Abstract
Despite the advanced age at onset, chronic lymphocytic leukemia (CLL) shortens the life expectancy of the majority of newly diagnosed patients. The management of elderly patients with CLL is more complex than that of younger patients due to the greater frequency of comorbidities and functional impairment as well as reduced organ function. Many of the recent advances in the care of CLL patients (prognostication, more intense combination therapy regimens) are of unclear relevance for elderly patients. This review addresses 5 key questions in the management of elderly patients with CLL: (1) why is classifying the “fitness” of CLL patients necessary; (2) what criteria should be used to classify patient fitness; (3) when should elderly patients be treated; (4) how should therapy be selected for elderly patients; and (5) which therapy is best (for this patient)?
Introduction
The last 2 decades have been a time of tremendous progress in the care of patients with chronic lymphocytic leukemia (CLL). Most patients are diagnosed with early-stage disease before developing symptoms after they are incidentally found to have lymphocytosis. Insights into the molecular biology and genetics of the leukemic CLL B cell have not only led to a better understanding of disease biology,1-3 but have also enhanced the accuracy of prognostication4,5 and identified potential new therapeutic targets.6 The development of chemoimmunotherapy (CIT) combining purine nucleoside analogs with the anti-CD20 monoclonal antibody rituximab has improved response rates, progression-free survival (PFS), and overall survival (OS).7,8 With such CIT approaches, a high proportion of patients achieve a minimal residual disease–negative disease state, a depth of remission rarely achieved with historical treatments.9,10
Unfortunately, these advances do not benefit all patients uniformly. CLL is a disease of the elderly, with a median age of onset of ∼ 70 years. According to the Surveillance Epidemiology and End Results (SEER) registry, ∼ 75% of patients are more than 65 years of age at the time of diagnosis. Despite the advanced age at onset, CLL shortens the life expectancy of the majority of newly diagnosed patients,11-13 with survival relative to the age-matched population reduced for those < age 75 at diagnosis, including those with Rai stage 0 disease.13 Although studies suggest that there has been an improvement in survival for CLL patients < age 70 at diagnosis over the past several decades, no improvement has been observed for older patients.11,12
Because most patients have early-stage disease at diagnosis and are observed for several years before starting treatment, the median age at the time therapy is initiated is closer to 75 years. Elderly patients have been underrepresented in previous clinical trials, which has resulted in uncertainty regarding the optimal treatment approaches for these individuals.14 Although select elderly individuals may be able to tolerate aggressive treatment approaches,8 standard fludarabine, cyclophosphamide, rituximab (FCR) CIT cannot be tolerated by the majority of CLL patients who have comorbid health conditions and begin treatment after the age of 70.15 Despite advanced age, efficacious treatment is needed for these individuals because a majority of patients beginning treatment for CLL will die as a direct result of the disease or its complications.16
This review addresses 5 key questions in the management of elderly patients with CLL: (1) why is classifying the “fitness” of CLL patients necessary; (2) what criteria should be used to classify patient fitness; (3) when should elderly patients be treated; (4) how should therapy be selected for elderly patients; and (5) which therapy is best (for this patient)?
Why is classifying the “fitness” of CLL patients necessary?
There are several purposes for classifying fitness level in patients with CLL. The first is to accurately categorize the patient's life expectancy unrelated to CLL (eg, due to other health problems). This information can then be used for patient counseling and to help define the importance of durable disease control when selecting treatment. An 18-month remission in a symptomatic CLL patient is acceptable for an individual with a life expectancy of 24 months due to other health problems, whereas it would be considered inadequate for an individual with a 10-year life expectancy unrelated to CLL.
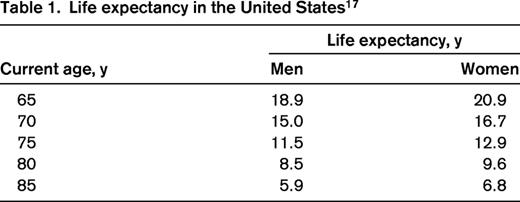
Physicians often underestimate the life expectancy of older adults. For example, the life expectancy of the average 70-year-old man in the United States according to the actuarial calculations of the United States Social Security Office is ∼ 15 years.17 The life expectancy for the average 80-year-old man is ∼ 8 years (Table 1).17 These data illustrate the limitations of using chronologic age alone to estimate survival in patients with CLL.
