Abstract
Outcome in diffuse large B-cell lymphoma (DLBCL) has improved over the last decade and will likely improve further with the introduction of novel agents. At the present time, clinical prognostic factors are limited in their ability to identify patients with sufficiently poor outcome to justify deviation of therapy away from R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) outside of a clinical trial. Similarly, with the exception of the concurrent translocation of MYC and BCL2, there are no validated biologic markers that can be used to guide initial therapy in routine practice. Recognition of the molecular heterogeneity of DLBCL is of paramount importance and must be taken into consideration when investigating new therapies. It will be vital for novel targeted agents to be evaluated in patient populations enriched for those who are most likely to benefit. The identification of prognostic and predictive biomarkers should be initiated during the early phase of drug development so that these tests can be validated within phase 3 trials. Although currently available techniques such as immunohistochemistry may still be used, gene-expression profiling and whole genomic analytic techniques will likely play a major role in the evaluation of patients in the future to determine optimal personalized treatment for DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL), accounting for approximately 30% of all newly diagnosed cases and more than 80% of aggressive lymphomas.1 It is readily curable, even in the most advanced cases. Whereas DLBCL can be one of the most satisfying lymphomas to treat, it can also be one of the most challenging. It has become increasingly recognized that DLBCL is not one entity, but rather is a heterogeneous group of disorders that, despite having similar morphologic appearance, can have a wide range of clinical presentations, immunologic characteristics, genetic features, and clinical outcomes. Recently, gene-expression profiling (GEP) studies have identified several molecular subtypes within DLBCL with distinct intracellular oncogenic pathways.2–4 The 2008 version of the World Health Organization (WHO) classification of lymphoid malignancies acknowledges this heterogeneity by recognizing a broad category termed DLBCL, not otherwise specified (which contains several molecular and immunohistochemical subgroups), a variety of DLBCL subtypes, and an increasing number of “other lymphomas of large B cells” that have been identified due to their unique clinical and pathologic features.1
Despite our improved understanding of the diversity of DLBCL, management strategies remain remarkably uniform. Standard therapy offers the majority of patients a favorable outcome, making it difficult to deviate toward potentially more toxic options. However, most patients who fail initial therapy will ultimately succumb to their disease. With an increasing number of treatment options available, improved prognostication will be crucial to allow for the possibility of individualized risk-adapted therapy. In addition, further insight into the biologic heterogeneity of DLBCL, together with the development of predictive biomarkers, will be required to select appropriate patients for novel targeted strategies with the goal of achieving a personalized cancer care approach.
A comprehensive review of all clinical and biologic markers reported to be prognostic in patients with DLBCL is beyond the scope of this review. Several excellent reviews on the biology of DLBCL have been published recently.2–4 The present review aims to summarize key findings that may have clinical implications regarding management and to highlight ongoing controversies in this rapidly changing field.
