoimmunity to platelets, these processes present tantalizing targets for therapy to reverse the ITP pathogenic processes and restore the immune balance.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Jill Johnsen, Puget Sound Blood Center Research Institute, 921 Terry Ave, Seattle, WA 98104; Phone: 206-568-2230; Fax: 206-587-6056; e-mail: jillj@psbc.org.