The second reason to classify fitness is to help determine the patient's ability to tolerate aggressive therapy. A 70-year-old woman with CLL in need of treatment may have an average life expectancy unrelated to CLL of greater than 15 years. Although this implies that the goal of treatment should be durable remission and prolongation of life, it does not necessarily indicate that she will be able to tolerate an aggressive therapeutic regimen that has been developed and tested in younger individuals. Rather than chronologic age, the patient's comorbid health conditions, organ function and the pharmacokinetics (absorption, metabolism, excretion) of the drugs to be used become key considerations in evaluating the suitability of a given treatment approach. This principle is well illustrated by the Cancer and Leukemia Group B (CALGB) 9011 trial, which found that creatinine clearance rather than age was the primary predictor of higher toxicity with fludarabine-based therapy in CLL patients.18
The third reason for classification of patient fitness is to allow more consistent stratification and selection of patients across clinical trials. Historically, trials have primarily used an exclusionary (rather than a stratification) approach declaring patients above an age cutoff or with any decrease in organ function or performance status ineligible.19 These criteria have excluded those with the characteristics typical of real-world CLL patients from participating.20 A stratification approach allows trials to be designed with more liberal eligibility and to use alternative dosing strategies or different treatments for those deemed unable to receive aggressive regimens. A consistent approach to stratification in CLL trials that can also be applied in practice would help oncologists more readily interpret the relevance of trial results to the patient they are evaluating and facilitate more rapid translation of trial results into routine clinical practice.
What criteria should be used to classify patient fitness?
There are currently no standardized criteria by which to define fitness in CLL patients. Although patients were traditionally classified based on age alone, it is now well recognized that chronologic age is not a reliable surrogate for physiologic age or fitness.21 Although organ function, muscle mass, and endurance show a general decline with age, there is wide variability at the level of the individual.
Comprehensive geriatric assessment (CGA) of functional status is one strategy used to assess fitness/frailty more accurately. CGA includes evaluation of function, coexisting health problems, level of social support, cognitive ability, nutritional status, and common syndromes that affect geriatric patients (dementia, delirium, falls, neglect, failure to thrive, depression, polypharmacy).21,22 Although there is limited information on the use of CGA to predict outcomes in patients with CLL, standardized scales to perform CGA are available23,24 and these assessments have been shown to reliably predict the ability of elderly patients to tolerate chemotherapy in other malignancies.25
The number and severity of comorbid health conditions also provides important information regarding patient fitness. A Mayo Clinic study of 373 unselected CLL patients found that 89% of newly diagnosed patients had at least one comorbid condition and 46% had at least one major comorbidity (eg, coronary artery disease, peripheral vascular disease, diabetes mellitus, pulmonary disease [COPD], etc).20 An additional 9% of patients reported having difficulty in performing activities of daily living independently (eg, eating, bathing, dressing, walking, toileting). Although the presence of a major comorbidity affected OS on univariate analysis, it was not a significant predictor on the multivariate analysis adjusting for CLL-specific characteristics (eg, stage).20 This finding underscores that CLL-related characteristics are often the major factor influencing survival even when substantial comorbidities are present. Although it enrolled only fit older patients (performance status 0-2, no severe organ disfunction), the presence of 2 or more comorbid health conditions was still associated with shorter PFS and shorter OS in the German CLL5 trial.16 There are a variety of methods to assess comorbidity,26 of which the Cumulative Index Rating Scale (CIRS)27 and the Charlson Comorbidity Index28 are among the most widely used.
