Abstract
Robust evidence remains scarce in guiding best practice in the prevention and treatment of venous thromboembolism in patients living with cancer. Recommendations from major consensus guidelines are largely based on extrapolated data from trials performed mostly in noncancer patients, observational studies and registries, studies using surrogate outcomes, and underpowered randomized controlled trials. Nonetheless, a personalized approach based on individual risk assessment is uniformly recommended for inpatient and outpatient thromboprophylaxis and there is consensus that anticoagulant prophylaxis is warranted in selected patients with a high risk of thrombosis. Prediction tools for estimating the risk of thrombosis in the hospital setting have not been validated, but the use of prophylaxis in the ambulatory setting in those with a high Khorana score is under active investigation. Symptomatic and incidental thrombosis should be treated with anticoagulant therapy, but little is known about the optimal duration. Pharmacologic options for prophylaxis and treatment are still restricted to unfractionated heparin, low molecular weight heparin, and vitamin K antagonists because there is currently insufficient evidence to support the use of target-specific, non-vitamin K-antagonist oral anticoagulants. Although these agents offer practical advantages over traditional anticoagulants, potential drug interaction with chemotherapeutic agents, gastrointestinal problems, hepatic and renal impairment, and the lack of rapid reversal agents are important limitations that may reduce the efficacy and safety of these drugs in patients with active cancer. Clinicians and patients are encouraged to participate in clinical trials to advance the care of patients with cancer-associated thrombosis.
Learning Objectives
To review the evidence and guideline recommendations on the use of anticoagulant therapy for the prevention and treatment of cancer-associated thrombosis
To highlight the evidence and limitations of target-specific oral anticoagulants for the prevention and treatment of cancer associated thrombosis
Introduction
Venous thromboembolism (VTE) is a leading cause of death in patients with cancer. The risk of VTE varies depending on patient-, cancer- and cancer-treatment-related risk factors and is effectively reduced with anticoagulant prophylaxis. Treatment of cancer-associated thrombosis also requires anticoagulant therapy, but the drug choices remain limited and are complicated by heterogeneous clinical settings, high risk of recurrent thrombosis and bleeding, and other comorbidities. This review uses a case scenario to illustrate the knowledge gaps and complexities in prescribing thromboprophylaxis in a hospitalized patient, preventing thrombosis in the ambulatory setting, and treating symptomatic VTE. The evidence available for target-specific non-vitamin K-antagonist oral anticoagulants (NOACs) is emphasized in these settings.
Scenario
A 68-year-old woman presents with a 1-month history of progressive anorexia, fever, weight loss, and night sweats. She was previously well and has a remote history of primary immune thrombocytopenia treated with splenectomy. She is not on any medication. Vital signs are unremarkable except for a low-grade fever at 38.2°C. She weighs 62 kg with a BMI of 25 kg/m2. Physical examination is significant for crackles and dullness at the right posterior lung base, lymphadenopathy in her left groin, and 1+ edema in both ankles. Her abdomen is mildly tender with bloating. Investigations show a leukocyte count of 4.5 × 109/L, normocytic anemia with a hemoglobin of 107 g/L, and thrombocytopenia with a platelet count of 68 × 109/L. Peripheral smear showed postsplenectomy changes, but no RBC fragmentation or abnormal lymphocytes. Her international normalized ratio and activated partial thromboplastin time are normal. Her estimated glomerular filtration rate is 45 mL/min with normal electrolyte results. She has elevated levels of gamma-glutamyltransferase and lactate dehydrogenase at 1.5–2× the upper limit of normal. Computed tomography showed a small right pleural effusion with basal atelectasis, mediastinal adenopathy, and retroperitoneal adenopathy in the portal and periaortic region, with a large mass in the left iliac fossa and inguinal region. She is admitted to hospital for diagnostic workup for probable lymphoma.
Question 1: Is our patient a candidate for inpatient thromboprophylaxis?
Cancer patients admitted to the hospital comprise a diverse group, ranging from patients undergoing surgery, having an acute medical illness, receiving scheduled antineoplastic therapy, or requiring investigative workup or end-of-life care. The risks of VTE and bleeding differ across these settings and, therefore, in-hospital anticoagulant prophylaxis may not be beneficial or necessary in every patient. Few studies have reported on the incidence of these risks and there are no validated risk assessment tools for this patient population. Older, post-hoc data show that ∼10% of cancer patients admitted with an acute medical illness develop thrombosis detected on routine screening,1-4 whereas contemporary data suggest that ∼2% of these patients receiving in-hospital prophylaxis develops major or clinically relevant nonmajor bleeding.5 However, it is not known if the bleeding risk is increased with thromboprophylaxis or if these findings from randomized controlled trials are representative of unselected patients. Therefore, robust evidence is lacking to determine the risk-benefit ratio of in-hospital thromboprophylaxis in cancer patients.
