Abstract
Several advances have occurred over the last 2 years in the clinical management of venous thromboembolism (VTE), as evidenced by several high-profile publications in top-tier medical journals. The translation of the knowledge gained into routine clinical practice is an important challenge so that VTE is managed optimally and established and new anticoagulants are used effectively and safely. This chapter reviews issues of VTE treatment from acute management to treatment of long-term complications, addressing new data gained in the last 2 years and putting them into a clinical context, with the goal of improved everyday VTE management.
Learning Objective
To be able to incorporate new clinical data into the decision making when treating a patient with VTE
Introduction
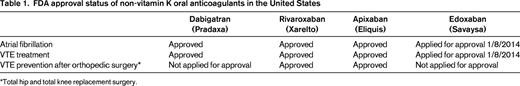
A variety of developments have occurred over the last 2 years that have had a significant impact on our clinical management of venous thromboembolism (VTE). The key developments are: (1) the creation of several treatment guidelines and guidance documents that provide clinicians with evidence-based recommendations on state-of-the-art VTE management1-7 ; (2) publication of a study (the PEITHO trial) that helps to define further the role of thrombolytic therapy in patients with pulmonary embolism (PE)8 ; (3) the ongoing ATTRACT trial, which will help to clarify which patients with deep vein thrombosis (DVT) to consider for thrombolytic therapy9 ; (4) the approval of several of the non-vitamin K oral anticoagulant (NOAC) drugs for the treatment of VTE10 (Table 1); (5) US Food and Drug Administration (FDA) approval of a 4-factor prothrombin complex concentrate (Kcentra) for major bleeding on warfarin and urgent warfarin reversal before surgery based on 2 recent studies11,12 ; (6) 2 studies (WARFASA and ASPIRE trials) suggesting that aspirin is mildly effective in decreasing the risk of recurrent VTE in patients with unprovoked VTE who have been treated with a standard course of anticoagulation13-15 ; (7) publication of a clinical trial (the SOX trial) showing that wearing compression stockings after an episode of acute DVT does not decrease the occurrence of postthrombotic syndrome (PTS)16 ; (8) the further recognition that progestin-releasing intrauterine devices (IUDs) appear to be contraceptive methods without thromboembolic risk and may thus be suitable contraceptive methods for women at increased risk for VTE17,18 and the recent approval by the FDA of a small, progestin-releasing IUD (Skyla) suitable for nulliparous women; and (9) the creation of tools that help health care professionals educate patients with VTE.19 These various treatment issues are discussed in this chapter in a clinical-practical context.
Guidelines
Several good, recent guidelines and guidance statements for the treatment of VTE exist, providing solid, evidence-based recommendations on the state-of-the art management of VTE. These include publications from the American College of Chest Physicians (ACCP; 2012),1 American Heart Association (AHA; 2011),2 International Society on Thrombosis and Haemostasis (ISTH; 2012),3 British Committee for Standards in Haematology (BSH; 2011),4 and British National Institute for Health and Clinical Excellence (NICE; 2012).5 Which of these guidelines to use in clinical practice is a matter of personal preference. I typically consult the ACCP 2012 guidelines.1,20,21 A user-friendly summary of the ACCP 2012 guideline, referred to as a “Quick Reference Guide,” has been published by the ACCP and is easily available online22 ; in addition, a succinct review has recently been published.23 Comprehensive best-practice advice has also been created regarding specific topics relevant to VTE, such as anticoagulation management,7 thrombophilia testing,6,24,25 management of thrombosis at unusual sites,26,27 inferior vena cava (IVC) filters,1,6 VTE in pregnancy,21,28,29 and VTE in patients with cancer.1,30,31 Easy and comprehensive internet access to these guidelines is available through a guideline portal of the web-based VTE information resource Clot Connect.32
Outpatient versus inpatient management
Once diagnosed with DVT or PE, safe outpatient management of many patients with DVT and PE is possible. Several scores have been developed (and were recently reviewed33 ) to assess PE patients' risk for poor outcome in the weeks after the PE diagnosis, but most were not developed for the determination of whom to treat on an outpatient or inpatient basis, but rather as tools to assess risk of good or poor outcomes from the PE. The HESTIA criteria, recently evaluated in a multicenter prospective cohort study, were developed for inpatient/outpatient management decisions and use common sense parameters to determine hospital admission need34 (Table 2). Noteworthy are the data suggesting that some patients with right ventricular dysfunction can be safely treated as outpatients.34 Therefore, right ventricular dysfunction by itself is not necessarily a reason to treat a patient as an inpatient. A rough practical guide for deciding whether to admit a patient or not may be this: if the patient with DVT or PE can walk into the office or emergency department, then he/she can walk out of it and be treated as an outpatient. Several common sense additional considerations flow into decision making, such as a patient's comorbidities, degree of social support, and ease of access to outpatient medical care.
