Abstract
Thrombotic complications associated with the use of central venous catheters (CVCs) are common and lead to distressing patient symptoms, catheter dysfunction, increased risk of infections, long-term central venous stenosis, and considerable costs of care. Risk factors for catheter-related thrombosis include use of larger, multilumen, and peripherally inserted catheters in patients with cancer receiving chemotherapy. Symptomatic catheter-related thrombosis is treated with anticoagulation, generally without removing the catheter. The intensity and duration of anticoagulation depend on the extent of thrombosis, risk of bleeding, and need for continued use of a CVC. To date, the clinical benefit of prophylactic doses of anticoagulant has been disappointing and these agents are not used routinely for this purpose. This chapter focuses on recent evidence, remaining controversies, and practical approaches to reducing the burden of thrombosis associated with CVCs.
Learning Objectives
To identify the types and consequences of catheter-related thrombotic complications in patients with CVCs
To develop an approach to the management of CVC-related thrombosis
To review the evidence on the prevention of CVC-related thrombosis
Introduction
Central venous catheters (CVCs) are indispensable components of therapy in many cancer patients and in those undergoing hemodialysis, parenteral feeding, plasmapheresis, or administration of certain drugs. They are used in most critical care patients, in more than 25% of hospitalized non-intensive care unit patients, and in many outpatients for the infusion of IV fluids, blood products, antibiotics, and chemotherapy agents, as well as for blood sampling. However, there is considerable uncertainty about the risks, treatment, and prevention of catheter-related thrombosis (CRT) because of substantial study heterogeneity and a paucity of rigorous clinical trials on its management. Furthermore, approaches to the prevention CRT have been disappointing. This chapter focuses on the association between CVC and thrombosis, especially on knowledge acquired since the 2012 ASH Education Book.1 Thrombosis associated with hemodialysis lines, noncatheter venous devices, and in pediatric patients are not discussed.
Pathophysiology of CRT
Thrombosis associated with a CVC can be classified into 3 types: pericatheter sheath (“fibrin sleeve”), thrombotic occlusion of the catheter lumen, and mural thrombosis, either superficial (SVT) or deep vein thrombosis (DVT). Insertion of a CVC produces local venous injury at the access site. Deposition of fibrin on the thrombogenic catheter surface and the subsequent in-growth of smooth muscle and endothelial cells are universal and begin within hours of insertion.2 This pericatheter sheath grows along the catheter from the venotomy site. Blood flow is reduced up to 60% around the CVC, which leads to further cellular adhesion to the catheter and vein walls.3 Ongoing movement of the catheter within the vein produces endothelial erosions and triggers the development of mural thrombi, which encroach on the lumen until there is occlusion of the vein. Occasionally, a catheter-tip thrombus will create a ball valve phenomenon that impedes withdrawal of blood from the catheter while instillation of fluids remains possible.
Incidence of CRT
The presence of an IV catheter is by far the most common cause of upper extremity DVT (UEDVT).4 The reported rates of CRT vary widely depending on study design, patient selection, type and location of the catheter, duration of follow-up, modality of detection [symptomatic only or routine screening with Doppler ultrasound (DUS) or contrast venography], and definition of events (DVT only or including SVT and catheter occlusion). Among 25 studies of CVC, the rates of asymptomatic DVT were 41% when venography was used to screen patients and 19% with DUS.5 Contrast venography in 114 patients 1 week after placement of a CVC detected DVT in 53%, but only 3% were occlusive.6 Over the past 2 decades, although the use of CVCs has increased dramatically, the risk of CRTs per catheter has decreased, perhaps related to less thrombogenic catheters and improved insertion techniques.7,8