In clinical practice, anticoagulant prophylaxis is given to most patients with cancer when they are admitted to the hospital if they do not have contraindications to anticoagulation.6 This approach reflects the broad recommendations from major evidence-based guidelines. The 2013 update of the American Society of Clinical Oncology (ASCO) VTE Guideline recommends anticoagulant prophylaxis in patients who have active malignancy and are admitted with an acute medical illness or reduced mobility.7 Alternatively, the American College of Chest Physicians (ACCP) 2012 Guideline advocates the use of the Padua prediction score for VTE risk stratification.8 According to this score, patients with active cancer must have one or more additional risk factors to warrant prophylaxis. These factors include older age (≥70 years), obesity (BMI ≥ 30 kg/m2), reduced mobility (bed rest with bathroom privileges for at least 3 days), previous history of VTE, use of hormone therapy, and acute medical comorbid conditions (eg, acute systemic infection).9 The Padua score has not been externally validated and it does not include well-established risk factors in cancer patients such as the tumor site, stage, or status and the type of antineoplastic treatment. Finally, the European Society of Medical Oncology (ESMO) Guideline has the most “restrictive” recommendation, stating that prophylaxis is indicated in hospitalized cancer patients only when they are confined to bed with an acute medical complication.10
For our patient, thromboprophylaxis is reasonable because: (1) the risk of thrombosis is high in patients with lymphoma11 ; (2) she has significant systemic symptoms that may indicate an infection; (3) she likely has reduced mobility; and (4) postsplenectomy status is associated with an increased risk of thrombosis, even beyond the postoperative period.12 The degree of thrombocytopenia and her borderline renal impairment are not severe enough to justify withholding anticoagulant prophylaxis.
Question 2: Which anticoagulant should she receive for in-hospital prophylaxis?
To date, the only evidence available on the efficacy and safety of anticoagulant prophylaxis in patients with cancer hospitalized for medical illnesses comes from post hoc, subgroup analyses of trials that included a small number of selected patients with cancer.1-4 A meta-analysis of these randomized, placebo-controlled trials found that, among patients with cancer, no statistical reduction in the overall incidence of VTE was demonstrated with low-molecular-weight heparin (LMWH) or fondaparinux.13 This negative finding might be due to insufficient power, a skewed selection of low-risk patients, or inadequate suppression of the hypercoagulable state using standard doses of anticoagulant prophylaxis. There are no studies evaluating the efficacy of unfractionated heparin (UFH) for prophylaxis in hospitalized medical cancer patients. Despite this lack of evidence in oncology patients, UFH and LMWH are the most commonly used pharmacologic regimens based on their efficacy in reducing symptomatic deep vein thrombosis (DVT) and fatal pulmonary embolism (PE) in hospitalized, medically ill noncancer patients.14
The direct oral anti-Xa inhibitors rivaroxaban and apixaban have been compared with enoxaparin for extended thromboprophylaxis in hospitalized medically ill patients in the MAGELLAN and ADOPT trials, respectively.5,15 These placebo-controlled trials compared an extended course of NOAC for 30–35 days with a short course of enoxaparin for 7–10 days. Both studies found comparable efficacy between treatment groups during the initial 7–10 days after hospitalization but demonstrated a higher risk of bleeding in the NOAC groups. Results for the cancer subgroup during the hospitalization phase of the studies have not been published. Therefore, the role of NOACs for thromboprophylaxis in hospitalized cancer patients requires further study.
Scenario
Our patient is given LMWH prophylaxis while in the hospital. Investigations confirm she has stage IIIB diffuse large B-cell lymphoma. Infectious workup was negative. Systemic chemotherapy with R-CHOP (rituximab + cyclophosphamide + hydroxydaunorubicin + vincristine + prednisone/prednisolone) is recommended. She wants to start therapy on an outpatient basis at a center closer to her sister. She is discharged after 3 days in hospital.
Question 3: Is extended prophylaxis indicated after hospital discharge in medical patients with cancer?
