Learning Objectives
To discuss approaches for central nervous system (CNS) prophylaxis in pediatric acute lymphoblastic leukemia (ALL)
To summarize the efficacy of various CNS prophylaxis methods in patients with pediatric T-cell ALL
Clinical vignette
An 18-month-old girl was diagnosed with T-cell acute lymphoblastic leukemia. Her initial leukocyte count was 250 000/μL and her diagnostic lumbar puncture showed 3 WBCs/μL and leukemic blasts on cytospin (CNS2). She achieved a minimal residual disease–negative remission after multiagent induction therapy. Her parents are concerned because cranial radiation therapy (CRT) will be included in her treatment and they ask you if any other therapies can be substituted for it.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy. Stepwise advances in diagnosis, risk stratification, therapy, and supportive care have led to rates of event-free survival (EFS) approaching 85%.1 A critical discovery for improving outcomes was the identification of the CNS as a sanctuary site for ALL. The addition of prophylactic CRT to systemic therapy led to a significant decrease in relapse rates. However, CRT is associated with significant side effects, including cognitive, endocrine, and growth dysfunction, as well as secondary malignancies.2-4 Studies have indicated that patients with B-precursor ALL who are CNS1 (<5 WBC/μL and no blasts on cytospin) or CNS2 (<5 WBC/μL and blasts on cytospin) may be successfully treated without CRT. In these trials, CRT is typically replaced with extra doses of intrathecal chemotherapy and/or medium- or high-dose intravenous methotrexate. For example, the Dana-Farber Cancer Institute (DFCI) ALL Consortium trial 95-01 randomized standard-risk patients [B-precursor, age 2-9 years, CNS1, no mediastinal mass, t(9;22) negative] to cranial radiation plus double intrathecal therapy (cytarabine and methotrexate) or more frequently dosed triple intrathecal therapy (hydrocortisone, cytarabine, and methotrexate) alone.5 Ten-year EFS was not significantly different between the 2 groups (88% CRT vs 80% intrathecal alone). Neuropsychological testing performed 6 years after diagnosis found that cognitive function was in the average range for both patient groups.2 Historically, T-cell ALL (T-ALL) patients have been considered to be at higher risk of CNS relapse and have received CRT as CNS prophylaxis, but some recent trials have omitted CRT for this patient population.
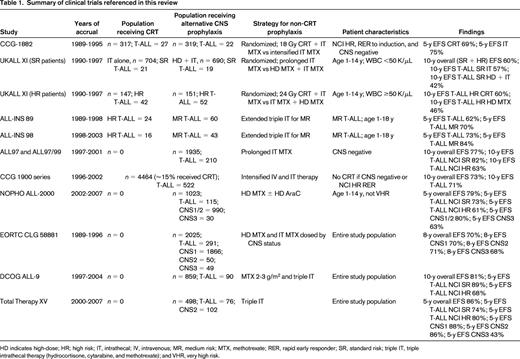
To examine the evidence supporting the omission of CRT in patients with CNS2-positive T-ALL with a high presenting leukocyte count, we reviewed the results of clinical trials conducted since 1990 in children and adolescents with ALL. We performed a PubMed search using the keywords “leukemia” and either “cranial irradiation” or “cranial radiation.” This yielded 1313 results. After limiting the search to the pediatric age group (birth to 18 years), clinical trials, and publication within the last 10 years, we were left with 62 results. We reviewed each publication to identify trials that were opened since 1990, had an objective of omitting CRT, and allowed T-ALL patients to be eligible for omission of CRT. Due to space limitations, we eliminated articles focused solely on neurocognitive function. We determined that 7 articles were appropriate for addressing our question, from which we included an additional 1 article from the bibliographies. In total, we included 8 articles and summarized 11 trials (Table 1). As a point of reference, we also included 2 articles from our PubMed search that reported trials in which CRT was administered to all T-ALL patients.
