Abstract
A 27-year-old man with sickle cell disease (HbSS) presents to the sickle cell clinic for follow-up after a screening echocardiogram revealed an increased tricuspid regurgitant velocity of 2.7 m/s. He has a history of 2 painful crises per year and has been hospitalized 3 times over the past 10 years for management of painful crises. He had one episode of acute chest syndrome at age 15 that was treated with an RBC exchange transfusion, supplemental oxygen, and intravenous antibiotics; he did not require mechanical ventilation. He has not had additional episodes of acute chest syndrome and does not have a history of stroke, retinopathy, or leg ulcers. The patient has never been treated with hydroxyurea. He wants to know whether hydroxyurea will prevent future pulmonary complications related to sickle cell disease.
Learning Objective
To critically appraise the available evidence from medical literature regarding the use of hydroxyurea to prevent pulmonary complications of sickle cell disease
Introduction
Hydroxyurea (HU) gained approval for the treatment of adults with sickle cell anemia from the US Food and Drug Administration in 1998. The positive clinical effects of HU are thought to be largely mediated by the medication's ability to induce the expression of fetal hemoglobin (HbF) in RBCs; low levels of HbF are one of the strongest predictors of morbidity and mortality in sickle cell disease (SCD).1 HU also has positive effects on RBC hydration and deformability that lessen the rate of hemolysis, and its ability to lower the number of circulating WBCs likely leads to decreased endothelial inflammation and vasoocclusion.2 HU decreases the expression of adhesion receptors on RBCs and endothelial cells.3-6 In addition, it releases a nitric oxide moiety that not only decreases platelet and coagulation activation, but may also lessen endothelial injury through its vasodilatory effects.7-9 HU reduces vasoocclusive complications of both children and adults with SCD. Long-term use of HU has been reported to reduce mortality and improve health-related quality of life in individuals with SCD.10-16
Pulmonary complications of SCD include acute chest syndrome (ACS), pulmonary hypertension (PH), pulmonary artery thrombosis, and pulmonary fibrosis, with an increased prevalence of reactive airways disease, increased tricuspid regurgitant jet velocity (TRV), sleep-disordered breathing, and nocturnal hypoxemia.17-19 ACS and PH are major causes of mortality in adults with SCD.20,21 ACS is defined as development of a new pulmonary infiltrate on chest x-ray that is accompanied by chest pain, fever, tachypnea, wheezing, or cough.14 PH is defined as a mean pulmonary arterial pressure (mPAP) of at least 25 mmHg obtained at right heart catheterization (RHC).22 In this review, we sought to examine the medical literature to evaluate the impact of HU on pulmonary complications. Bcause the majority of studies of pulmonary complications in SCD have focused on ACS, PH, and increased TRV, we targeted our review to these 3 conditions.
To examine the current best evidence for the impact of HU on pulmonary complications associated with SCD, we performed a PubMed search in July 2014 using the Medical Subject Headings (MeSH) “sickle cell disease” AND “hydroxyurea” (646 hits). We then restricted the search to include only articles with “acute chest syndrome” OR “pulmonary” as text words (Figure 1). We reviewed the abstracts of each of these studies and excluded publications that did not evaluate and report the relationship between HU and acute chest syndrome or PH/increased TRV in at least 2 patients with HbSS disease or HbSβ0-thalassemia. We then reviewed the full texts of the remaining articles and excluded additional studies based on the same criteria. Finally, we added articles identified as relevant from the reference lists of the included studies, bringing the total number of publications included in this review to 27. The study designs, sample characteristics, and results of the studies are presented in Tables 1 and 2.
PubMed search strategy and results. Articles were excluded if they did not report the relationship between hydroxyurea and acute chest syndrome or pulmonary hypertension. MeSH = medical subject heading, ACS = acute chest syndrome, PH = pulmonary hypertension, TRV = tricuspid regurgitant velocity, RHC = right heart catheterization.
PubMed search strategy and results. Articles were excluded if they did not report the relationship between hydroxyurea and acute chest syndrome or pulmonary hypertension. MeSH = medical subject heading, ACS = acute chest syndrome, PH = pulmonary hypertension, TRV = tricuspid regurgitant velocity, RHC = right heart catheterization.