Although the risk of VTE among hospitalized medical patients is known to persist for weeks after hospital discharge, clinical trials have failed to show a net benefit for extending prophylaxis beyond hospitalization. The EXCLAIM trial, which assessed extended thromboprophylaxis with enoxaparin compared with placebo, reported a reduction of VTE (2.5% vs 4.0%), but found a significant increase in major bleeding episodes (0.3% vs 0.8%).16 In the MAGELLAN and ADOPT trials, the extended use of rivaroxaban or apixaban for 30 or 35 days, respectively, was also associated with more bleeding.5,15 In fact, subgroup analyses from MAGELLAN suggested that better efficacy was achieved with enoxaparin than rivaroxaban among patients with active cancer. In this high-risk patient group, 9.9% of whom received rivaroxaban for 35 days versus 7.4% of whom received enoxaparin for 10 days had a thrombotic event during the 35-day treatment period. Rivaroxaban was also associated with a statistically significant higher risk of major and clinically relevant nonmajor bleeding compared with enoxaparin in cancer patients (5.4% vs 1.7%). This unfavorable safety result, along with the lack of improved efficacy, raises concerns about the use of rivaroxaban and possibly other NOACs in cancer patients. Cancer-patient-specific data from ADOPT have not been published. Therefore, the routine use of extended thromboprophylaxis in cancer patients after discharge from hospital for medical illness is not recommended.
Scenario
The patient is discharged without continuing prophylaxis. Before starting chemotherapy, her blood work shows a leukocyte count of 5.6 × 109/L, hemoglobin of 78 g/L, and platelet count of 77 × 109/L. There is evidence of hemolysis. She reports marked fatigue and has an Eastern Cooperative Oncology Group status of 2. She feels better after getting RBC transfusion. She is informed of the potential side effects of chemotherapy, including the risk of thrombosis.
Question 4: What is the risk of thrombosis while our patient is receiving chemotherapy?
Ambulatory patients receiving outpatient chemotherapy have significant risks of thrombosis.17 A simple risk assessment score has been validated and recommended by the 2013 ASCO Guideline to estimate the risk of thrombosis in the ambulatory setting.7 Using 5 clinical factors (tumor type, leukocyte count, hemoglobin or use or erythropoiesis stimulant agent, platelet count, and BMI), the Khorana score is able to stratify patients into having a low, moderate, or high risk of VTE while receiving outpatient chemotherapy.18,19 Multiple other cohort studies have validated this score, although the absolute rates of VTE vary depending on the tumor population and duration of follow-up.20
One major criticism of the Khorana score is that it does not take into account treatment-related risk of VTE.17 Specific chemotherapeutic regimens are associated with very high rates of VTE. For example, thrombosis occurs in 18% of patients during treatment with cisplatin-based therapy and up to 34% of patients receiving thalidomide-based regimens containing high-dose steroid or chemotherapy for multiple myeloma.21,22 Therefore, routine primary prophylaxis with acetylsalicylic acid or LMWH is recommended in this latter patient group.7,8
Question 5: How effective and safe is outpatient primary thromboprophylaxis? What are the anticoagulant options?
The efficacy and safety for primary prophylaxis in ambulatory patients receiving systemic chemotherapy has been investigated in controlled randomized trials. The largest and most recent trials, PROTECHT and SAVE-ONCO, demonstrated that anticoagulant prophylaxis reduced the relative risk of symptomatic VTE by 49%–64% without increasing the risk of major bleeding in patients with locally advanced or metastatic solid tumors.23,24 However, the risk of VTE in the control groups who received placebo was only ∼4%, and most oncologists do not consider this a high enough risk to justify primary prophylaxis. In contrast, the risk of VTE in patients with advanced pancreatic cancer is up to 25%. Two open-label trials in patients with advanced pancreatic cancer reported dramatic reductions in clinically relevant VTE when therapeutic or half-therapeutic doses of LMWH were given in conjunction with standard chemotherapy. In one study, the risk of fatal PE was also lowered.25 Bleeding was not increased with LMWH. The efficacy of using higher doses of LMWH raises an interesting question about the adequacy of standard prophylaxis dosing in cancer patients. Previous biomarkers studies have shown that higher doses of LMWH result in greater suppression of coagulation activation markers in cancer patients, suggesting that higher doses are needed to attenuate the prothrombotic state related to cancer.26
The NOACs offer an attractive alternative to LMWH prophylaxis. The ABLE study was designed to evaluate the acceptability and tolerability of apixaban in patients with advanced or metastatic malignancies without thrombosis.27 In this phase 2, double-blind, randomized trial, apixaban or placebo was given to patients receiving first- or second-line chemotherapy. Patients were randomized to receive placebo or apixaban at 5, 10, or 20 mg once daily beginning within 4 weeks of the start of chemotherapy and for the duration of 12 weeks. The primary outcome of major or clinically relevant nonmajor bleeding occurred in 6.5% of the patients who received apixaban and in 3.4% of those in the placebo group. There were no fatal bleeding events. Three patients (10.3%) in the placebo group and none in the apixaban groups developed symptomatic DVT or PE.
