Abstract
An essential component of a cancer patient's comprehensive care is addressing potential threats to his or her reproductive health. Providers should discuss the risk of infertility with newly diagnosed patients and offer the chance to consult with a reproductive specialist as early as possible. Standard fertility preservation options include embryo or oocyte cryopreservation for women and sperm banking for men; all options for pre-pubertal children are experimental. Patients with hematologic malignancies are a distinct population in whom standard options may present special challenges, and alternative management strategies are being explored. Unique approaches in hematologic malignancy patients include experimental techniques, such as hormonal therapy, referrals to reproductive specialists after cancer treatment, or discontinuation of tyrosine kinase inhibitor therapy in appropriate chronic myelogenous leukemia patients. Importantly, expedited communication between hematologists and reproductive specialists may greatly enhance the quality of care for these patients. Facilitation of referrals will both improve the quality-of-life and expand the prospect of parenthood in survivors. There are ample opportunities to advance the field of oncofertility through additional research, especially in hematologic malignancy patients.
Learning Objectives
Understand the risks of infertility associated with cancer chemotherapy, radiation therapy, and targeted agents
Recognize that young patients with hematologic malignancies should have fertility addressed as soon as possible upon diagnosis, and should be referred expeditiously to reproductive specialists
Identify unique characteristics of patients with hematologic malignancies that may present special challenges in fertility preservation, and recognize patients in whom standard options may not be appropriate
Recognize that patients with hematologic malignancies may benefit from post-treatment referrals to reproductive specialists, particularly in high-risk or pre-transplant settings
Progress in cancer therapy and in supportive care has led to substantial improvements in outcomes and increasing numbers of cancer survivors. Satisfactory quality-of-life after cancer includes the ability to become a parent; reproductive concerns after cancer treatment are associated with depression and distress.1-3 Hence, addressing potential threats to fertility is an essential part of the comprehensive care of the cancer patient.4,5 This review will focus on patients with blood cancers who require cytotoxic therapies for cure (acute leukemia, Hodgkin and some non-Hodgkin lymphoma) as well as those with chronic myelogenous leukemia (CML). Hematopoietic cell transplantation (HCT), a therapy almost universally associated with permanent amenorrhea and azoospermia, will also be addressed. Patients with myelodysplastic syndrome, chronic lymphocytic leukemia, and multiple myeloma will be excluded from this review because these diseases are incurable with the exception of patients undergoing allogeneic HCT, and typically affect older adults.6
There are >1 million survivors of acute leukemia and Hodgkin (HL) and non-Hodgkin (NHL) lymphoma in the United States and an estimated additional 130 000 new cases of these blood cancers will be diagnosed each year.6 Although acute myeloid leukemia (AML) and NHL predominantly impact older adults, a substantial number of cases (∼20% of AML and 17% of NHL) occur in patients under the age of 50, with cure rates ∼50%-60% for AML and ∼60% for diffuse large B-cell lymphoma. On the other hand, patients under the age of 50 predominate in Hodgkin lymphoma (64%) and acute lymphoblastic leukemia (ALL; 75%), with cure rates ∼80% for HL and between 40%-90% for ALL.6 In addition, there were an estimated 108 900 HCT survivors in the US in 2009 with 242 000 HCT survivors anticipated by 2020, representing 2% of all cancer survivors7 ; 45% of allogeneic HCT recipients and 25% of autologous HCT recipients are under the age of 40 at the time of transplant.
Why do cancer treatments jeopardize fertility?