HESTIA criteria34

If at least one of the questions is answered with “yes,” then the patient should be admitted to hospital.
HIT indicates heparin-induced thrombocytopenia.
Thrombolytic therapy
DVT
A recent study of 209 patients with proximal DVT showed that catheter-directed thrombolytic therapy decreases PTS, but at the expense of major bleeding.35 An ongoing study (the ATTRACT trial) will further clarify the benefit and risk of catheter-delivered mechanical thrombectomy with or without thrombolytic therapy.9 Until the study results are published (668 of planned 692 patients were enrolled as of September 29, 2014; completion of enrollment is expected in the second half of 2014; end of patient follow-up 2 years later), it seems fair to conclude that pharmacomechanical thrombectomy may be useful in patients with proximal lower extremity DVT who have significant leg symptoms, duration of symptoms of <14 days, and are at low risk for bleeding. However, individualized decisions need to be made.
PE
The anatomical extent of PE on imaging studies and physiological consequences (ie, right ventricular dysfunction or myocardial injury) often do not correlate well; it is the latter that is the predictor of a poor outcome and is therefore used for risk stratification. PE is categorized into 3 groups: (1) “massive PE,” PE with hemodynamic instability; (b) “submassive PE,” PE with right heart dysfunction based on imaging (cardiac echocardiography or CT angiography) or elevated cardiac enzymes (troponin or brain natriuretic peptide; and (3) “low-risk PE,” PE with normal cardiac enzymes and normal right ventricular function.2 Thrombolytic therapy is clearly indicated only in patients with hemodynamic compromise/instability (ie, hypotension). In all others, the benefit of thrombolytic therapy is questionable. The recent PEITHO trial of the sickest of patients with submassive PE (normotensive, yet with right ventricular dysfunction plus myocardial injury by positivity of serum cardiac enzymes) showed no mortality benefit of thrombolytics by day 30.8 In addition, thrombolytics led to a higher rate of intracranial and other major bleeding.8 Unfortunately, no assessment of long-term pulmonary damage [chronic thromboembolic pulmonary hypertension (CTEPH)] was included in the study report, so it is not known whether thrombolytics have a positive effect on that outcome. One can speculate that thrombolytics may be useful in patients with submassive PE who are at low risk for bleeding, but this is not known at this point. Therefore, at present, it seems fair to limit thrombolytic to patients with PE and hemodynamic compromise and consider it, non-evidence based, for patients with submassive PE (right heart strain plus cardiac enzyme positivity) who are at low risk for bleeding.
Anticoagulant drug choice
Two anticoagulant drug management choices exist once a diagnosis of DVT or PE has been made: (1) warfarin with a parenteral anticoagulant for at least the first 5 days and until the international normalized ratio (INR) is therapeutic or (2) one of the NOACs. Both options should be discussed with the patient; a table listing pros and cons as a discussion aid is available.19 A NOAC is a good choice in many patients, but not all (Table 3). It is typically one of convenience of drug therapy, not of superior efficacy. An additional possible benefit of apixaban and rivaroxaban is less major bleeding; furthermore, a lower composite of major bleeding and clinically relevant non major bleeding was seen with apixaban compared with low-molecular-weight heparin (LMWH)/warfarin in the initial treatment of VTE (AMPLIFY). However, it is unclear whether the safety and efficacy data from these controlled clinical trials translate to a similar benefit (ie, effectiveness) in real world practice. Therefore, the conclusion of the author is that choosing a NOAC over LMWH/warfarin is typically a choice of patient convenience.
Use of NOACs in patients with VTE

*In the apixaban trial (AMPLIFY), major bleeding and composite of major bleeding and clinically relevant nonmajor bleeding was less with apixaban than with LMWH/warfarin; in the rivaroxaban trial (EINSTEIN), major bleeding with rivaroxaban was less than with warfarin in the PE trial, but the same between the two treatment arms in the DVT trial; in the dabigatran trial (RECOVER), major bleeding with dabigatran and LMWH/warfarin was similar.