Standard therapy and anticipated outcome
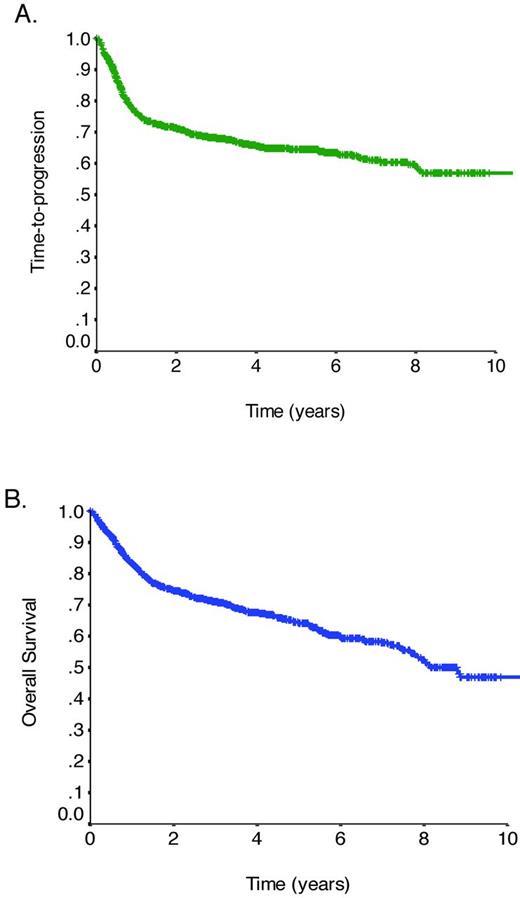
Therapy for DLBCL relies on multiagent chemotherapy incorporating an anthracycline, an alkylating agent plus other agents. The CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) regimen has been the most commonly used combination, because attempts to improve outcomes with more intensive chemotherapy or additional agents have failed to show additional benefit.5 Approximately one decade ago, the addition of the anti-CD20 mAb rituximab to this chemotherapy backbone resulted in a dramatic improvement in outcome.6 Long-term follow-up of the initial phase 3 trial performed in elderly patients (≥ 60 years of age) with advanced-stage DLBCL demonstrated a 16% absolute improvement in the 10-year overall survival (OS) rate with the addition of rituximab.7 Additional trials confirmed the benefit of rituximab, establishing R-CHOP (rituximab plus CHOP) as the standard of care.8–10 Within the province of British Columbia (BC), the introduction of rituximab has improved progression-free survival (PFS) and OS significantly for unselected patients with DLBCL in routine clinical practice.11 Figure 1 demonstrates the time-to-progression (TTP) and OS curves for all patients with DLBCL (N = 1366) treated with R-CHOP with curative intent in the province of BC between 2001 and 2011, revealing an overall cure rate of approximately 60% (unpublished data using the BC Cancer Agency Lymphoid Cancer Database).
TTP and OS in DLBCL. (A) TTP for all patients with DLBCL treated with R-CHOP with curative intent in BC between 2001 and 2011 (N = 1366). (B) OS for all patients with DLBCL treated with R-CHOP with curative intent in BC between 2001 and 2011 (N = 1366).
TTP and OS in DLBCL. (A) TTP for all patients with DLBCL treated with R-CHOP with curative intent in BC between 2001 and 2011 (N = 1366). (B) OS for all patients with DLBCL treated with R-CHOP with curative intent in BC between 2001 and 2011 (N = 1366).
Whereas outcomes in DLBCL have improved with the addition of rituximab, approximately 10%-15% of patients exhibit primary refractory disease (nonresponse or relapse within 3 months of therapy) and an additional 20%-25% relapse after initial response to therapy. Most relapses occur within the first 3 years; however, the slope of the TTP curve continues to trend downward, with approximately 10% of all progressions occurring more than 5 years after treatment.7 High-dose chemotherapy and autologous stem cell transplantation (ASCT) has been shown to provide the best chance of cure for patients with chemotherapy-sensitive relapse12 ; however, due to advanced age and comorbidities, only half of all patients are eligible for such an intensive approach and even when it can be delivered, only a modest minority of patients is cured. The outcome for elderly patients who fail R-CHOP is dismal, with a median survival after progression in the range of 0.7 months.7
The addition of rituximab to the initial management of DLBCL appears to be associated with poorer outcomes after secondary therapies. The Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL) trial, one of the largest studies to date in relapsed DLBCL, compared 2 salvage regimens, R-DHAP (rituximab, dexamethasone, Ara-C, cisplatin) versus R-ICE (rituximab, ifosfamide, carboplatin, etoposide) before ASCT, followed by maintenance rituximab versus observation.13 Although there was no significant difference in outcome between the 2 salvage regimens, the response rate to salvage therapy was much lower in patients previously exposed to rituximab compared with rituximab-naive patients (51% vs 83%, P < .001), which affected 3-year event-free survival similarly (21% vs 47%, P < .001). This difference was particularly notable for patients who relapsed within 12 months of initial diagnosis. Patients with primary refractory disease after R-CHOP present the greatest challenge. A recent review of patients with primary refractory DLBCL after R-CHOP treated at the BC Cancer Agency revealed that less than 10% of patients achieve durable remissions with secondary therapies.14
The minimal difference seen between the TTP and OS curves emphasizes the limitations of salvage therapies for the overall population of patients with DLBCL (Figure 1). There is an important need to identify patients in whom R-CHOP will prove insufficient so that alternate treatment strategies can be considered, and in particular to identify those who may benefit from novel targeted therapies. The question of whether reliable prognostic or predictive markers exist and should be used to guide initial treatment choice deserves consideration.