Outcome of clinical trials on which all T-ALL patients received CRT
On the ALL-BFM 95 protocol (1995-2000), which was conducted by the Berlin-Frankfurt-Munster (BFM) clinical trials group, all T-ALL patients 1 year of age or older received 12 Gy CRT (18 Gy if CNS3).6 The 6-year EFS for the 277 T-ALL patients enrolled on that trial was 75%. All T-ALL patients enrolled on the DFCI ALL Consortium protocol 95-01 (1996-2000) also received CRT (18 Gy), with a 5-year EFS of 82%.7
Randomized comparisons of CRT versus no CRT that included T-ALL patients
Children's Cancer Group (CCG) study 1882 (1989-1995) enrolled 636 high-risk patients (by National Cancer Institute [NCI] criteria) who were either CNS1 or CNS2 at diagnosis and had achieved a rapid early response to induction therapy.8 Patients were randomized to either 18 Gy CRT plus intrathecal methotrexate or intensified intrathecal methotrexate alone. An interim analysis showed a significant difference in 3-year EFS favoring the CRT group. However, with longer follow-up, the 5-year EFS was not significantly different between the 2 groups (69% for the CRT arm, 75% for the no CRT arm).9 In addition, none of the CNS2 patients randomized to the intrathecal-only arm experienced a CNS relapse. Only 49 of the 636 randomized patients had a T-cell phenotype; the outcome of these patients was not reported.
The United Kingdom Medical Research Council protocol UKALL XI (1990-1997) enrolled patients 1-14 years of age and randomized those with presenting WBC <50 000/μL to prolonged intrathecal methotrexate or high-dose intravenous methotrexate plus prolonged intrathecal methotrexate.10 A second randomization allocated patients with WBC ≥50 000/μL to 24 Gy CRT plus intrathecal methotrexate or high-dose intravenous methotrexate plus prolonged intrathecal methotrexate. The overall 10-year EFS for the trial was 60%. For patients with WBC <50 000/μL, there was no difference in outcome based on randomization; T-ALL patients in this WBC group had a significantly worse EFS than the B-ALL patients in this group due to an increase in both CNS and non-CNS relapses. For patients with WBC ≥50 000/μL, there was no significant difference in EFS based on randomization (CRT vs extended intrathecal chemotherapy). The rate of CNS-involved relapses (isolated or combined) was lower in the CRT group, but this was offset by a nonsignificant increase in the number of non-CNS relapses, similar to the CCG 1882 study. For patients with higher presenting WBC counts, there was no difference in outcome by phenotype. The overall 10-year EFS for the 205 T-ALL patients enrolled on UKALL XI was 50%.
Nonrandomized omission of CRT in subsets of T-ALL patients
Stark et al reported the results from 2 consecutive, nonrandomized Israeli National Studies (INS) for patients with T-ALL conducted between 1989 and 2003.11 Both ALL-INS 89 and ALL-INS 98 omitted CRT in favor of extended triple intrathecal therapy in patients with medium-risk disease (prednisone good responders) and administered CRT in high-risk patients (prednisone poor responders and/or M2/M3 BM after induction). The cumulative risk of any CNS relapse for both studies was 3.8%. The overall 5-year EFS rates were 62% and 73% for ALL-INS 89 and ALL-INS 98, respectively. Presenting leukocyte count was not a significant predictor of outcome, although on the ALL-INS 98 trial, patients with initial WBC >100 000/μL received CRT. The outcome of CNS2 patients was not reported.
On the UK ALL97 and 97/99 protocols (1997-2001), only CNS3 patients received CRT.10 The 10-year EFS for the overall study population was 77% and the cumulative risk of any CNS relapse (isolated or combined) was 7%. The incidence of isolated CNS relapse in the ALL97/99 phase of the trial was 4.8% for patients with presenting leukocyte counts >100 000/μL and 3.8% for those with T-ALL phenotype. The UK ALL97 and 97/99 protocols included a randomized comparison of dexamethasone and prednisone. The overall EFS was significantly better with dexamethasone due to a reduction in both CNS and non-CNS relapses, with no evidence of differing effects in any patient subset. Patients randomized to dexamethasone had half the risk of an isolated CNS relapse compared with those randomized to prednisone.
Between 1996 and 2002, 522 T-ALL patients were treated on the CCG 1900 series of trials.9 Risk group status on these studies was based on NCI age/leukocyte count criteria and not immunophenotype. CRT (18 Gy) was administered only to patients with CNS leukemia (CNS3) at diagnosis, or NCI high-risk (NCI-HR) patients who were slow early responders (M3 BM at day 7). Approximately 80% of T-ALL patients were treated as high risk, and the rest as standard risk. Thirty-two T-ALL patients (6%) experienced an isolated CNS relapse. The 10-year EFS for T-ALL patients treated on the CCG-1900 series of trials was 71%.