The 6 acute VTE phase 3 trials (EINSTEIN-DVT and EINSTEIN-PE for rivaroxaban; RECOVER I and II for dabigatran; AMPIFY for apixaban; and Hokusai-VTE for edoxaban) have recently been discussed in detail elsewhere.10,36,37 Rivaroxaban and apixaban were used in the phase 3 clinical trials without the need for initial LMWH bridging. Dabigatran and edoxaban, however, were used only after initial 5-10 days of LMWH therapy, so should not be used immediately from day 1 onward in acute VTE treatment. The cost of the drug therapy chosen depends on a patient's insurance status and should be clarified before prescribing LMWH/warfarin or a NOAC.
The NOACs as a possible treatment alternative should be mentioned to and discussed with any patient on long-term warfarin for a history of VTE, but is a particularly attractive option in the patient with widely fluctuating INRs and one who reports a high “warfarin hate factor” (further discussed below in the section entitled “Patient preference or ‘warfarin hate factor’: the author's approach”). Again, choosing a NOAC over warfarin is typically a choice of convenience, not superior efficacy or safety. There are 4 published phase 3 VTE extension trials (comparison of NOAC with placebo: EINSTEIN-EXTENSION for rivaroxaban; RESONATE for dabigatran; and AMPIFY-EXTENSION for apixaban). A comparison of NOAC with warfarin (REMEDY for dabigatran) has recently been discussed in detail elsewhere.10,36,37 Because no head-to-head comparison between the NOACs exists, it is a matter of personal choice between physician and patient which drug to choose. A patient's renal function, preference of once daily (rivaroxaban) over twice daily (dabigatran, apixaban) dosing, FDA approval status of the drug, familiarity/comfort of a health care professional with the drugs, and cost of the drug for the patient factor into which of the individual NOACs to use.
NOAC management
Interruption for procedures
It is not known at what residual drug level surgeries can be safely performed without undue bleeding risk in patients taking one of the NOACs. In the absence of clinical data directing providers how soon before surgery to stop anticoagulation, the anticoagulant drug's half-life is often a parameter used to decide when to stop the drug: 2-3 half-lives for surgical procedures with standard risk for bleeding or 4-5 half-lives for high-risk bleeding procedures. The literature reports half-lives of 14-17 hours for dabigatran, 7-11 hours for rivaroxaban, 8-14 hours for apixaban, and 5-11 hours for edoxaban.10 However, given that these half-life data are mainly derived from healthy volunteers and may have a wider spread in patients and the fact that wide interindividual NOAC plasma drug level ranges occur with some, if not all of the NOACs,38 it is not clear at this point when exactly to stop the drugs before surgical procedures. Close attention needs to be paid to renal function because an impaired glomerular filtration rate leads to a prolonged half-life of the NOACs, particularly of dabigatran. Published recommendations, such as to stop NOACs 24 hours before procedures with a standard risk of bleeding and 2-4 days before high-risk bleeding procedures in patients with normal renal function,39 may be appropriate for now, but may have to be modified in the future as we learn more about interindividual differences in drug half-lives and plasma drug levels. At this point, it seems reasonable to stop a NOAC 24-36 hours before procedures with standard risk of bleeding and 2-4 days before high-risk bleeding procedures in patients with normal renal function, with possibly longer discontinuation before procedures for dabigatran given its longer half-life and known wide interindividual plasma drug level spread.
Major bleeding
No meaningful clinical data exist on the basis of which evidence-based recommendations for the management of major bleeding on NOACs can be made. A management approach is presented in a condensed, practical way in the 2014 ASH Quick Reference Guide on Antithrombotic Therapy,40 which includes usual hemostatic measures, giving oral charcoal for recently ingested drug, and considering nonactivated prothrombin concentrate (PCC), activated PCC (Feiba), or recombinant factor VIIa. Hemodialysis can be considered in the dabigatran-treated patients; apixaban, rivaroxaban, and edoxaban are not dialyzable. Whether antifibrinolytic drugs (aminocaproic acid, tranexaminc acid) are beneficial is not known.
Several antidotes for the NOACs are in clinical development. Andexanet (PRT064445) is a recombinant, modified factor Xa molecule used to directly reverse factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban). It binds the anticoagulant drug, thus making the patient's own factor Xa molecule available to participate in the hemostatic process. A phase 2 healthy volunteer study is ongoing to evaluate Andexanet's ability to reverse the effects of several anticoagulant drugs on laboratory tests (www.ClinicalTrials.govidentifier#NCT01758432). Idarucizumab (BI 655075) is a humanized mouse monoclonal antibody fragment directed against dabigatran generated from mouse monoclonal antibody against dabigatran. A phase 3 study of patients on dabigatran with major bleeding or needing emergency surgery is under way (www.ClinicalTrials.govidentifier#NCT02104947). Aripazine (PER977) is a synthetic small molecule (D-arginine compound) that appears to have broad activity against various new oral anticoagulants (dabigatran, rivaroxaban, apixaban, and edoxaban) and other anticoagulants (heparins). A first study investigating the drug's effect on edoxaban-treated human volunteers has been completed (www.ClinicalTrials.govidentifier#NCT01826266).