Clinical prognostic factors
The International Prognostic Index (IPI) was developed as a multicenter international effort before the availability of rituximab, and has been the primary clinical tool used to predict outcome for patients with aggressive NHL.15 Based on the number of negative prognostic features present at the time of diagnosis (age > 60 years, stage III/IV disease, elevated serum lactate dehydrogenase level, Eastern Cooperative Oncology Group [ECOG] performance status ≥ 2, > 1 extranodal site of disease), 4 discrete outcome groups were identified, with 5-year OS ranging from 26%-73%. The utility of the IPI has been reassessed in patients treated with rituximab-based therapy.16,17 Whereas the IPI remains prognostic, the range of outcomes has narrowed substantially, with the 3-year event-free survival ranging from 50% in patients with 4-5 factors to approximately 90% in patients with no risk factors. Currently, the IPI has limited ability to identify a subgroup with sufficiently poor outcome to consider deviating from R-CHOP, because all risk categories have at least a 50% chance of cure.
Recently, additional clinical factors have been identified that may also contribute to outcome after rituximab-based therapy. Maximum tumor diameter greater than 10 cm appears to negatively affect outcome in young patients with good-prognosis DLBCL.18 Male sex may also affect outcome negatively due to more rapid clearance of rituximab compared with female sex.19 A comprehensive review of the implication of BM involvement in DLBCL identified the presence of large cells in the BM (but not discordant small cells) to be an independent predictor of outcome after controlling for the IPI.20 Finally, elevated serum free light chains were identified as an independent predictor of outcome in DLBCL and may reflect tumor burden.21 Although the inclusion of additional clinical factors within future prognostic indices may allow for wider differentiation of outcome, clinical factors remain surrogates for the underlying biologic differences between patients. Whereas clinical tools may be easily applied in routine practice and enable stratification on clinical trials, they do not provide the necessary biologic insight to allow for tailored therapy approaches with novel targeted agents.
Molecular prognostic factors
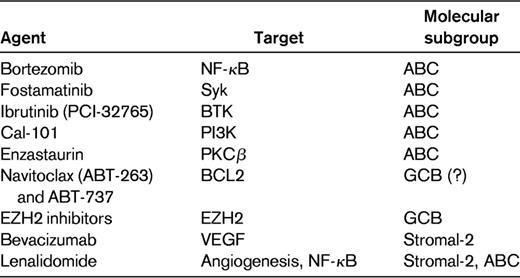
The heterogeneity of DLBCL is highlighted by the variable expression of a variety of molecular aberrations, some of which have been correlated with outcome. Although studies of individual biomarkers have improved our understanding of the pathogenesis of DLBCL, many studies have yielded conflicting results. Reasons for these discrepancies include the retrospective nature of most studies, small patient sample sizes, lack of uniformity in technique, and failure to control for other simultaneous biologic processes that may be confounding outcome. The relevance of biologic markers may be affected by the treatment received, and therefore should be assessed in a population of patients receiving uniform therapy in keeping with the current standard of care. Table 1 lists some of the molecular prognostic markers reported recently in DLBCL patients treated with R-CHOP, most of which still require independent validation.