Organ function is a third factor in assessing patients' suitability for aggressive treatment. It is well recognized that renal function decreases with age.29,30 Analysis of > 15 000 adults participating in the National Health and Nutrition Examination Survey (NHANES) found that 38% of individuals > age 70 had an estimated glomerular filtration rate < 60 mL/min/1.73 m2 as measured using the Modification of Diet in Renal Disease (MDRD) equation.30 Common methods to estimate renal function, such as the Cockcroft-Gault method, are heavily influenced by age and lose precision among elderly patients. For example, a study of ∼ 100 000 individuals from Japan found that > 80% of those > age 70 had a calculated creatinine clearance < 60 mL/min/1.73 m2 when estimated by the Cockcroft-Gault method.31 In the Mayo Clinic series of unselected, newly diagnosed CLL patients, ∼ 4% had decreased organ function (ie, total bilirubin > 2 mg/dL or a serum creatinine > 1.5 times the upper limit of normal).20
It should be emphasized that functional status (CGA), performance status, comorbidities, and organ function are related but distinct dimensions. Measuring only one of these dimensions can lead to an inaccurate assessment of overall patient fitness.32,33 Although Eastern Cooperative Oncology Group (ECOG) performance status, the most widely used assessment of patient fitness in oncology practice, has a moderate correlation with more formal CGA instruments,32 it is an inadequate measure of function for most older adults.33,34
Although not yet defined, the optimal method to assess fitness for patients with CLL will likely involve a combination of CGA, comorbidity evaluation, and assessment of organ function (Table 2). Given the importance of assessing fitness/frailty in the management of CLL patients, the International Workshop on CLL (IWCLL) recently launched an international initiative to bring greater consistency to this assessment. It is hoped that this effort will identify standard criteria by which both fit and less fit CLL patients may be selected for trials and that can be used to stratify patients within a trial to evaluate how fitness relates to response and toxicity. This effort will also explore how such criteria may be used to inform therapy selection in routine clinical practice.
When should elderly patients be treated?
The indications to initiate therapy for elderly patients with CLL are generally the same as those for younger patients.35 These indications include cytopenias due to BM failure (hemoglobin < 11.0 g/dL, platelets < 100 × 109/L), massive/symptomatic lymphadenopathy or organomegaly, and disease-related constitutional symptoms (disabling fatigue, unintentional weight loss, drenching night sweats, fevers unrelated to infectious complications).35 Care should be used when applying these criteria in elderly patients because they are at higher risk for having other disease processes that may contribute to fatigue or weight loss (eg, second cancer, malnutrition, neglect, other organ dysfunction). Up to 50% of older patients have been found to have depression,34,36 and patients should be specifically screened for depression and other disorders associated with fatigue (eg, hypothyroid, sleep apnea, anemia from a cause unrelated to CLL) before attributing fatigue to CLL. Although a lymphocyte doubling time of less than 6 months is considered an acceptable indication for treatment,35 treatment on this basis alone is rarely indicated in the absence of other indications for therapy. CLL experts appear to have a greater tolerance for delaying therapy when it is not indicated,37 particularly in elderly patients.13
It is also worth emphasizing several features that are not acceptable reasons to initiate therapy. The degree of elevation in the absolute lymphocyte count, regardless of magnitude, is not an acceptable reason to initiate therapy in the absence of other indications.35 It is also not necessary to initiate treatment for asymptomatic lymph node enlargement or slow, progressive lymphadenopathy. Because treatment increases rather than decreases the risk of infection, frequent or recurrent infections are also not a reason to initiate therapy.35 Rather, patients with frequent or recurrent infections should undergo assessment for hypogammaglobulinemia or other causes of immunocompromise.38,39
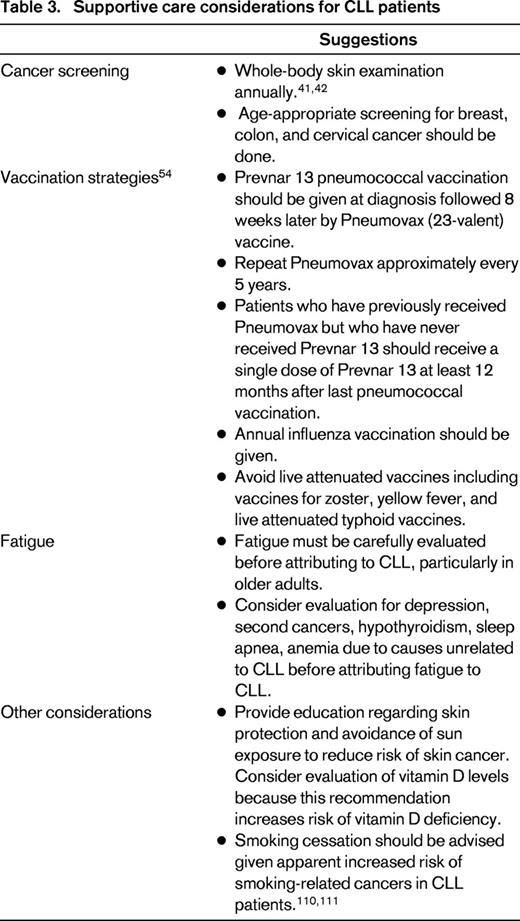