IVC filters
The only clear indication for an IVC filter is presence of an acute DVT in a patient who cannot be fully anticoagulated due to a high risk for bleeding.1 In this situation, a retrievable filter should be placed and should be removed once the patient can be safely anticoagulated because the presence of a filter slightly increases the risk for recurrent DVT. The ACCP 2012 guidelines do not recommend IVC filters for primary VTE prophylaxis, not even in patients at high risk for VTE. Whether an IVC filter should be placed in the patient who has a recurrent VTE in spite of therapeutic anticoagulation is controversial. The potential risks of IVC filters (device migration, filter fracture, embolization of the entire filter or fracture fragments to heart or lungs, perforation of the IVC, and difficulty removing the device) have recently been highlighted by the FDA and led to the FDA's recommendation to remove the filter as soon as protection from PE is no longer needed.41 Furthermore, IVC filters have been shown to lead to a higher risk of recurrent DVT and are therefore a thrombogenic risk factor.42 Therefore, given the downsides/risks of IVC filters, it seems appropriate that all hospitals and practices that place IVC filters have: (a) an institutional policy regarding appropriate IVC filter use; (b) removable filters on the formulary, not just permanent IVC filter types; and (c) an institutional pathway/policy in place to have filters removed when they are no longer needed.
Warfarin management
Dosing, reversal, bleeding
Management aides for several important and commonly encountered clinical issues are provided in a 2014 ASH Quick Reference Guide on antithrombotic therapy, adapted in part from the ACCP 2012 guidelines.40 A nonactivated PCC plus vitamin K in addition to routine hemostatic measures are the treatment of choice in warfarin-associated major bleeding. The year 2013 saw FDA approval of a 4-factor nonactivated PCC (Kcentra) for reversal of major bleeding on warfarin and for urgent reversal of warfarin anticoagulation at times of surgeries based on 2 clinical trials.11,12 However, warfarin-associated major bleeding in clinical practice is generally poorly managed, with marked underutilization of vitamin K, PCCs, and, where PCCs are not available, fresh frozen plasma. This was recently demonstrated in a publication about treatments given for major bleeding in the warfarin arm of a large phase 3 NOAC trial.43
Anticoagulation clinics, INR self-testing
Patients on warfarin need to be followed in a systematic way to optimize safety and efficacy.44 Although smaller-volume physician practices may well have appropriate structures in place, formal anticoagulation clinics with their expertise and setup are often a good place to send patients for warfarin management. Information about the location of anticoagulation clinics can be found at www.acforum.org, the website of the nonprofit Anticoagulation Forum. In appropriately selected patients on warfarin, INR self-testing is reliable, safe, and effective.45 It is reimbursable for the patient by Medicare and Medicaid and many other insurance carriers. The devices generally yield reliable INR results. Details how patients and physicians can go about obtaining a device are available.46,47
Length of anticoagulation therapy
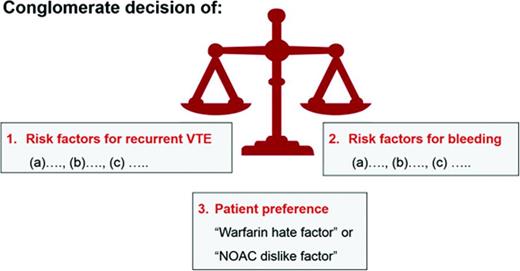
The length of anticoagulant therapy depends on 3 factors: (1) the risk for recurrent VTE, (2) the risk for major and life-threatening bleed, and (3) a patient's own values and preferences (Figure 1). For the discussion and decision making on whether a patient should remain on or discontinue anticoagulation, it is helpful to clearly define each venous thrombotic episode a patient has had in respect to anatomy (ie, was it a superficial thrombophlebitis or a DVT?; was it a distal or proximal DVT?) and to clarify the VTE risk factors for each thrombotic event. Similarly, a listing of all bleeding risk factors (fluctuating INRs, age, etc) is needed. However, it is fair to state that existing formal bleeding risk scores predict major bleeding in patients with VTE only modestly well.48 In addition, it is unknown whether the bleeding prediction rules apply to patients treated with the NOACs. Finally, patient treatment preference needs to be taken into consideration (see “Patient preference or ‘warfarin hate factor’: the author's approach” section below).