Role of BCL2
The role of BCL2 provides a nice example of the difficulty of assessing the significance of individual biomarkers in DLBCL. BCL2 is an anti-apoptotic protein that is important in normal B-cell development and differentiation. BCL2 overexpression has been reported in approximately 40%-60% of patients with DLBCL, and has been associated with poorer survival. However, no correlation with survival was seen in patients receiving chemotherapy and rituximab, implying that the addition of rituximab had overcome its negative influence.22 More recently, the prognostic significance of BCL2 expression was evaluated within the context of DLBCL molecular subtypes in patients treated with R-CHOP.23 BCL2 expression was predictive of poorer outcome within the germinal center B-cell (GCB) but not the activated B-cell (ABC) subtype of DLBCL, which is the opposite of what had been noted in patients treated with CHOP alone.24 This finding may be explained by the differential mechanism by which BCL2 expression occurs within the molecular subtypes and the mode of action of rituximab.2 In GCB DLBCL, the presence of a t(14;18) translocation accounts for the overexpression of BCL2 in most (but not all) cases, and the BCL2-associated resistance induced may not be overcome by rituximab. In ABC DLBCL, the t(14;18) translocation is not seen, but rather BCL2 is up-regulated due to gene amplification or the constitutive activation of the NF-κB pathway, which may be down-regulated by rituximab. Therefore, the mechanism of BCL2 expression and its association with other biologic events may ultimately determine its value as a prognostic, as well as a predictive, biomarker.
Limitations of IHC
Immunohistochemistry (IHC) provides a platform with multiple advantages that make it a desirable approach for biomarker evaluation in routine patient care. First, it can be readily performed on paraffin-embedded tissue and on large numbers of patients simultaneously in the context of tissue microarrays. IHC is used primarily to measure protein expression, the end-product of oncogene dysregulation that is likely the major determinant of effect. Finally, the technology is universally available and performed at reasonable cost. However, significant limitations exist due to variability in technical aspects, such as antibodies used, staining techniques, scoring methodology, and cut points selected. A validation study performed by the Lunenburg Lymphoma Biomarker Consortium (LLBC) revealed poor reproducibility among pathologists in assessing routine IHC markers in lymphoma, although this could be improved by optimizing and standardizing procedures.25
More recently, the LLBC evaluated the prognostic significance of a series of IHC biomarkers (BCL2, BCL6, CD5, CD10, MUM1, Ki67, and HLA-DR) in a large cohort of patients with DLBCL treated with R-CHOP.26 Of the IHC markers tested, only CD5 and Ki67 were associated with survival on univariate analysis, and BCL2 had marginal significance (P = .09). The impact of biologic markers was largely overshadowed by the influence of the IPI in predicting outcome. The addition of BCL2 and Ki67 within a combined model of biologic markers and IPI factors allowed for greater discrimination of outcome within the low and low-intermediate/high-intermediate risk groups, but did not allow for better identification of high-risk patients.
Role of MYC
MYC oncogene rearrangement is a hallmark of Burkitt lymphoma and can be identified in approximately 5%-10% of patients with typical DLBCL morphology. Increased expression of the MYC oncoprotein promotes cellular growth and proliferation. Several recent studies have found the presence of a MYC rearrangement to be associated with a poorer outcome in DLBCL patients treated with R-CHOP, with PFS rates at 3-5 years in the range of 30%-35%, which was half that seen in patients without a MYC rearrangement.27,28 The negative impact of a MYC rearrangement was independent of the IPI, and in one study was associated with a higher risk of CNS relapse.28 The presence of a MYC rearrangement could not be predicted based on clinical factors or proliferation rate as measured by Ki67.27,28
Whereas the presence of a MYC rearrangement appears prognostic in DLBCL, recent studies suggest that its clinical significance is influenced strongly by BCL2. Lymphomas that harbor both a MYC and BCL2 translocation have been termed “double-hit,” and have been associated with an extremely poor prognosis, with a median survival of less than 1 year.29 However, most reports have included cases with variable histology, the majority of which were transformed lymphoma or B-cell lymphoma, unclassifiable, with features intermediate between Burkitt lymphoma and DLBCL. The implication of MYC and BCL2 deregulation has been recently examined in DLBCL patients treated with R-CHOP.30 The presence of concurrent MYC and BCL2 translocations occurred in only 5% of cases, but was associated with an extremely poor outcome, with a median survival of approximately 8 months. Overall, 11% of patients in that study had MYC translocations; however, when measured by IHC, 33% overexpressed MYC oncoprotein due to up-regulation by other mechanisms. The negative prognostic impact of MYC protein expression was only seen in patients who coexpressed the BCL2 protein. Patients with dual expression of MYC and BCL2 protein had a poor outcome, with a 5-year survival of less than 40%, which was independent of the IPI. This finding was also demonstrated in a similar study showing that patients with an IHC double-hit score of 2 (dual expression of the MYC and BCL2 proteins) had a significantly poorer outcome compared with patients with a double-hit score of 0 or 1 (expression of neither or only one protein).31
Based on these findings, patients with DLBCL should be assessed for concurrent MYC and BCL2 deregulation at diagnosis. Patients with dual translocations have the worse outcome and should be considered for alternate therapies. Patients with dual protein expression of MYC and BCL2 represent a larger cohort with an unfavorable prognosis; however, as yet, an IHC antibody for MYC has not been validated for clinical use. Optimal therapy for patients with dual expression of MYC and BCL2 should be evaluated within the context of clinical trials in which standardized procedures for MYC and BCL2 measurement are used.