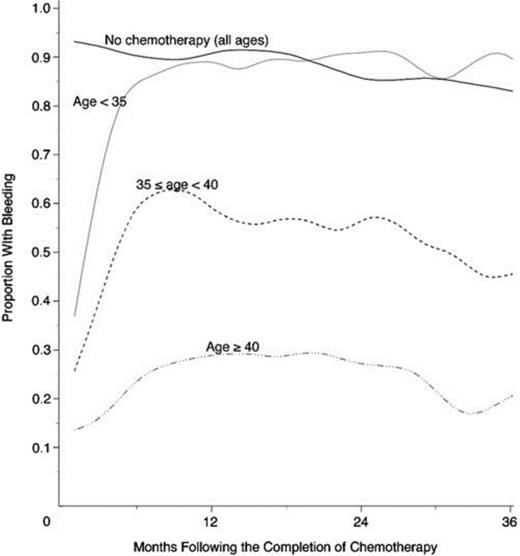
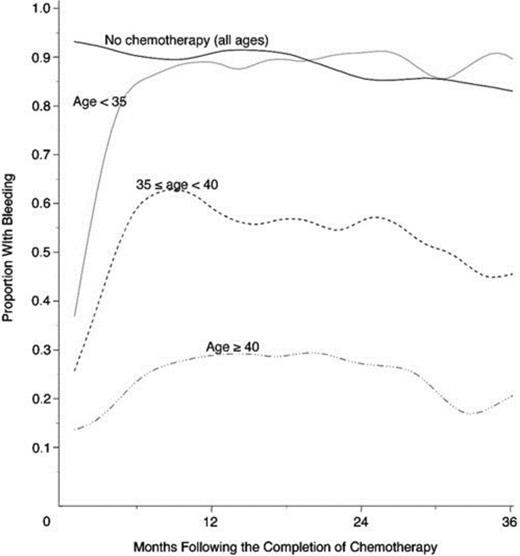
The most gonadotoxic therapies are alkylating agents and radiation; damage is dose-dependent, and in women, the risk of inducing amenorrhea increases with age (Figure 1). Clinically these exposures have translated into markedly reduced pregnancy rates in survivors of cancer: the Childhood Cancer Survivor Study reported survivors' pregnancy rates to be 20% lower than their siblings and female patients with radiation to the pelvis exceeding 10 Gy were 82% less likely to report a pregnancy.8 Survivors are 48% more likely to report infertility; in the cohort of survivors <20 years of age at treatment, infertility was nearly 3-fold more common.9 In the BMT Survivor Study, transplant recipients were 36 times more likely than siblings to report no conception.10
Age-dependence of chemotherapy-induced amenorrhea. Proportion of women with early-stage invasive breast cancer treated with alkylator-based chemotherapy who reported menstrual bleeding after therapy, by age.70
Age-dependence of chemotherapy-induced amenorrhea. Proportion of women with early-stage invasive breast cancer treated with alkylator-based chemotherapy who reported menstrual bleeding after therapy, by age.70
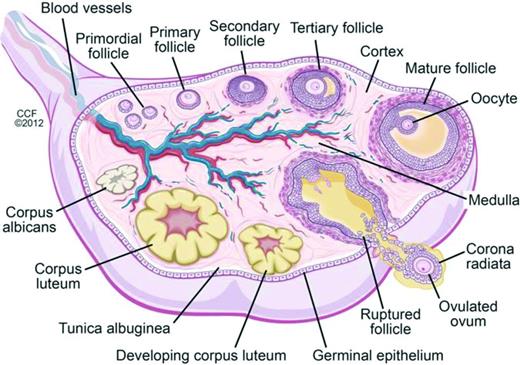
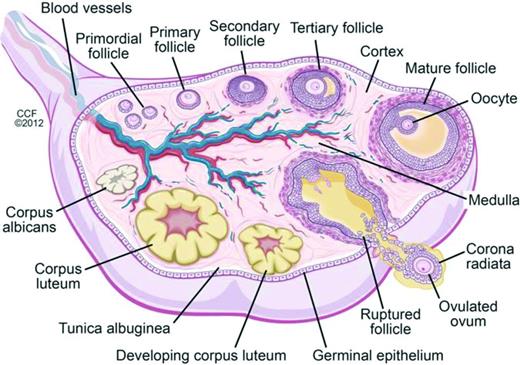
Oocytes begin forming in female embryos in utero at ∼15 weeks gestation. By 20 weeks gestation there are 6-7 million oocytes, and pre-antral follicles begin to develop (Figure 2). Pre-antral, or secondary, follicles are maturing follicles that become dependent on hormonal stimulation for growth, with formation of the zona pellucida and proliferation of granulosa cells surrounding the ovum. Follicular atresia begins before birth, and girls/women are continually losing follicles and oocytes over the course of their lifetimes. There are in 1-2 million oocytes at birth, ∼25 000 at the age of 37 years, and 1000 at the age of 51 years, the average age of natural menopause in the United States; menopause occurs when oocytes and follicles are depleted.11,12 Chemotherapy has differential effects on primordial, dormant follicles and growing, larger ovarian follicles. Chemotherapy targets actively dividing cells, and therefore, destroys mature ovarian follicles, specifically by inducing apoptosis in granulosa cells. However, the dormant, primordial follicles, which represent the stockpiles that represent future fertility, are harmed through other mechanisms, which are poorly understood. Theories include chemotherapy-induced apoptosis of the primordial follicles themselves; induction of fibrosis of the ovarian cortex, which may lead to diminished blood flow to the primordial follicles; and depletion of primordial follicles via premature activation triggered by a decrease in granulosa cells (which secrete factors that inhibit primordial cell recruitment).13 As these primordial follicles develop into maturing follicles, they undergo atresia and may also become more susceptible to chemotherapy-induced damage.13 Radiation is directly toxic to oocytes, both in the dormant primordial follicle and in the larger antral follicle. A dose of just 2 Gy to the ovaries results in loss of 50% of oocytes.14
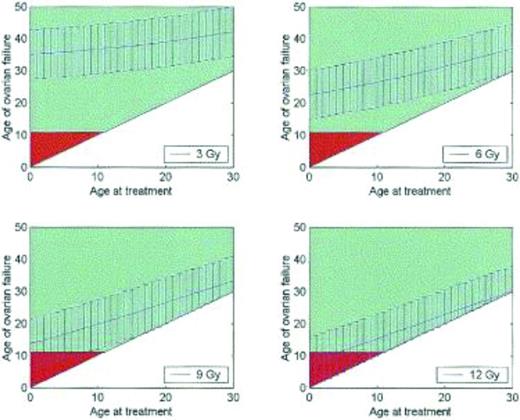
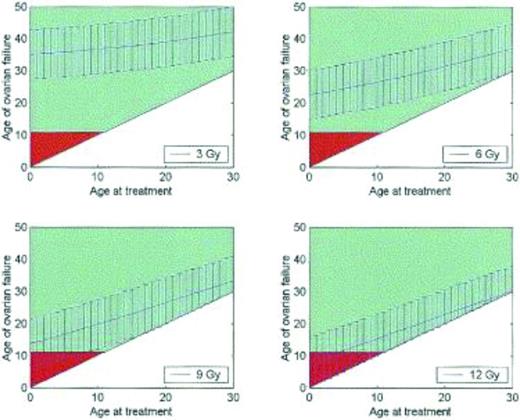