VTE recurrence prediction models
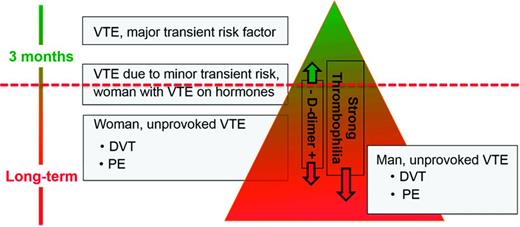
Several models or scoring systems have been developed in an attempt to predict recurrent VTE and make the translation of existing guidelines into clinical practice easier: the HERDOO-2 score,49 the DASH score,50 and the Vienna model.51,52 However, all 3 have significant limitations: none has been validated; some criteria used in the VTE recurrence prediction models are discrepant between the different scoring systems (eg, old age predicts a higher risk of recurrence in women in the HERDOO-2 score, but in the DASH score young age predicts a higher recurrence risk); and none incorporates into the decision making process a bleeding risk assessment or an assessment of patient treatment preference. A simple web-based VTE recurrence risk calculator has been developed based on the Vienna model.53 I do not use these prediction scores/models for solid clinical decision making, but I do use the HERDOO-2 score and DASH score for curiosity's sake in patients whom I assess as having an intermediate risk of recurrence (Figure 2) to see whether both scores come to the same conclusion as to whether to stop or continue anticoagulation.
“Risk of recurrence” triangle: how long to anticoagulate?: the author's approach.
“Risk of recurrence” triangle: how long to anticoagulate?: the author's approach.
Length of anticoagulation based on ACCP 20121,22,23
Reversible VTE risk factor.
In patients whose first proximal DVT or PE event was associated with surgical or nonsurgical transient VTE risk factor (hospitalization, immobility, cast immobilization, contraceptives), 3 months of anticoagulation are recommended.
Unprovoked (idiopathic) VTE.
Patients with idiopathic VTE should be treated with at least 3 months of anticoagulation. Long-term anticoagulation is recommended in such patients if they have no bleeding problems or risk factors and tolerate warfarin well.1 The guideline states that other factors (D-dimer off anticoagulation, antiphospholipid antibodies, inherited thrombophilias, gender, residual thrombosis on Duplex ultrasound) predict risk of recurrence, but not strongly or consistently enough to influence recommendations on duration of therapy once the primary factors (VTE due to transient risk factor vs unprovoked event, presence of cancer) and secondary factors (proximal vs distal DVT; was this a first VTE event or recurrent episode?) have been considered.
Cancer and DVT or PE.
For patients with cancer and DVT or PE, LMWH for 3-6 months is recommended; thereafter, LMWH or warfarin with a target INR of 2-3 indefinitely or until the cancer has resolved.
Distal DVT.
For severely symptomatic distal DVT, anticoagulation for 3 months is recommended. For asymptomatic or only mild to moderately symptomatic DVT without risk factors for DVT extension (eg, no continued immobility or cancer), serial follow-up ultrasounds rather than anticoagulation are recommended. For distal DVT that is asymptomatic or with nonsevere symptoms but with persistent risk factors for extension, anticoagulation is recommended.
Patient preference or “warfarin hate factor”: the author's approach
A patient's values and personal preference play important roles in the decision making on how long to treat with anticoagulation for an episode of VTE.54 I use a “Warfarin Hate Factor” or, now that the NOACs are in clinical use, a “Blood Thinner Hate Factor” scale for that assessment. I ask the patient to give me a number on a scale from 0 to 10 how much he/she “hates” being on warfarin, taking into consideration the following: INR fluctuations, the inconvenience of the need for monitoring, dietary restrictions, fear of major bleeding or recurrent clot, impact of the anticoagulant on his/her lifestyle, and cost of the drug and INR monitoring. A “hate factor” of “zero” reflects: “I don't mind at all being on warfarin—it's just a pill I take”; “10” means: “I hate warfarin so much, I need to come off; I don't care about another clot.” This warfarin hate factor question often opens up the discussion on the burden of being on an anticoagulant. It provides me as the physician with the patient value/preference perspective needed for decision making on length of anticoagulation (Figure 1). Although I find the warfarin hate factor concept to be clinically helpful and would encourage physicians to try to use it, it has to be said that this clinical tool has not been validated.