Gene expression profiling
GEP is a powerful genomics technique that uses DNA microarrays to measure simultaneously the expression of thousands of genes, providing a molecular profile of RNA within a biopsy specimen. GEP studies have identified at least 2 molecularly distinct subtypes within DLBCL, not otherwise specified, ABC and GCB, with approximately 15% of patients remaining unclassifiable.32,33 This molecular distinction has prognostic implications. Whereas rituximab has improved the outcome of both GCB and ABC DLBCL, patients with the ABC subtype have an inferior outcome after treatment with R-CHOP (3-year PFS approximately 40% versus 75%, P < .001).34 Due to the lack of a standardized commercially available test and the requirement for fresh-frozen tissue specimens (although paraffin-based methodologies are under development), GEP is not routinely available in patient care. Several IHC-based algorithms have been developed as a practical tool intended to mimic the molecular designation obtained by GEP.35 The Hans algorithm is used most commonly and designates patients as GCB versus GCB-like based on the presence of 3 IHC markers: CD10, BCL6, and MUM1.36 In a recent comparison of several published IHC algorithms, including the Hans algorithm,36 the Choi algorithm,37 and others, the Tally algorithm (based on FoxP1, GCET1, CD10, MUM1, and LMO2) was suggested to be the most robust.35 Unfortunately, these IHC algorithms remain an imperfect substitution for microarray-based GEP,38 because of their inherent oversimplification, the poor reproducibility of IHC, and the fact that unclassifiable patients are also dichotomized. This has resulted in highly discrepant results within the literature regarding their prognostic utility in DLBCL, and at this time, these algorithms should not be used to guide choice of therapy outside of a clinical trial.
More important than its potential prognostic relevance, the molecular subtypes designated by GEP reflect lymphomas that originate from lymphocytes at different stages of differentiation (cell-of-origin) and are associated with distinct oncogenic pathways that may be exploited differentially by novel targeted therapies.2,4 GCB DLBCLs express genes normally detected in GCB cells, such as CD10, LMO2, and the transcriptional repressor BCL6. Approximately 40% of GCB DLBCL cases have a t(14;18) translocation, 30% have c-rel amplification, and 20% have mutations of the histone methyltransferase EZH2, all of which are never seen in ABC DLBCL.
In contrast, ABC DLBCLs typically express genes that are normally expressed in plasma cells. The pathogenetic hallmark of ABC DLBCL is the constitutive activation of the NF-κB signaling pathway, which promotes cell survival, cell proliferation, and inhibition of apoptosis. This is largely due to constitutive activation of the CBM signaling complex (formed by CARD11, BCL10, and MALT1), which in normal lymphocytes is only transiently active after antigen stimulation. In ABC DLBCL, the CBM complex can be activated by different genetic aberrations; approximately 10% harbor activating mutations of CARD11, and in the remaining cases, chronic active B-cell receptor (BCR) signaling engages the CBM pathway. Chronic active BCR signaling is mediated through the BCR (which in 20% of cases, harbors mutations in CD79A or CD79B) and downstream kinases, which include spleen tyrosine kinase (Syk), PI3K, Bruton tyrosine kinase (BTK), and protein kinase C β (PKCβ).