On the ALL-2000 protocol conducted by the Nordic Society of Paediatric Haematology and Oncology (NOPHO) group (2002-2007), CRT was omitted for all but the highest-risk patients.12 In that study, T-ALL patients with WBC ≥100 000/μL, mediastinal mass, and/or CNS leukemia were allocated to 18-24 Gy CRT (as long as they were 5 years of age or older); patients with WBC >200 000/μL, age >10 years, and/or adverse cytogenetics were eligible for allogeneic stem cell transplantation in first complete remission. The 5-year EFS was 79% for the overall study population, 80% for patients who were CNS1 or CNS2 at diagnosis, and 63% for those who were CNS3. The 5-year EFS for the 115 T-ALL patients was 64%, which was significantly lower than B-ALL patients. Nearly 75% of the T-ALL patients were NCI-HR (age ≥10 years and/or WBC ≥50 000/μL); for these patients, the 5-year EFS was 61%.
Outcome of trials in which CRT was omitted for all T-ALL patients
On the European Organization for Research and Treatment of Cancer (EORTC) Children Leukemia Group (CLG) study 58881 (1989-1996), CRT was omitted in all patients.13 All patients received high-dose intravenous methotrexate and intrathecal methotrexate, and the number of courses of both intravenous and intrathecal methotrexate were stratified by CNS status. Of the 2025 enrolled on the trial, 291 had the T-lineage immunophenotype. T-ALL patients were more likely to present with blasts in the CSF (28% versus 13%). The overall 8-year EFS for all 2025 patients was 70%. There was no significant in difference in EFS based on presence or absence of blasts in the CSF at diagnosis. The outcome of T-ALL patients was not separately reported.
The Dutch Childhood Oncology Group (DCOG) ALL-9 protocol (1997-2004) omitted CRT for all patients, using instead medium- or high-dose intravenous methotrexate plus triple intrathecal therapy as CNS-directed therapy.14 Dexamethasone was also used instead of prednisone. The overall 10-year EFS was 81% with a cumulative risk of any CNS relapse of 3.3%. The 10-year EFS in high-risk patients [defined as WBC ≥50 000/μL, T-cell phenotype, presence of a mediastinal mass, leukemic involvement of the CNS or testicles, t(9;22), or MLL rearrangement] was 68%. Ninety patients on DCOG ALL-9 had T-ALL, 88 of whom were considered high-risk. The 5-year EFS for the T-ALL patients was 72%, which was significantly lower than that for B-ALL patients (P < .01).
Pui et al reported the results of the St. Jude Children's Research Hospital (SJCRH) Total Therapy XV protocol (2000-2007), which enrolled 498 evaluable patients between the ages of 1 and 18 years.15 No patient on that trial received CRT. The overall 5-year EFS was 86% and the cumulative risk of any CNS relapse was 2.7%. There were 76 T-ALL patients on that trial, with a 5-year EFS of 78%. There was no difference in the outcome of T-ALL patients by NCI age/WBC risk criteria. In this study, clinical features associated with a significantly higher risk of isolated CNS relapse included T-cell phenotype, the t(1;19) translocation, or the presence of any blasts in the CSF at diagnosis; however, only high end-induction minimal residual disease and CNS3 status at diagnosis were independent predictors of inferior EFS.
Summary
A review of clinical trials conducted since 1990 suggests that CRT may be safely omitted in at least some T-ALL patients without a significant decrement in EFS, particularly those with non-high-risk presenting features and favorable early response to therapy. In general, CNS2 status is not associated with an increased risk of relapse when CRT is omitted; however, in most published reports, it is not clear if this is true if only T-ALL patients are considered. Heterogeneity between treatment regimens and the paucity of large randomized trials make it difficult to state definitively that CRT can be safely omitted in T-ALL patients with higher-risk features, such as very high presenting leukocyte counts and/or CNS3 status. In addition, although several studies demonstrated relatively favorable EFS in T-ALL patients treated without CRT, intensified intrathecal and/or systemic chemotherapy is not without adverse effects.4 Based on our review, we conclude that there is insufficient evidence to strongly recommend that CRT be omitted for T-ALL patients with higher-risk features (grade 2B). Additional clinical trials are necessary to determine whether omission of CRT reduces toxicity without compromising efficacy in these high-risk T-ALL patients.
Disclosures
Conflict-of-interest disclosures: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Edward Allan R. Sison, Pediatric Hematology/Oncology, Texas Children's Cancer and Hematology Centers, 6701 Fannin St, Suite 1510, Houston, TX 77030; Phone: 832-824-4029; Fax: 832-825-1503; e-mail: ersison@txch.org.