“Risk of recurrence triangle”: the author's approach to decision making
This is how I discuss the “length of anticoagulation” topic with an individual patient. First I draw for the patient the “recurrence triangle” (Figure 2), marking where approximately in the triangle the patient falls regarding his/her risk of recurrent VTE. I use the ACCP 2012 guidelines1 as the major evidence-based building block for this triangle, concluding the following. First, a patient with VTE due to a major surgery has a low risk of recurrence once anticoagulation is stopped after 3 months and therefore is in the top of the triangle. Second, patients with unprovoked VTE have a higher risk of recurrence and fall in the lower part (the base) of the triangle. Based on conglomerate published data, men with unprovoked VTE have the highest risk for recurrence and therefore are in the bottom of the triangle; women with unprovoked, non-hormone-associated VTE are still in the lower part of the triangle but are above where men fall.55 Finally, patients with VTE associated with minor risk factors (contraceptives, estrogen replacement therapy or pregnancy, minor surgery, minor immobility such as long-distance travel) appear to have an intermediate risk of recurrence and therefore fall in the middle area of the triangle.56,57 However, for several conditions, such as VTE associated with longer or shorter airline travel or minor surgeries (such as outpatient arthroscopic surgery), the risk of recurrence is not known or only poorly defined.
A positive D-dimer or the presence of a strong thrombophilia in the intermediate-risk patient leads me to move the patient downward, further toward the broad base of the triangle, indicating a higher risk recurrence. Presence of a mild thrombophilia (eg, heterozygous factor V Leiden, heterozygous prothrombin G20210A mutation) does not change or only slightly changes the position of the patient downward in the triangle because the heterozygous prothrombin G20210A mutation is not a risk factor for recurrent VTE and the heterozygous factor V Leiden mutation is only a mild risk factor for recurrence.59
The horizontal dashed line (Figure 2) indicates the approximate risk above which short-term anticoagulation (3 months) seems appropriate. Scientifically, it is the position above which the case fatality from recurrent VTE is lower than the one from bleeding on warfarin anticoagulation.58 However, case fatality from bleeding in patients on long-term warfarin for secondary VTE prevention, as well as in patients on the NOACs, is not accurately known.58 As discussed above, formal bleeding risk prediction models only moderately well predict major bleeding in patients on long-term warfarin.48 The dashed line's position is neither in the same position for all patients nor static over time: it gets moved up for the patient at low risk for bleeding and down in the one at high risk for bleeding because the case fatality rate from bleeding changes and thus the acceptance to treat with long-term anticoagulation. Similarly, in the patient with a strong preference not to be on anticoagulation—that is, a patient with a high warfarin hate factor, the line would move down; in the patient with a low warfarin hate factor, the line would move up.
“Long-term” anticoagulation is also referred to as “extended” or, in clinical practice terminology, as “indefinite” or “life-long,” meaning for years to come. However, periodic reevaluation is indicated with new risk-benefit assessment of anticoagulation, taking into consideration new clinical trial data, updates on the status of new anticoagulants, and reassessment of a patient's bleeding risk and treatment preference.
Thrombophilia workup: the author's approach
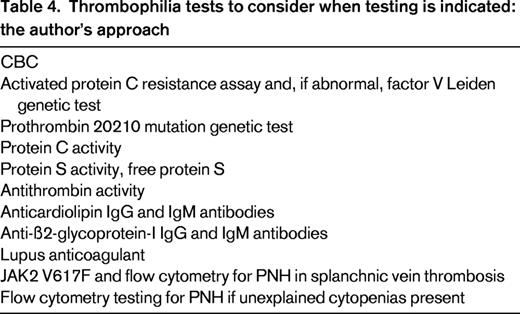
No full agreement exists as to who should be tested for thrombophilia and who should not. What is clear, however, is that patients with VTE associated with a major risk factor (ie, patients in the tip of the recurrence triangle) should not undergo thrombophilia testing.6 The ACCP 2012 guideline concludes that inherited thrombophilias do not strongly or consistently enough predict VTE recurrence to influence recommendations on duration of anticoagulant therapy.1 However, given that strong thrombophilias are risk factors for VTE recurrence,59,60 I consider thrombophilia testing in the patient who is in the intermediate risk of recurrence area of the recurrence triangle because finding a strong thrombophilia pushes the patient down into the broader base of the triangle and is one of the reasons to consider long-term anticoagulation. I consider 7 thrombophilias as being “strong”: (1) homozygous factor V Leiden, (2) homozygous prothrombin G20210A gene mutation (II20210), (3) double heterozygous state (heterozygous factor V Leiden PLUS heterozygous II20210), (4) protein C deficiency, (5) protein S deficiency, (6) antithrombin deficiency, and (7) antiphospholipid antibody syndrome. The tests I would consider, if testing is indicated, are listed in Table 4.