In addition to the molecular distinction by cell-of-origin, GEP studies of DLBCL have identified molecular signatures related to the microenvironment that, when integrated within a statistical model, were shown to be correlated with outcome.34 A prognostically favorable stromal-1 signature reflects extracellular matrix deposition and infiltration of the tumor by macrophages. The less favorable stromal-2 signature identifies tumors associated with a high level of angiogenesis and a high density of blood vessels. The stromal signatures were variably present in both GCB and ABC DLBCL, suggesting that the biologic attributes of the microenvironment could be associated with either subtype. The identification of molecular signatures correlated with nonmalignant cells of the lymphoma raises the possibility that selectively targeting this aspect may offer therapeutic benefit.
Are there effective alternatives to R-CHOP?
At the present time, based on the limitation of clinical factors to identify a very poor risk group and the lack of validated biologic prognostic markers for routine clinical use, deviation away from R-CHOP is difficult to justify outside of a clinical trial. Moreover, a therapeutic alternative that is better than R-CHOP and without substantial additional toxicity has not been firmly established.
Whereas CHOP administered on a 2-weekly schedule (CHOP-14) had demonstrated promise in elderly patients, 2 recently performed randomized phase 3 trials comparing standard 3-weekly R-CHOP-21 and R-CHOP-14 have shown no advantage for R-CHOP-14 and have suggested a higher frequency of toxicity.39,40 A recent population-based study suggested a survival benefit with the addition of etoposide to R-CHOP (R-CHOEP) for young patients with high-risk DLBCL; however, this benefit was not seen in a subgroup analysis of a phase 3 trial in young patients with good-risk DLBCL.41,42 The infusional regimen dose-adjusted EPOCH-R has yielded promising results in a phase 2 trial43 ; however, confirmation of its merit must await results of a completed phase 3 trial comparing it with R-CHOP. In a recent randomized trial, the dose-intensive regimen R-ACVBP was associated with a better OS compared with R-CHOP (3-year OS was 92% vs 84%, respectively, P = .007) in a select population of patients less than 60 years of age with only one IPI risk factor.44 Despite apparent benefit, this regimen has not been used frequently, because its value in a general population of patients with DLBCL remains unclear and because of concern for both acute and delayed toxicity.44,45 In addition, vindesine is not routinely available in some countries.
The value of consolidative ASCT in the initial management of DLBCL has been inconclusive in historic trials and remains controversial.46 Recent studies evaluating its role after response to rituximab-based chemotherapy have yielded mixed results. A large randomized trial comparing standard-dose R-CHOP-14 versus ASCT in the initial management of young patients with DLBCL demonstrated no difference in outcome.47 In a separate trial, conventional-dose R-CHOEP-14 resulted in better outcomes than a high-dose therapy approach (R-Mega-CHOEP) for young high-risk patients with aggressive B-cell lymphoma.48 A recently reported US/Canadian intergroup phase 3 study evaluated the benefit of ASCT after standard-dose CHOP (with or without rituximab) in patients with high-intermediate and high-risk aggressive NHL.49 Whereas the overall trial results suggested an improvement in PFS, but not OS, with ASCT, an exploratory subgroup analysis demonstrated that the benefit was confined to those with a high-risk IPI, in whom both an improved PFS and OS were seen. In view of the benefit of ASCT in the salvage setting, it is likely that a subgroup of patients may benefit from its use upfront. However, the ability to identify this subgroup based on clinical or biologic indicators is limited, and therefore consolidative ASCT cannot be recommended routinely.
Currently, the one subgroup of patients with DLBCL who should be considered for alternate initial therapy outside of a clinical trial is the subset with a concurrent translocation involving BCL2 and MYC (double-hit). Although it remains unclear which alternate approach will prove beneficial, these patients have a remarkably poor outcome after R-CHOP, with a median survival of less than 1 year.30 Consideration of intensive Burkitt-type regimens followed by ASCT, infusional strategies such as dose-adjusted EPOCH-R, or a clinical trial involving novel agents would seem appropriate.