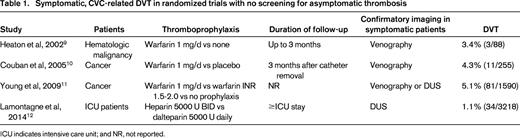
Although the majority of asymptomatic CRT cases remain subclinical, symptomatic DVT occurs in 1%-5% of patients with a CVC (Table 1).9-12 A prospective study of 444 cancer patients with a CVC reported symptomatic, ipsilateral DVT in 4% at a median of 30 days after insertion.13 This corresponds to an incidence of 0.3 per 1000 catheter-days. Another prospective study in 2014 patients with a peripherally inserted central catheter (PICC) identified DVT at a similar rate of 3%.14 Among 3218 critical care patients randomized to low-dose heparin or low-molecular-weight heparin (LMWH) as thromboprophylaxis, only 1% developed symptomatic catheter-related DVT during their intensive care unit stay.12
Central venous implanted ports
Although implanted ports are commonly used in cancer patients for administration of long-term chemotherapy, few studies have addressed the risk of DVT associated with such devices. Among 400 cancer patients with a newly implanted port followed for a median of 1 year without any thromboprophylaxis, symptomatic ipsilateral DVT was diagnosed in 4.5%.15
PICCs
The use of PICCs has increased substantially in recent years because they are easy to insert and remove at the bedside by nurse CVC teams, the risk of early complications is very low, and they can be readily used outside of the hospital.16 However, PICCs are associated with an even greater DVT risk than centrally placed catheters.14,17-21 A review of 11 studies in almost 4000 patients found that PICCs were associated with a 2.6-fold greater risk of thrombosis than other types of CVC (P < .0001).19 This may be related to their longer length, greater catheter-to-arm vein diameter, and increased mobility of the catheter with subsequent endothelial injury.3 In patients with a PICC (as with other CVCs), there is a major disconnect between asymptomatic DVT detected by screening and symptomatic DVT. Among 332 patients randomized to 1 of 2 PICCs who underwent DUS at catheter removal or at 28 days, thrombosis was detected in 72%, whereas symptomatic DVT occurred in only 4%.20 In another study of 2056 PICCs placed over 1 year, symptomatic UEDVT and SVT were detected in only 2.6% and 2.0%, respectively.18
Risk factors for CRT
Risk factors for CRT can be divided into those related to the catheter or its insertion and factors related to the patient (Table 2).14,17-19,22-25 Features of the CVC associated with increased rates of CRT include: PICC > centrally inserted catheter > implanted port,14,17-19 jugular > subclavian,24 size of the catheter relative to the size of the vein,3,14,20 and position of the catheter tip.1,26 Among 184 critical care patients, placement of a CVC in the jugular vein was associated with a 6.8-fold higher rate of CRT per 1000 catheter-days than subclavian access.24 A prospective study demonstrated that the use of a multilumen PICC was associated with a significantly greater rate of CRT than a single-lumen catheter (3.0% vs 1.9%).16 Patients whose CVC tip was above the proximal superior vena cava (SVC) were 7 times more likely to develop CRT than those whose tip was in the SVC near the right atrium.26 Patient factors associated with increased rates of CRT include the presence of cancer,14,19,20,25 previous history of DVT,14,17 and systemic or catheter-related infection.5 Cancer, particularly extensive or metastatic disease, has consistently been shown to be one of the strongest risk factors for CRT.19,20,25 A systematic review of 64 studies evaluated the risk of UEDVT in 29 503 adults who had a CVC.19 The mean rates of arm vein thrombosis were 4.9% overall, 6.7% in patients with cancer, and 13.9% in patients admitted to critical care. A systematic review of 10 studies of 1000 cancer patients with a CVC found that those with Factor V Leiden or prothrombin gene mutation had a >4-fold greater odds of CRT.27
Risk factors for CRT*
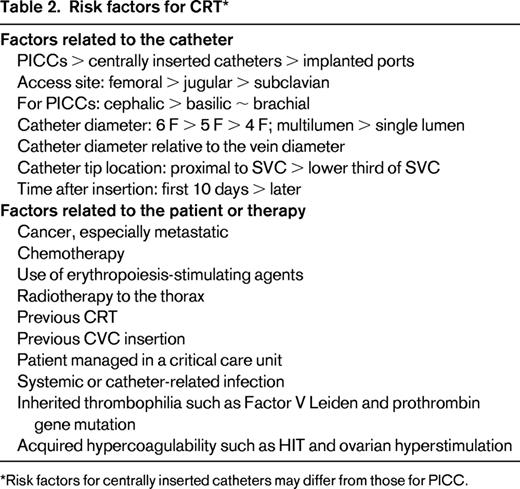
Risk factors for centrally inserted catheters may differ from those for PICC.