Aspirin to prevent VTE recurrence
Aspirin appears to have some, although only a modest, beneficial effect on decreasing the risk of recurrent VTE. Two randomized trials, the WARFASA and ASPIRE trials, were published in 2013 that investigated the benefit of aspirin (100 mg/d) over placebo in preventing recurrent VTE in patients with a history of unprovoked VTE who had been treated with a standard length of anticoagulation.13,14 The smaller WARFASA study (402 patients enrolled) showed a strong benefit of aspirin in preventing recurrent VTE [hazard ratio (HR) = 0.58; confidence interval (CI), 0.36-0.93; P = .02), the much larger ASPIRE study (822 patients enrolled) did not show a statistically significant benefit of aspirin, although a trend toward benefit (HR = 0.74; CI, 0.52-1.05; P = .09).
Reasons for the somewhat discrepant findings—the marked benefit of aspirin in the smaller WARFASA trial versus only a trend toward benefit in the larger ASPIRE trial—may be that patients at higher risk for recurrent VTE were studied in the WARFASA trial, so it was easier to detect a beneficial effect of aspirin in the smaller study with higher-risk patients. The fact that the VTE recurrence rate in the placebo arm was 11% per year in the WARFASA trial and only 6.5% in the ASPIRE trial indicates that, indeed, a higher-risk population was enrolled into the WARFASA trial. Because the trial design of the studies was similar, a pooled analysis of both trials was performed.13,14 It showed a statistically significant benefit of aspirin over placebo on the rate of recurrent VTE (HR = 0.68; CI, 0.51-0.90; P = .007); that is, a 32% risk reduction. My conclusion is that aspirin appears to have some beneficial effect, but that it is modest at best, clearly less than the benefit of continued anticoagulation with warfarin or one of the NOACs.
Therefore, aspirin is not an alternative to anticoagulation, but rather an option in the patient in whom anticoagulation is discontinued because the risk of recurrence is considered to be low enough that long-term anticoagulation is not chosen or the patient has a high anticoagulation hate factor. Although aspirin in the WARFASA and ASPIRE studies did not lead to an increased risk of major bleeding, it has, in other studies and patient populations, increased the risk for major bleeding. Choosing long-term aspirin should therefore always be a judicious decision incorporating an individual patient's risk factors for bleeding.
Long-term complications after VTE
Postthrombotic syndrome
Prevention.
A recent well-designed randomized, doubled-blinded study (the SOX trial) published in 2013 showed that compression stockings worn for 2 years after an episode of acute DVT did not prevent PTS.16 Therefore, one can conclude that patients do not HAVE to wear them. Although 2 previous studies had shown that wearing elastic compression stockings decreased the risk of developing PTS, these studies were limited by their small size and the fact that they were single-center trials. To further clarify whether stockings do play any role in preventing PTS, a randomized study (IDEAL study; clinicialtrials.gov: NCT01429714) is ongoing that compares individually tailored duration of elastic compression therapy based on signs and symptoms according to the Villalta scale after an initial therapeutic period of 6 months, with elastic compression therapy with a standard duration of 24 months.
Treatment.
Although, with our current knowledge from the SOX trial, one can conclude that PTS is not prevented with stockings, patients' leg symptoms (swelling, pain, etc) may be improved in the acute setting or with established PTS while a stocking is worn. Therefore, a stocking should be offered to any patient with bothersome leg symptoms. These should be graduated compression stockings with a compression pressure of 35 mmHg at the ankle and 25 mmHg at the midcalf, also sometimes referred to as “grade 2.” TED (thromboembolism-deterrent) stockings are not sufficiently tight because they deliver only <20 mmHg of pressure. A practical, comprehensive informational handout on stockings for patients (what stocking is right for me?; how do I get one?) is available online.61 In the patient with pronounced established PTS and only mild venous obstructive changes on Duplex ultrasound of the leg, pelvic MRI-venogram or CT-venogram should be considered to evaluate for possible pelvic vein stenosis or occlusion, which might be amenable to angioplasty or venous stenting. In addition, use of a home compression pump worn on the extremity for 30-60 minutes once or twice daily may be beneficial.