While under observation, older patients should receive aggressive supportive care measures to minimize disease-related complications. This includes age-appropriate cancer screening and regular evaluation for skin cancer specifically.40,41 Given the heightened risk of both melanoma and nonmelanoma skin cancer, patients should also be counseled regarding sun protection and avoidance.42 Approximately 50% of adults are vitamin D deficient43-45 and counseling to avoid sun exposure can exacerbate this problem. Studies from both the United States and Italy suggest that low vitamin D levels may be associated with more rapid CLL disease progression and shorter survival,46,47 a finding that has also been found in other malignancies.48-50 It is unknown whether screening for vitamin D deficiency and/or vitamin D replacement reverses this association. Nonetheless, vitamin D assessment in patients at risk can be considered good general care for elderly patients due to bone health51 as well as to reduce falls52,53 and we routinely perform vitamin D assessment at the time of diagnosis.46,47
Vaccination strategies should also be employed in CLL patients to try to reduce infectious complications.38,39 In the United States, the Center for Disease Control vaccination guidelines (updated in 2012) suggest that all immunocompromised patients, such as those with leukemia, receive initial pneumococcal vaccination with the Prevnar 13 vaccine followed 8 weeks later by the pneumococcal 23 vaccine.54 The pneumococcal 23 vaccine should then be repeated at 5-year intervals. Adult patients who have previously had the pneumococcal 23 vaccine should receive the Prevnar 13 vaccination at least once during their lifetime (administer 12 months after the most recent pneumococcal 23 vaccine).54 Although annual influenza vaccination is also recommended, live attenuated vaccines, including Zostavax, are contraindicated in patients with CLL (Table 3).
Although the genetic and biologic characteristics of CLL B cells have been found to predict outcome in younger CLL patients, there is limited information on the utility of these molecular biomarkers for predicting outcome in older CLL patients.55,56 The Mayo Clinic evaluated the utility of prognostic testing based on age at diagnosis in a cohort of ∼ 2500 CLL patients, including 748 patients age 65-74 and 433 patients age ≥ 75.13 After adjusting for disease stage, IGHV, ZAP-70, and CD38 remained powerful predictors of time to first treatment in patients of all ages, including those age ≥ 75. In contrast, prognostic testing had minimal to no value for predicting OS among patients age ≥ 75 after adjusting for stage.13 These findings suggest that these molecular biomarkers remain relevant to the biology and natural history of CLL in elderly patients, but that competing causes of death may decrease their direct relevance for predicting OS with increasing age.
How to select therapy for older patients
In younger patients without comorbidities, genetic characteristics of the clonal B cells (del[17p13], TP53 gene mutations, del[11q23]) are the primary factor influencing therapy selection. Deep remission (even minimal residual disease–negative remission) is the goal of treatment57 and the treatment strategies offering the best PFS and OS should be prioritized. Based on a series of phase 3 trials,8,58-64 most patients (with exception of those having TP53 defects) should receive an FCR8 or FCR-like65,66 treatment regimen. The German CLL Study Group (GCLLSG) CLL10 trial will determine whether bendamustine and rituximab (BR)67 is an acceptable alternative in these patients. Inclusion of an alkylating agent appears to be important for patients with deletion 11q23.68 Although associated with PFS, this cytogenetic defect does not appear to effect OS when these patients are treated with FCR-like platforms.69 As outlined in the accompanying chapter in this publication by Gribben and Riches,70 suitable young patients with del(17p13) in need of treatment should be debulked and proceed to reduced intensity allogeneic stem cell transplantation.71,72
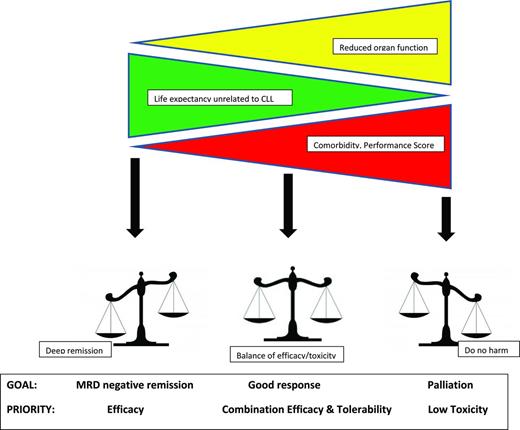
As discussed, the selection of therapy in older patients is more complex because they are more likely to have coexistent health conditions that affect organ function and the ability to tolerate treatment. Establishing the goals of treatment is the first step in therapy selection (Figure 1). Patient characteristics (rather than the characteristics of the treatment regimen) determine the goals of therapy. The combination of performance status, comorbidity assessment, CGA (such as independence with activities of daily living), and organ function (primarily creatinine clearance) are more important factors than age in determining treatment goals.