Overall, the findings suggest that anticoagulant prophylaxis is effective in reducing thrombotic complications in ambulatory patients receiving chemotherapy without increasing the risk of bleeding.28 However, meaningful benefit is likely limited to patients with a high risk of thrombosis and higher doses of anticoagulants may be needed in some patients. The 2013 ASCO Guideline does not recommend the routine use of thromboprophylaxis in ambulatory cancer patients receiving chemotherapy, but it can be considered on a case-by-case basis for high-risk patients without contraindications for anticoagulation.7 The ACCP 2012 Guideline suggests using LMWH or low-dose UFH in outpatients with solid tumors with any of the following additional risk factors provided they have a low risk of bleeding: previous VTE, immobilization or the use of hormonal therapy, angiogenesis inhibitors, thalidomide, or lenalidomide.8 This Grade 2B recommendation, based on little to no evidence, translates into considering primary prophylaxis to a large number of patients (eg, women on tamoxifen for adjuvant therapy for breast cancer). Ongoing randomized trials in patients with a high Khorana score will provide strong evidence if primary prophylaxis offers net benefit in these patients with a higher risk of thrombosis.
Scenario
Given her anemia, preexisting thrombocytopenia, and the cost of LMWH, our patient and her oncologist decide against primary prophylaxis. After 3 cycles of chemotherapy, a restaging CT scan reveals a good disease response, but incidental PE involving several segmental and subsegmental pulmonary arteries is reported. The patient reports shortness of breath for 2 weeks but denies symptoms of DVT. Anticoagulation with LMWH is recommended. She is wants to know if alternative therapy is possible.
Question 6: Are NOACs acceptable treatment options for her cancer-associated thrombosis?
Based primarily on the results of 3 open-label, randomized controlled trials comparing LMWH with vitamin K-antagonist (VKA) therapy, monotherapy with LMWH for the treatment of cancer-associated thrombosis is recommended by major evidence-based guidelines.7,10,29 Among the 3 trials, the CANTHANOX trial investigated enoxaparin, the CLOT trial investigated dalteparin, and the LITE trial investigated tinzaparin; only the CLOT trial found statistically superior efficacy with dalteparin over VKA therapy. The relative risk reduction of recurrent symptomatic thrombosis with LMWH therapy is ∼50%.30
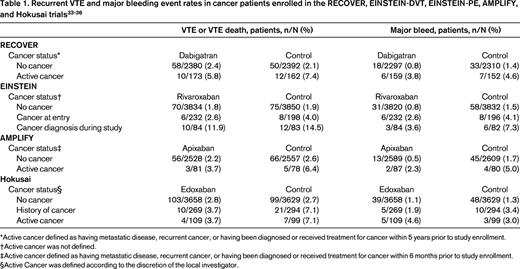
However, the disadvantages of LMWH, including the need for daily injections and the high cost, have prompted off-label use of NOACs for treating cancer-associated thrombosis. Whether NOACs are efficacious and safe for this indication is currently unknown. Dabigatran, rivaroxaban, apixaban, and edoxaban have all been shown to be noninferior to conventional therapy with a heparin followed by VKA for treatment of symptomatic proximal DVT and PE, but only a small proportion and highly selected patients with cancer were enrolled.31,32 The detailed characteristics of this subgroup from each trial have not been published, but it is apparent that the definition of cancer and active cancer differed among these trials (Table 1). For example, the RECOVER trial defined active cancer as having metastatic or recurrent cancer or being diagnosed with cancer or having received treatment for cancer within 5 years before study enrollment, whereas in the Hokusai trial, patients with any history of cancer were included in the cancer cohort along with patients who were considered to have active cancer according to local investigators. Table 1 summarizes the published data from cancer patients treated with NOACs compared with VKA.33-36 Given the small number of patients with active cancer enrolled, these studies were underpowered to show noninferiority or superiority of the NOAC in the cancer patient subgroup.31 Importantly, without information on the time-in-therapeutic range for the VKA-treated patients and the baseline prognostic characteristics such as tumor type and stage, treatment duration, and mortality, it is not appropriate to interpret the available information as evidence of efficacy or safety for NOACs relative to VKA-based therapy.