Clinical manifestations of CRT
The majority of thrombi associated with CVC are asymptomatic.20 Patients with CRT may have localized swelling, pain, tenderness, and erythema along the course of the involved arm or neck vein, especially when the thrombosis arises in a superficial vein. For patients with DVT, there may be ipsilateral swelling of the arm and tenderness over the course of the affected brachial, axillary, or internal jugular vein. If the thrombosis occludes the innominate vein or SVC, there is often face and neck swelling, headache, and hoarseness. Visible collaterals involving the upper chest wall or shoulder area frequently develop with occlusion of the subclavian or innominate vein. If thrombosis involves the catheter tip, it may not be possible to withdraw blood and/or to infuse fluids and there may be leaking at the access site. Infection at the insertion site should be considered because this increases the risk of concomitant CRT and influences management.5
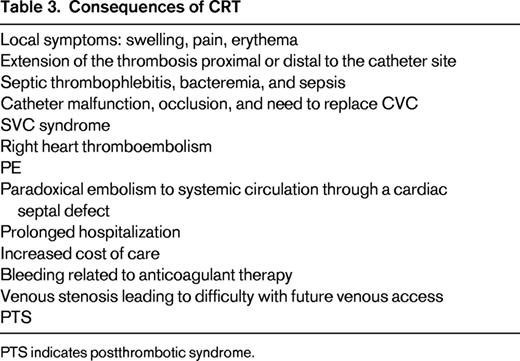
Consequences of CRT (Table 3)
Clearly, CRT may lead to arm or neck symptoms, and catheter occlusion produces catheter malfunction. Although pulmonary emboli (PE) have been reported in patients with CVC and in those with CRT, there is no direct evidence that CVCs are associated with an increased rate of PE compared with seriously ill patients without a CVC. I believe that the risk of clinically important PE due to CRT is very low and rarely results in hemodynamic compromise. Among 12 studies comparing PICCs and CVCs in almost 4000 patients, no PEs were reported in any study.19 Among 252 patients with proven CRT from 4 studies, no PEs were diagnosed during follow-up.23 However, chronic venous stenosis associated with CVCs is common and compromises future vascular access.28 This is particularly important in patients with cancer and in those requiring long-term parenteral nutrition or hemodialysis.
The biofilm layer around and in the lumen of CVCs provides a nidus for bacterial growth. Microorganisms from a skin source or due to bacteremia from another site often colonize the pericatheter sheath and CVC lumen.5 The presence of CRT predisposes to bacterial colonization and to catheter-related sepsis.29,30 This is particularly important in patients with cancer or other serious disorders. Conversely, it appears that sepsis also predisposes to CRT. Ultrasound screening detected CRT in 71% of patients with staphylococcal bacteremia.31 Although postthrombotic syndrome (PTS) has been reported to occur frequently after a UEDVT, PTS appears to be less common after CRT and most patients recover without any sequelae. Severe PTS is uncommon in adults with CRT and is usually related to persistent occlusion of the subclavian or innominate vein.32 In my experience, both PE and PTS related to CVCs are rare events.
Investigation of suspected CRT
Because asymptomatic CRT is relatively common and the benefit of treating such thrombi is uncertain, patients should not be routinely screened for CRT or investigated for minor arm symptoms. The physical examination alone is neither sensitive nor specific for CRT.4 The diagnostic modality of choice for most patients with suspected CRT is compression DUS, which has high sensitivity and specificity.4,33 Compression of the subclavian and innominate veins is not possible; therefore, direct visualization of thrombus around the catheter and absence of color flow with distal compression or respiratory variation are necessary to identify DVT in these veins. The use of contrast CT or MRI should be considered in patients with a high clinical suspicion of intrathoracic CRT and normal or nondiagnostic DUS.
Treatment of CRT
The primary objectives of treatment of CRT are to reduce symptoms, prevent extension into more central veins or into the intracranial venous sinuses, and to prevent chronic venous occlusion. However, there are no randomized trials of acute or long-term therapies for CRT.23,34,35 The first 2 issues to consider in patients with CRT generally are whether thrombolytic therapy should be used to reestablish central venous flow and whether the CVC should be removed. Most patients with CRT do well with anticoagulation alone.36 However, chronic occlusion of the central veins compromises future venous access and may have a substantial negative impact on the quality of life for such patients.28 Therefore, our threshold for considering catheter-directed, local thrombolysis is lower in patients with intrathoracic venous occlusion and those who will need long-term central venous access such as those requiring hemodialysis, parenteral nutrition, or ongoing chemotherapy. Although there are no prospective studies, catheter-directed thrombus reduction therapy for extensive, acute UEDVT is technically simple, highly successful, and safe.4,23,34,37 In patients with an acute CRT, even if the line is removed, anticoagulation is still required and reinsertion of another CVC at a new site is usually needed. There is no evidence that removal of the catheter improves outcomes.23,34,36 Therefore, it is not our practice to have the catheter removed unless it is no longer needed, is nonfunctional, or may be infected.