In patients with a long life expectancy unrelated to CLL and good functional status, deep remission remains an appropriate goal regardless of age. For those with short life expectancy due to other health conditions, palliation is appropriate. Most older patients with CLL fall somewhere in between and require that clinicians balance a variety of considerations when selecting therapy. It must also be kept in mind that, with the exception of allogeneic stem cell transplantation, the current treatments for CLL are noncurative. Although achieving the longest remission duration possible is an appropriate strategy in younger patients, intermediate durations of remission (eg, 3-4 years) with salvage therapy at relapse may be more appropriate for some elderly patients if this approach offers less toxicity and better quality of life. Selecting a too aggressive approach that requires extensive dose reduction or results in discontinuation of treatment may be less beneficial than choosing a better tolerated treatment that allows administration of a full course of therapy.15,73
Which therapy is best (for this patient)?
The fact that a majority of CLL patients have comorbid conditions and greater than half have at least one major comorbidity underscores that therapy selection must be individualized. There is unlikely to be a single treatment regimen that will be an optimal fit for all older patients. After determining the goals of treatment, the best strategy to achieve these goals must be determined. Here the characteristics of the treatment regimen (efficacy, toxicity, mechanism drug elimination/metabolism) and the patient's underlying organ function become substantial considerations. Tables providing an extensive cataloging of the experience of elderly CLL patients treated in past and ongoing clinical trials have been published by multiple authors and will not be reproduced here.14,74-76
The GCLLSG has used the combination of the CIRS assessment of comorbidities and creatinine clearance as useful parameters to identify older patients fit for aggressive treatment approaches. Those with a CIRS score ≤ 6 and preserved creatinine clearance (≥ 70 mL/min) have generally been considered candidates for aggressive therapy independent of age, whereas patients with higher CIRS scores are considered to be less fit and are therefore candidates for less intense treatment strategies.74 A recent report from the Australian CLL Study Group in a cohort of fit CLL patients age ≥ 65 (median age, 72) found no difference in the frequency of grade ≥ 3 adverse events among individuals with a CIRS score of 0-2, 3-4, or 5-6, providing some justification that a CIRS score ≤ 6 identifies elderly patients able to tolerate more aggressive CIT treatment strategies.77
In contrast to the findings of 4 randomized trials in younger patients,58-61 several recent observations have challenged the notion that purine analogs are superior to chlorambucil in all age groups.63,75,78 The CLL5 trial of the GCLLSG randomized patients age ≥ 65 to fludarabine monotherapy or single-agent chlorambucil.16 Although a higher overall (72% vs 51%, P = .003) and complete response rate (7% vs 0%, P = .11) were observed for the fludarabine arm, no difference in the median PFS was observed between groups (19 months for fludarabine and 18 months for chlorambucil, P = .7). Time to treatment failure, however, did favor the fludarabine arm (18 months vs 11 months, P = .004) because ∼ 30% of patients randomized to chlorambucil crossed over to fludarabine before disease progression. No difference in grade 3-4 neutropenia or infection was observed between the arms and a higher proportion of patients in the chlorambucil arm discontinued therapy due to treatment side effects. Greater improvements in quality of life were observed in the fludarabine arm at both 6 and 12 months. Although no difference in OS was observed, interpreting this finding is confounded because patients randomized to chlorambucil were markedly more likely to received salvage therapy compared with those randomized to fludarabine (50% vs 77%, P = .006). Nonetheless, in the absence of evidence suggesting an OS advantage, either a fludarabine- or chlorambucil-based strategy could be considered an appropriate platform from which to build.63,74,78-80 Some favor administering the most active treatment first (when the patient is younger and may be better able to tolerate it), whereas others favor administering the less toxic treatment first (even if it is less efficacious) and salvaging patients at the time of recurrence. It should also be noted that there are a variety of doses and schedules of chlorambucil that have been evaluated and that higher dosing strategies appear to have greater efficacy.81 Higher-dose strategies may also have greater side effects, indicating a trade-off between efficacy and toxicity.