In addition to the lack of supportive data, NOAC use is problematic in cancer patients for other reasons. Given that cancer patients have higher risks of recurrence and bleeding than patients without cancer, the efficacy and safety outcomes observed in noncancer patients should not be applied to cancer patients. There is also a lack of experience on how to manipulate these agents around invasive procedures and in the setting of thrombocytopenia. Oral administration is not ideal in patients with significant nausea, vomiting, or diarrhea, which are common in those receiving chemotherapy. Renal impairment and hepatic metastases may affect drug clearance. Most importantly, potential drug interactions with chemotherapeutic agents can be complicated. Although these agents have far fewer drug interactions than warfarin, their uptake and clearance are critically dependent on the P-glycoprotein transport and CYP3A4 metabolic pathways. Because many chemotherapeutic agents are moderate or strong inhibitors or inducers of these pathways, therapeutic anticoagulant levels of NOACs might not be achieved.37 In addition, because the anticoagulant effect of NOACs cannot be reliably measured and their therapeutic ranges have not been established, it is difficult to determine whether a significant drug–drug interaction has occurred and if that could lead to thrombosis or bleeding. Lastly, head-to-head comparison with LMWH has not been performed, but one randomized trial comparing rivaroxaban and dalteparin has just started (the select-d study: http://controlled-trials.com/ISRCTN86712308). Indirect evidence from the negative results associated with target-specific inhibition to prevent VTE in the setting of mechanical heart valves (thrombin inhibition with dabigatran was associated with higher risks of thrombosis and bleeding compared with warfarin) and catheter-related thrombosis (factor Xa inhibition with fondaparinux was associated with higher risk of thrombosis compared with enoxaparin) does raise the provocative question of whether Xa and IIa inhibition by LMWH offers superior efficacy over target-specific inhibition of only one of these proteases.38,39 Therefore, until cancer-specific randomized controlled data are available, it is prudent to avoid NOACs for the treatment of cancer-associated thrombosis.7,40
Our patient is receiving R-CHOP. Doxorubicin induces P-glycoprotein and is a weak inhibitor of CYP3A4, vincristine and cyclophosphamide are weak inhibitors of CYP34, and prednisone is a weak to moderate inducer of CYP3A4. Therefore, it is difficult to know whether concomitant administration of these chemotherapeutic agents with a NOAC will lead to a net increase or decrease in the plasma levels of the anticoagulant. Her anemia and thrombocytopenia further heighten concern when a rapid reversal agent is not available if she presents with bleeding. LMWH remains the best therapeutic option for our patient based on the available evidence.
Summary
Much remains uncertain about best practice in the prevention and treatment of VTE in cancer patients because of the lack of cancer-patient-specific data. Published guidelines offer recommendations that must be interpreted and implemented with careful consideration of the individual patient's risk of thrombosis, bleeding, cancer status, and treatments, as well as patient preference. Clinical and translational research is ongoing to bridge the knowledge gaps. Until cancer-specific studies in VTE prevention and treatment are available to provide sound evidence of efficacy and safety, the use of NOACs in patients with cancer is strongly discouraged.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Bristol-Myers Squibb, Pfizer, and Eisai; has consulted for Bayer, Bristol-Myer Squibb, Daiichi Sankyo, LEO Pharma, Pfizer; and has received honoraria from Avivia, Bayer, Boehringher Ingelheim, Bristol-Myer Squibb, LEO Pharma, Pfizer, and Sanofi Aventis. Off-label drug use: Target-specific oral anticoagulants for the prevention and treatment of cancer-associated thrombosis.
Correspondence
Agnes Y.Y. Lee, MD, MSc, FRCP(C), Vancouver Coastal Health Vancouver General Hospital, British Columbia Cancer Agency, Department of Medicine, University of British Columbia, 2775 Laurel St, 10th FL, Vancouver, BC V5Z 1M9, Canada; Phone: 604-875-4592; Fax: 604-875-4696; e-mail: alee14@bccancer.bc.ca.