Among cancer patients with CRT, the use of LMWH is attractive because there is evidence of its effectiveness in other cancer-associated VTEs, LMWH allows flexibility in the dose to try to balance effectiveness and safety, and does not require laboratory monitoring.23,34 Kovacs et al prospectively studied treatment of symptomatic CRT in 74 cancer patients using full-dose dalteparin with conversion to warfarin (target international normalized ratio, 2-3).36 During 3 months of follow-up, no patient had new VTE and 57% of catheters were still functional (although 5% developed major bleeding). For patients without cancer, treatment options include LMWH overlapping with an oral vitamin K antagonist, LMWH alone or, increasingly, an oral inhibitor of factor Xa or thrombin. The oral direct inhibitors of factor Xa (apixaban, rivaroxaban) and thrombin (dabigatran), shown to be effective and safe in the treatment of lower extremity DVT and PE, have not been evaluated in CRT. However, they are also likely to be effective in these patients although clinicians should carefully consider renal and liver function, possible drug interactions and need for interruption for invasive procedures or thrombocytopenia in decision-making particularly in cancer patients with CRT.
For CVC-related SVT, application of a topical anti-inflammatory agent such as diclofenac is likely to be effective in reducing symptoms. An intermediate dose of LMWH (50%-75% of a therapeutic dose) is very likely to prevent extension of SVT into the deep veins with a low risk of bleeding.
The optimal duration of anticoagulation for CRT has not been subjected to clinical trials. Factors to consider in the treatment of CRT include: (1) the thrombosis has been provoked by the CVC; (2) the risk of progression remains high as long as the CVC remains in place; (3) patients with previous CRT are at increased risk for recurrent venous thrombosis if anticoagulant is stopped and a new CVC is placed; and (4) if the CVC is no longer needed and is removed, the risk of recurrent venous thrombosis is low if some (unknown) period of anticoagulation is provided. The most recent ACCP guidelines recommend anticoagulation for 3 months if the CVC has been removed and for as long as the catheter is in place if longer than 3 months.34 For thrombi restricted to the arm, my practice is to anticoagulate, usually with intermediate doses of LMWH, for approximately 2 weeks after the catheter has been removed. For axillary, subclavian or more proximal DVT, I generally treat for 3 months if the CVC has been removed. If the CVC remains in place, I continue anticoagulation at full- or intermediate-doses until after the catheter has been removed.
Management of thrombotic occlusion of a CVC
For patients with thrombotic occlusion of the CVC who have an ongoing need for central venous access, the management options include removal and replacement of the nonfunctional catheter or instillation of a thrombolytic agent to relieve the occlusion.30 The second approach is more attractive and less costly. A mechanical cause of catheter occlusion such as kinking should be excluded first. Instillation of a thrombolytic agent into the catheter is highly effective in clearing CVC occlusions without causing bleeding.30,37-39 For example, one or 2 doses of alteplase 2 mg or tenectaplase 2 mg instilled into the occluded catheter with a dwell time of 1-2 hours will relieve 80%-90% of catheter occlusions.37-39
Prevention of CRT
Multiple reviews of strategies to try to prevent CRT are available.1,8,23,35,39-41 There is evidence that selecting the smallest catheter for the purpose and placement of the catheter tip in the SVC just above the right atrium reduces the risk of CRT.16,17,23 Flushing CVCs with heparinized saline has not been shown to reduce line occlusion, CRT or catheter-related infections compared with saline flushing or no flushing.42,43 Several modifications in catheter composition, coating and flushing solution are currently being investigated to determine whether CRT and infection can be decreased.
Anticoagulant thromboprophylaxis has been evaluated in multiple randomized trials of patients with CVC, leading to several recent systematic reviews.8,23,39-41 A meta-analysis of 15 randomized trials comparing anticoagulant thromboprophylaxis to no prophylaxis in 1714 patients with CVC found a significant benefit of anticoagulant prophylaxis both for asymptomatic (13.5% vs 27.4%, P < .0001) and symptomatic CRT (3.1% vs 5.4%, P = .04).40 However, a more recent systematic review, restricted to CVC in 3611 cancer patients, found that both prophylactic heparin and low dose vitamin K antagonist showed only a nonsignificant trend toward a decrease in symptomatic DVT.8 The largest study of thromboprophylaxis in CVC randomized 1590 cancer patients undergoing chemotherapy to adjusted-dose warfarin (international normalized ratio, 1.5-2.0), fixed-dose warfarin (1 mg/d), and no prophylaxis.11 Symptomatic CRT was less frequent in the patients given adjusted-dose warfarin than in those who received no prophylaxis (2.7% vs 5.9%, P = .019), but major bleeding was more common (3.4% vs 0.2%, P < .001). Warfarin 1 mg/d was not protective but was still associated with increased risk of major bleeding. Analyses that pooled trials of prophylactic LMWH found a nonsignificant trend toward greater efficacy in preventing asymptomatic DVT compared with no prophylaxis without increased bleeding.9,26 The most recent study randomized patients receiving chemotherapy through a CVC to warfarin 1 mg/d, 1 of 3 LMWHs, or no prophylaxis for 3 months; DUS was performed at 90 days.44 Anticoagulant prophylaxis was associated with fewer asymptomatic DVTs (8.1% vs 14.8%, P = .04) and symptomatic DVTs (1.1% vs 6.7%, P = .003), but more bleeding, and there were no significant differences between warfarin and LMWH.