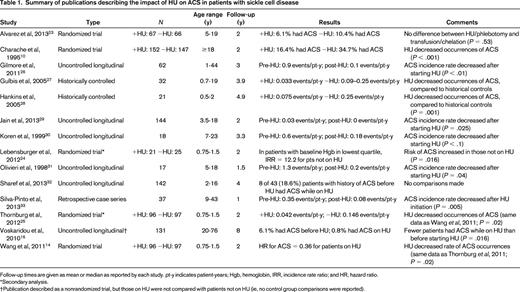
For ACS, there were 5 publications reporting the results of 3 multi-institutional, randomized controlled trials (RCTs).10,14,23-25 Two of these RCTs (one in adults, one in children) compared HU with placebo; the other compared HU and phlebotomy with chronic transfusions and chelation in children with SCD and a history of stroke. The remaining publications on ACS described uncontrolled longitudinal studies, retrospective case series, or prospective cohort studies using historical controls.16,26-33 For PH, 10 studies estimated pulmonary artery systolic pressure using echocardiography-derived TRV, whereas only 2 studies reported pulmonary artery pressures measured during RHC.20,34-43 Five of the publications on increased TRV and both publications on RHC-confirmed PH described prospective cohort studies,20,34-37,42,43 but only the study by Desai et al37 included an analysis of the impact of HU on TRV over time. The remaining publications described either cross-sectional studies or case series. Steinberg et al15 reported on long-term follow-up of patients who had previously participated in an RCT of HU, but reported all pulmonary complications as a single exposure variable when examining patients' causes of death. Voskaridou et al16 described a nonrandomized trial of HU, but the report did not include any evaluation for PH other than as a cause of mortality and did not specify how PH was diagnosed.
ACS
We found moderate-quality evidence showing that HU reduces the occurrence of ACS in patients with SCD. The 2 randomized, placebo-controlled trials that evaluated the effects of HU compared with placebo were well performed and suffer from no major weaknesses.10,14 However, ACS was not the primary study outcome in either trial. The Multicenter Study of Hydroxyurea (MSH) trial, conducted in the early 1990s, found that significantly fewer patients taking HU developed ACS compared with patients treated with placebo (16.4% vs 34.7%, respectively; P < .001). The Pediatric Hydroxyurea in Sickle Cell Anemia (BABY HUG) study enrolled 299 children aged 9-18 months between 2003 and 2007.14 The rate of ACS was significantly reduced in patients receiving HU (hazard ratio = 0.36, 95% confidence interval, 0.15-0.87). A subset analysis of the BABY HUG trial's data that examined patients based on their baseline hemoglobin levels showed that the beneficial effect of HU on ACS incidence in young children was most pronounced in patients with baseline hemoglobin values in the lowest quartile.24 Follow-up studies of the BABY HUG cohort will determine whether this differential effect persists as children age.
The Stroke With Transfusions Changing to Hydroxyurea (SWiTCH) trial was an RCT that compared the use of HU and phlebotomy with chronic transfusion and chelation in children with SCD who had a history of stroke.23 The study showed no difference in the proportion of patients experiencing ACS between the 2 groups (6.1% in the HU group vs 10.4% in the transfusion group; P = .53). Given the low rates of ACS observed in the trial, the number of patients was likely not sufficient to determine whether there was a true difference between ACS rates in the 2 arms.
In addition to the aforementioned RCTs, we found 9 studies of various designs that also showed that HU decreased the occurrence of ACS.16,26-33 Although the designs of these studies provide lower-quality evidence of HU's effect on ACS, in aggregate, they include a large number of patients and provide results that are consistent with the findings of the RCTs. Overall, there is moderately strong evidence that HU significantly reduces the risk of ACS in patients with SCD.
Pulmonary hypertension
Ten of 12 studies of PH in SCD were limited by their use of echocardiograms, which have a positive predictive value for PH in SCD of only 25%–31%.42,43 Echocardiography-derived TRV is often used as either a surrogate measure of PH or as a screening test for PH, given the invasiveness and costs associated with RHC. Studies examining HU's impact on increased TRV are presented in Table 2. As a whole, the studies we identified that examined the relationship of HU use to increased TRV provided inconsistent evidence of HU's effect.20,34-41,44 Given echocardiography's lack of reliability for diagnosing PH, we have focused the remainder of this discussion only on studies examining PH diagnosed by RHC.
There were 2 studies that initially evaluated all patients with an echocardiogram and later performed RHC in patients with TRV ≥2.5 m/s.42,43 Each study examined the proportion of patients taking HU at the time of study entry and compared these proportions between various groups of patients based on the results of PH evaluations. Fonseca et al42 performed 2 separate comparisons: 1 for patients with TRV <2.5 m/s versus those with TRV ≥2.5 m/s and 1 for patients with mPAP <25 mmHg versus patients with mPAP ≥25 mm Hg. Parent et al43 made 1 comparison of 3 groups: those with TRV <2.5 m/s, those with TRV ≥2.5 m/s but mPAP <25 mmHg, and those with mPAP ≥25 mm Hg. Neither study showed a difference between or among any of the groups in terms of proportions of patients taking HU at the time of evaluation. As in the studies of increased TRV, these studies of PH as diagnosed by RHC do not provide evidence that HU prevents PH. However, the lack of randomization and prospective follow-up makes further interpretation of these results difficult. It is possible that a large treatment effect could have been masked by selection bias in these studies.