The data on life expectancy at the population level (Table 2) raises concerns that many elderly CLL patients are undertreated. The PFS rates with both fludarabine and chlorambucil monotherapy (< 18 months) are disappointing and inadequate for most patients.16,58,63 A retrospective analysis of 5 CALGB trials found that the addition of rituximab to fludarabine improved OS irrespective of age,79 arguing for the inclusion of rituximab for elderly patients regardless of which chemotherapy backbone is selected. Rituximab has been added to both chlorambucil and fludarabine platforms in efforts to improve disease control.82 A pivotal phase 3 trial by the GCLLSG has now demonstrated that the addition of rituximab to chlorambucil in elderly CLL patients (median age 73) doubles the overall response rate and prolongs PFS (16 months vs 11 months) without increasing the rates of infection.83 This trial also suggested that the third-generation type II anti-CD20 antibody obinutuzumab may have even greater efficacy, with a PFS of 23 months for the obinutuzumab-chlorambucil combination compared with 11 months for chlorambucil alone.83 The fludarabine-rituximab (FR) combination appears to result in even more impressive overall and complete response rates and a median PFS of ∼ 42 months.84,85 The British Columbia experience with FR confirmed this PFS in a community setting in a cohort of 98 patients (27% were age ≥ 70).85 Age did not affect the number of FR cycles administered, the need to stop treatment early, or OS.85
Although it represents a minority of older patients, the German CLL8 experience indicates that select, fit elderly patients with preserved organ function may be able to tolerate aggressive FCR-type therapy.8 On subanalysis, FCR resulted in superior PFS (but not OS) relative to FC therapy among patients age ≥ 65, although it was also associated with greater hematologic toxicity and bacterial infections. The LRF4 trial also found that the more intense FC regimen provided better outcomes than fludarabine or chlorambucil monotherapy in all patient age groups, including those age > 70.63 Data from the Australian CLL study group also support that older patients with preserved organ function and low comorbidity can be treated aggressively.77
Alternative or reduced-intensity versions of FCR-like regimens have also been developed. FCR-LITE decreases fludarabine and cyclophosphamide doses and increases the amount of rituximab administered.86 Although pilot data suggest that this regimen is efficacious, the median age of patients in the pilot trial was 58 years, and 3 of the 7 patients age ≥ 70 had to discontinue treatment early, making the suitability of this regimen for elderly patients unclear.86 Investigators from the Czech Republic have developed an alternative reduced-intensity FCR type strategy with even greater reduction in the fludarabine dose, which was tested in a cohort of fit, older patients (median age 70, median CIRS score = 4). Although these investigators reported the regimen was reasonably well tolerated, the overall response rate was only 70% and data on PFS and OS data are not yet available.87 The pentostatin, cyclophosphamide, rituximab (PCR) regimen is another highly efficacious CIT approach that appears to be well tolerated in elderly patients and those with moderately decreased renal function (CrCl 35-70 mL/min), where age does not appear to affect the number of cycles administered, the number of adverse events experienced, or the efficacy of therapy.73
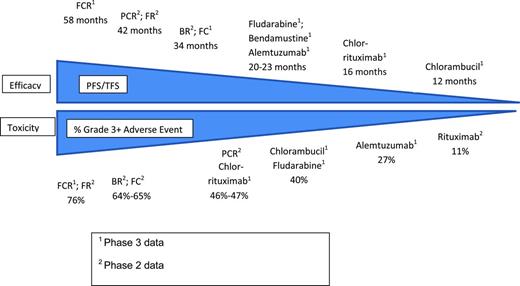
On balance, there remains a fairly strong correlation between efficacy and toxicity with historical CLL treatment regimens (Figure 2). Regimens offering deeper remission and greater PFS tend to induce greater myelosuppression and have more grade 3-4 toxicity. As outlined in the accompanying chapter in this publication by Hallek,88 it is hoped that novel targeted therapies currently in clinical testing (eg, ibrutinib,89 and GS110190 ) may uncouple the link between efficacy and toxicity and provide a wider menu of effective therapy options for older and more frail patients.