Novel targeted therapies
Whereas prognostic markers offer little utility in guiding the choice of therapy for DLBCL at this time, predictive markers will become paramount in making treatment decisions. GEP studies have led to the discovery of potential therapeutic targets in DLBCL, and this has translated into the development of novel agents with the prospect of higher tumor specificity and lower generalized toxicity. These agents hold substantial promise and may ultimately improve outcome when combined with initial therapy, provide an alternative to initial therapy for patients who are too frail for R-CHOP, or offer effective alternatives for relapsed/refractory patients. However, in view of the molecular heterogeneity of DLBCL and the selective activity of these drugs, it will be vital to evaluate them rationally in patient populations enriched for those who are most likely to benefit. Table 2 lists some of new agents being evaluated in DLBCL, with their postulated target and the preferential subgroup.
Therapies targeting the NF-κB pathway would seem particularly attractive in ABC DLBCL because it is expressed constitutively in this subtype. Direct inhibitors of various components of this pathway are under development. An indirect way to inhibit NF-κB is to block the degradation of IκBα (an inactivating protein of NF-κB) in proteasomes with the use of bortezomib. As proof of principle, patients with relapsed DLBCL were treated with bortezomib combined with EPOCH-R, and a significantly higher response (83% vs 13%, P < .001) and median OS (10.8 vs 3.4 months, P = .0003) were seen in ABC DLBCL compared with GCB DLBCL patients.50 Ongoing trials are currently evaluating the addition of bortezomib to R-CHOP compared with R-CHOP alone for untreated DLBCL, including a phase 3 trial in the United Kingdom that will analyze outcome according to molecular subtype and several randomized phase 2 trials that will enroll exclusively patients with the non-GCB subtype of DLBCL.
Due to the reliance of many B-cell lymphomas on the BCR signaling pathway, novel therapies targeting multiple components of this cascade are under evaluation. Fostamatinib disodium, an orally available Syk inhibitor, demonstrated clinical responses in more than 20% of patients with refractory DLBCL in a phase 1/2 trial.51 PCI-32765 (ibrutinib) is an orally available inhibitor of BTK that demonstrated responses in 29% of patients with DLBCL in a phase 1 trial.52 CAL-101, an oral selective inhibitor of PI3K-delta, is also under evaluation in various lymphomas, although limited data are available in DLBCL.53 PKCβ is downstream from the BCR signaling pathway and has been demonstrated to be highly expressed in fatal/refractory DLBCL.54 Enzastaurin is a selective inhibitor of PKCβ with demonstrated activity in DLBCL.55,56 A phase 3 trial evaluating enzastaurin as maintenance therapy after R-CHOP in high-risk DLBCL has been completed and is awaiting analysis. The trials noted above have not evaluated outcome according to molecular subtype; however, due to its dependence on chronic BCR signaling, ABC DLBCL may be expected to benefit preferentially from these agents. Given the complexity of this signaling cascade with parallel pathways, it is likely that combinations of these agents may yield the best results in appropriate patients as determined by GEP.
Whereas both ABC and GCB DLBCL frequently overexpress BCL2, the mechanism of BCL2 up-regulation is different between the subtypes (see “Role of BCL2”), and a recent trial has suggested that BCL2 remains a negative predictor of outcome after R-CHOP in the GCB subgroup only.23 This raises the possibility that agents targeting BCL2 (eg, ABT-263 and ABT-737) may provide greater benefit in GCB DLBCL.57 Recently, mutations involving EZH2, which encodes a histone methyltransferase, were identified exclusively in 22% of GCB DLBCL cases (and not in ABC DLBCL cases).58 EZH2 inhibitors may represent a novel targeted therapy for patients with this aberration.