Pulmonary hypertension
The current definition for pulmonary hypertension (PH) is a mean pulmonary artery pressure of ≥25 mmHg at rest by right heart catheterization, with normal pulmonary wedge pressure.62,63 The definition of PH with a pulmonary artery pressure of ≥30 mmHg with exercise is not supported by published data. PH typically occurs within the first 2 years after a PE, affecting 3.8%–4.8% of PE patients.2,64 Regardless of whether PH is present as a result of one episode of PE or of recurrent PEs, it is referred to as CTEPH. For the patient with a history of large PE or significant residual shortness of breath, fatigue, and exercise intolerance that does not improve further or deteriorates, the following screening for CTEPH is appropriate after 3 or more months of adequate anticoagulation63 : (1) pulse oximetry at rest and after climbing stairs (or formal 6-minute walk test in a pulmonary function laboratory); (2) cardiac echocardiogram with focus on right heart and estimation of pulmonary artery pressure; (3) VQ scan to assess for chronic PE (CT angiogram of the chest is NOT sensitive enough to detect CTEPH); and (4) right heart catheterization with pulmonary artery pressure measurements and pulmonary arteriography if any of the aforementioned screening tests raise the suspicion for the presence of CTEPH.
Referral to a specialized PH clinic for objective documentation of PH (ie, right heart catheterization) and coordination of possible surgical pulmonary endarterectomy and medical therapy is appropriate. The year 2013 saw the FDA approval of a new oral drug, riociguat (Adempas), to treat pulmonary hypertension. The drug is a guanylate cyclase stimulator that leads to arterial dilatation and improves exercise tolerance and is indicated for patients with CTEPH after surgical pulmonary endarterectomy or those who cannot undergo surgery.
Contraceptives and VTE
It is well established that combination contraceptives (containing estrogens and progestins) increase the risk for VTE and that the type of progestin in these contraceptives has an impact on the VTE risk. Relatively few data, however, have been published on progestin-only contraceptives and VTE risk. A 2012 systematic review and meta-analysis of trials evaluating the VTE risk associated with progestin-only contraceptive use identified 8 suitable studies for the analysis and found that there was no association between progestin-only pills and VTE (relative risk = 0.90; CI, 0.57-1.45), no association between progestin IUDs and VTE (relative risk = 0.61; CI, 0.24-1.53), and an increased VTE risk with injectable progestins (relative risk = 2.67; CI, 1.29-5.53).17 The investigators appropriately point out that the strength of the conclusions is limited by the paucity of literature on the topic and suggest that until more data become available, noninjectable forms of progestin-only contraception be used for the highest-risk women. This conclusion matches my approach in the discussion with patients. However, it is worthwhile to point out that the analyzed studies have investigated the impact on progestin-contraceptives on a first (ie, incident) VTE event. It is not known whether progestin-only contraceptives are safe in women who have had a previous VTE or have a thrombophilia or family history of VTE. I am only aware of one published study of women with thrombosis or with a family history of thrombosis who took a progestin-only pill.65 This study showed that there was no increased risk of thrombosis with the progestin-only pill. However, the progestin contraceptive evaluated in the study is not available in the United States.
The advice I presently give to a woman with known strong thrombophilia or with a history of VTE, particularly when additional VTE risk factors (eg, obesity) are present, is to advise against the use of injectable contraceptives (Depo-Provera, Implanon rod). I state that a progestin-releasing IUD (Mirena or Skyla IUD) appears to be a good, effective, and likely safe contraceptive choice if a hormonal method is desired.
Patient education
As a resource primarily for patients with VTE, but also for health care professionals looking after such patients, comprehensive information has been made available on the Clot Connect website (www.clotconnect.org), a nonprofit project of the University of North Carolina School of Medicine. A recent publication in the Circulation “Patient Page” can also be used as an educational handout for patients with VTE, addressing many of the questions that patients with VTE have.19
Disclosures
Conflict-of-interest disclosure: The author has consulted for Janssen Pharmaceuticals, Boehringer-Ingelheim, Daiichi Sankyo, CSL Behring, and Roche Diagnostics and has received honoraria from Janssen Pharmaceuticals. Off-label drug use: Edoxaban (by Daichi) is not FDA approved for VTE as of 9/25/2014; Daiichi has applied for FDA approval for their drug; by the time of the manuscript completion and the ASH 2014 presentation, this drug may or may not be FDA approved for VTE.
Correspondence
Stephan Moll, MD, Professor of Medicine, Division of Hematology-Oncology, Department of Medicine, University of North Carolina School of Medicine, CB 7035, Chapel Hill, NC 27599; Phone: (919)966-3311; Fax: (919)966-7639; e-mail: smoll@med.unc.edu.