Pulmonary complications
Two studies reported results from long-term follow-up of patients with SCD who had participated in HU trials. Steinberg et al15 described the most frequent causes of death for 299 patients 17.5 years after they had been enrolled in the MSH trial.10 After the MSH trial was halted, subsequent HU use by enrollees in the trial was based on patient and physician discretion. At the time of the follow-up publication, 24% of all deaths were due to pulmonary complications and 87% of these occurred in individuals with <5 years of exposure to HU during the study and follow-up periods. The Laikon Study of Hydroxyurea in Sickle Cell Syndromes (LaSHS) was a nonrandomized trial of HU in patients with SCD (HbSS, HbSβ0-thalassemia, and HbSβ+-thalassemia) that assigned patients to receive HU based on disease severity criteria.16 After 5-8 years of study follow-up, the investigators failed to find a reduction in deaths due to PH in patients taking HU; however, the number of deaths due to PH was small and the method for diagnosing PH as a cause of death was not reported. These 2 studies provide modest evidence that HU may reduce overall mortality in adults with SCD and that long-term exposure to HU appears to reduce the likelihood of deaths due to pulmonary complications of SCD.
Recommendations
The following graded recommendations follow the conventions recommended by the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group, which are described in Table 3.45 Given the moderate-quality evidence supporting the use of HU to prevent ACS, we would strongly recommend that the above patient be treated with HU given his history of ACS (grade 1B). Because ACS is a relatively frequent and potentially life-threatening complication in SCD, the benefits of reducing the frequency of ACS with HU outweigh the typically mild and manageable adverse effects of HU use for prevention of this disease outcome. Although the MSH and BABY HUG trials did not evaluate ACS as a primary study outcome, the reduction in ACS incidence observed in patients on HU was of sufficient magnitude to warrant a strong recommendation for HU use to prevent ACS.10,14
For patients with increased TRV who do not have other indications for HU use (ie, frequent acute painful crises or history of ACS), we strongly recommend that further evaluation be undertaken to determine whether PH is present (grade 1B). Other evaluations for PH, including the presence of cardiopulmonary symptoms, 6-minute walk distance, and measurement of N-terminal pro-brain natriuretic peptide levels, could be incorporated into the decision-making process regarding the necessity of establishing the diagnosis of PH with RHC. In patients found to have mPAP ≥25 mmHg, we make a weak recommendation based on very low-quality evidence to start HU in addition to specific interventions targeting precapillary or postcapillary PH (grade 2D). Although direct evidence supporting the use of HU in patients with isolated TRV ≥2.5 m/s is lacking, given the increased risk of death in patients with increased TRV and the potential benefit and relatively low risk of HU therapy, HU should also be considered in patients found to have persistently elevated TRV.22
Despite the limited data on HU in patients with increased TRV and PH, it is unlikely that placebo-controlled studies will be conducted in this setting due to a lack of clinical equipoise. However, it would be worthwhile to consider TRV and/or PH as end points in ongoing studies of HU in children. In addition, registry studies will be a useful resource to evaluate the effect of HU on TRV and PH in adults with SCD. These studies will require sufficiently long follow-up times to allow for evaluation of the long-term effects of HU on these complications.
Acknowledgments
T.W.B. is supported by a National Hemophilia Foundation/Baxter Healthcare Clinical Fellowship Award and a Hemostasis and Thrombosis Research Society/Novo Nordisk Clinical Fellowship Award in Hemophilia and Rare Bleeding Disorders. K.I.A. is supported by the National Institutes of Health (Grants R01HL111659 and U01HL117659) and the North Carolina State Sickle Cell Program.
Disclosures
Conflict-of-interest disclosures: T.W.B. declares no competing financial interests. K.I.A. has received research funding from HemaQuest Pharmaceuticals and Selexys Pharmaceuticals, has consulted for Pfizer, and has received honoraria from Pfizer, Adventrx, HemaQuest Pharmaceuticals, and Selexys Pharmaceuticals. Off-label drug use: None disclosed.
Correspondence
Tyler W. Buckner, MD, MSc, Division of Hematology/Oncology, University of North Carolina at Chapel Hill, Physicians' Office Bldg., 3rd Floor, CB# 7305, 170 Manning Drive, Chapel Hill, NC 27599; Phone: (919)966-3856; Fax: (919)966-6735; e-mail: tyler_buckner@med.unc.edu.