Molecular profiling has demonstrated the importance of the microenvironment in DLBCL, suggesting another component that could be targeted for therapeutic benefit. A subgroup of patients with high expression of the stromal-2 signature with high tumor-associated blood vessel density may benefit from strategies targeting angiogenesis.34 Bevacizumab, a mAb targeting VEGF, was associated with limited single-agent activity in relapsed/refractory DLBCL, with some patients exhibiting stability of their disease.59 Unfortunately, a phase 3 trial evaluating the addition of bevacizumab to R-CHOP in untreated DLBCL was closed prematurely due to excessive cardiotoxicity. Lenalidomide, an immunomodulatory agent with pleiotropic activity, including antiangiogenic properties and inhibition of NF-κB, has demonstrated an overall response rate of 35% in relapsed/refractory aggressive lymphoma.60,61 A recent study evaluated retrospectively clinical outcomes after lenalidomide treatment in a cohort of patients with relapsed/refractory DLBCL according to cell-of-origin.62 Interestingly, patients with non-GCB DLBCL had a higher response rate to lenalidomide compared with GCB DLBCL patients (52% vs 9%, P = .006). Several ongoing studies are investigating the role of lenalidomide in untreated DLBCL, including a large phase 3 trial assessing lenalidomide as maintenance therapy in elderly patients responding to R-CHOP.
The evolving literature of novel targeted therapy in DLBCL highlights the potential for a differential effect of these agents in patients whose tumors rely on distinct oncogenic processes. An updated analysis of the CORAL study revealed that patients with GCB DLBCL (but not non-GCB DLBCL) appeared to benefit preferentially from salvage therapy with R-DHAP rather than R-ICE.63 That study demonstrateed that even presumed “non-targeted” agents could have selective benefit in different subtypes of DLBCL based on their mechanism of action.
Conclusions
Outcome in DLBCL has improved over the last decade and will likely improve further with the introduction of novel agents. At the present time, clinical prognostic factors are limited in their ability to identify patients with sufficiently poor outcome to justify deviation of therapy away from R-CHOP outside of a clinical trial. Similarly, with the exception of the concurrent translocation of MYC and BCL2, there are no validated biologic markers that can be used to guide initial therapy in routine practice. Ultimately, outcome in DLBCL is influenced by a complex interplay among the malignant cells, immune cells of the microenvironment, and host constitutional genetics. A better understanding of these individual components and the development of validated tools to measure their relative contributions will be required before more reliable prognostic and predictive indices are available routinely.
Recognition of the biologic heterogeneity of DLBCL is of paramount importance and must be taken into consideration when investigating new therapies. It will be vital for novel targeted agents to be evaluated in patient populations enriched for those who are most likely to benefit. The identification of prognostic and predictive biomarkers should be initiated during the early phase of drug development so that these tests can be validated within phase 3 trials. Whereas the distinction between the molecular subgroups of GCB and ABC DLBCL will likely factor prominently in therapeutic prioritization, there are many shared pathways between the 2 subgroups and off-target effects of agents that will require consideration. Although currently available techniques such as IHC may still be used, GEP and whole-genomic analytic techniques with the capability of identifying individual somatic mutations will likely play a major role in the evaluation of patients in the future to determine optimal personalized treatment.58,64,65 With the large number of agents under investigation, it will be necessary to prioritize drugs that affect key driver pathways in oncogenesis and to combine them rationally to optimize their benefit. Finally, it will be important to foster a culture of enthusiasm among patients in clinical trials so that increased participation will propel further progress.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Roche/Genentech; has consulted for Roche/Genentech, Lundbeck, Celgene, and Amgen; and has received honoraria from Roche/Genentech, Lundbeck, Celgene, and Amgen. Off-label drug use: Possibly will include (uncertain at this time).
Correspondence
Laurie H. Sehn, MD, MPH, Division of Medical Oncology, British Columbia Cancer Agency, Vancouver Clinic, 600 West 10th Avenue, Vancouver, BC, Canada V5Z 4E6; Phone: 604-877-6000; Fax: 604-877-0585; e-mail: lsehn@bccancer.bc.ca.