There is no evidence that any antithrombotic therapy reduces catheter-related infection or death in cancer patients. There are also no randomized trials of anticoagulant prophylaxis in patients with PICCs or studies using the new oral anticoagulants as thromboprophylaxis in patients with CVC.
In summary, research suggests that the use of prophylactic doses of LMWH is not very effective at preventing CRT, but is safe, and recent clinical practice guidelines recommend against the routine use of any anticoagulant thromboprophylaxis in patients with a CVC.23,35,39,41 However, for patients at particularly high risk for CRT, for example, those with previous CRT, consideration can be given to using higher doses of anticoagulant as prophylaxis, although there are virtually no data to support this approach, which may also increase bleeding risk.11
My personal approach to the detection and management of CRT
■ Patients with a CVC are not screened for asymptomatic DVT.
■ Patients with proven CRT do not have the catheter removed unless: (1) the catheter is no longer required, (2) it is not functional, or (3) there is suspicion of line-related infection.
■ Patients with superficial arm vein thrombosis (cephalic or basilic vein) are generally treated with intermediate-dose LMWH (50%-75% of a therapeutic dose) for as long as the line remains in place plus an arbitrary 2 weeks after removal.
■ Patients with a more extensive CRT (axillary, subclavian, innominate, SVC) who do not have a high bleeding risk are treated very much like a provoked leg DVT, with therapeutic anticoagulation for at least 3 months and longer if the CVC remains in place.
■ Patients with active cancer and adequate renal function are generally given LMWH at the full therapeutic dose or a reduced dose, depending on the extent of the thrombosis and current and anticipated bleeding risk.
■ For noncancer patients with CRT, I generally use the oral factor Xa inhibitor rivaroxaban rather than LMWH alone or LMWH overlapping with warfarin.
My personal approach to the prevention of CRT
■ Avoid a CVC unless the benefits outweigh the risks.
■ Select the optimal CVC type for the patient based on the expected duration of need, frequency of access, and infection risks.
■ Select the smallest catheter and the least number of lumens appropriate for its intended function.
■ Minimize catheter insertion trauma by using an experienced vascular access team that follows a central line checklist and uses ultrasound guidance, if possible.
■ Place the catheter tip just above the cavo-atrial junction.
■ Use thromboprophylaxis appropriate for the patient's VTE risk (but generally not specifically trying to prevent CRT).
■ In patients at particularly high risk of recurrent CRT, I consider using an anticoagulant at a dose greater than the usual prophylaxis dose if this is acceptable based on the patient's bleeding risk.
■ Practice meticulous infection prevention.
■ Remove the CVC when it is no longer needed.
Conclusion
CRT is a relatively common and potentially serious complication associated with the widespread use of CVCs. The principal adverse effects of CRT are arm symptoms, catheter dysfunction, catheter-related infection, and chronic central venous occlusion. There is little evidence that CRT can be prevented with usual prophylactic doses of anticoagulant. DVT related to CVC is managed with anticoagulation for at least as long as the catheter is in place and longer if the intrathoracic veins are involved. Among hospital physicians, there are substantial knowledge gaps related to optimal CVC use and to the management of CRT. Each hospital should develop a local CVC practice guideline and implement active quality improvement strategies to reduce the burden of CVC complications.
Disclosures
Conflict-of-interest disclosure: The author has consulted for Bayer Healthcare, Boehringer Ingelheim, and Leo Pharma and has received honoraria from Bayer Healthcare, Leo Pharma, Pfizer, Sanofi, and Glaxo SmithKline. Off-label drug use: I will discuss the role of anticoagulants and thrombolytic agents in the management of CRT and the possible role of anticoagulants in the prevention of CRT.
Correspondence
William Geerts, MD, Thromboembolism Program, Sunnybrook Health Sciences Centre, 2075 Bayview Ave, Room D6 74, Toronto, ON M4N 3M5, Canada; Phone: (416)480-4427; Fax: (416)480-4186; e-mail: william.geerts@sunnybrook.ca.