The tight link between efficacy and toxicity with historical CLL therapy. This figure is a summary illustrating the link between efficacy and toxicity with historical CLL treatments. It cannot be used to compare regimens directly because results are drawn from across trials with different patient characteristics. In some cases, multiple trials testing the same regimen found slightly different PFS and rates of grade 3/4 toxicity; the representative experience is indicated in such cases.
The tight link between efficacy and toxicity with historical CLL therapy. This figure is a summary illustrating the link between efficacy and toxicity with historical CLL treatments. It cannot be used to compare regimens directly because results are drawn from across trials with different patient characteristics. In some cases, multiple trials testing the same regimen found slightly different PFS and rates of grade 3/4 toxicity; the representative experience is indicated in such cases.
The apparent relative equivalence of fludarabine and chlorambucil for elderly patients74,78 raises the question of whether other therapies that have proven superior to chlorambucil in randomized, controlled trials may also be good platforms upon which to build for elderly individuals. Both bendamustine91 and alemtuzumab92 have demonstrated higher overall and complete response rates as well as longer PFS in randomized comparisons with chlorambucil. Additional studies with these drugs in elderly patients are ongoing.
Nonchemotherapy regimens such as alemtuzumab-rituximab,93-95 lenalidomide monotherapy,96 lenalidomide in combination with rituximab,97 rituximab monotherapy,98 rituximab–GM-CSF,99 and high-dose methylprednisolone ± rituximab100 have also been explored in clinical trials. With the exception of the alemtuzumab-rituximab regimen for patients with nonbulky disease and/or del(17p13) and the methylprednisolone-rituximab regimen, there are currently inadequate data to recommend the routine use of these regimens.
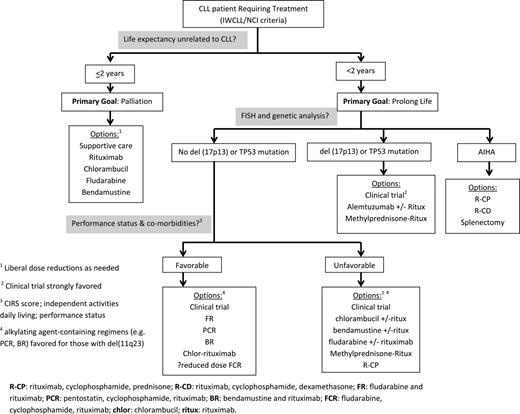
An approach to selecting therapy for elderly patients that considers life expectancy, the result of genetic testing, and performance status/comorbidities is provided in Figure 3. Although a personal interpretation, all regimens listed are considered standard treatment options for CLL patients by current National Comprehensive Cancer Network (NCCN) guidelines. When selecting among different CIT regimens (FR, PCR, BR, reduced-intensity FCR strategies) for more fit elderly patients, subtle trade-offs among tolerability, toxicity, and durability of remission may lead different providers to favor subtly different approaches. All of these strategies could be considered a high-activity approach, where some variation in practice is acceptable.
An approach to selecting first-line therapy for CLL patients age ≥ 70 years.
Conclusion
The management of elderly patients with CLL is more complex than that of younger patients due to the greater frequency of comorbidities and functional impairment as well as reduced organ function. The actuarial life expectancy of 70- to 80-year-old individuals is longer than most physicians estimate. CLL will ultimately become the cause of death for a majority of elderly patients once they progress to the point that they require treatment. Although they may not be able to tolerate regimens of the same intensity as younger CLL patients, fit elderly adults should be treated with highly active CIT approaches with the goal of achieving a reasonable depth of remission and a several-year PFS. Several rituximab-containing CIT platforms fit this profile and are appropriate treatment options. Cytogenetic abnormalities are an important consideration in treatment selection, even among elderly patients. Clinical trials designed specifically for elderly patients are under way and will help to better define optimal management for these individuals. Priority should be given to enrolling patients in these studies, particularly because several novel, targeted therapies currently in testing have the potential to transform the care of CLL patients in the near future.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Genentech, Glaxo-Smith-Kline, Celgene, Cephalon, Hospira, and Polyphenon E International. Off-label drug use: Off-label use of fludarabine, pentostatin, rituximab, ofatumuamb, lenalidomide, cyclophosphamide, ibrutinib, GS1101, and methylprednisolone for treatment of CLL.
Correspondence
Tait Shanafelt, MD, Mayo Clinic, 200 First St, Rochester, MN 55902; Phone: 507-266-1154; Fax: 507-266-4972; e-mail: shanafelt.tait@mayo.edu.