Fertility is difficult to measure, and correlates poorly with the occurrence of menses. Alternative measurements of ovarian function and ovarian reserve have been identified, including antral follicle count and anti-Mullerian hormone levels, although there are no universally accepted measures.12,15-17 Cancer therapies diminish ovarian reserve and result in premature ovarian failure. For example, women who receive 3 Gy of radiation to the pelvis at age 20 can expect to enter menopause at approximately age 35; women who receive 12 Gy can expect to enter menopause almost immediately (Figure 3).18 In a study of teenage cancer patients who were surveyed 5 years after treatment and who were all menstruating at age 21, 42% were menopausal by age 31. The risk of menopause by age 25 was 9-fold higher in women who had received alkylator therapy, 3.6-fold higher with radiation to the abdomen, and 27-fold higher if both modalities had been received.19 Cancer therapy results in accelerated ovarian aging, such that women ages 15-39 treated with high-dose cancer therapies have ovarian reserves comparable to healthy women in their 40s.16
Premature ovarian failure after radiation therapy. Graphs demonstrating the age at which a patient may be predicted to experience ovarian failure (with 95% confidence intervals) at total body irradiation doses of 3, 6, 9, and 12 Gy. Patients over the age of 11 years are shown in green, whereas under age 11 is in red; age 11 is the earliest age that ovarian failure may be detected biochemically.18
Premature ovarian failure after radiation therapy. Graphs demonstrating the age at which a patient may be predicted to experience ovarian failure (with 95% confidence intervals) at total body irradiation doses of 3, 6, 9, and 12 Gy. Patients over the age of 11 years are shown in green, whereas under age 11 is in red; age 11 is the earliest age that ovarian failure may be detected biochemically.18
Men are also susceptible to chemotherapy- and radiation-induced infertility. Sperm form continuously from a pool of primordial germ cells, or spermatogonial stem cells, in the seminiferous tubules. An immature germ cell requires ∼74 days20 to develop into a mature spermatozoa. Once mature, the sperm is transported to the epididymis for storage until released. Alkylators and platinum-based agents are most toxic to sperm, and they are also exquisitely sensitive to damage by radiation.21 Because sperm are “preformed,” a decline in sperm counts is delayed by ∼2-3 months from the beginning of chemotherapy. However, DNA damage to formed sperm occurs almost immediately.22,23 Probability of recovery of spermatogenesis is related to the degree of damage to spermatozoal stem cells, and thus is strongly correlated to dose of alkylators and radiation. The risk of impaired spermatogenesis is low when the cyclophosphamide equivalent dose is <4000 mg/m2, but is still dose-dependent, with odds of azoospermia increasing by 22% and oligospermia by 14% for every 1000 mg/m2 increase in cyclophosphamide equivalent dose.24 Nevertheless, sperm recovery may occur in 20%-25% of patients after even the most intensive therapies including myeloablative HCT,20 although it may be delayed by years after therapy.25-27 Despite this, male cancer survivors are ∼50% less likely than their siblings to father children, and this risk was greatest in men receiving radiation to the testes >7.5 Gy or alkylating agents at the highest dose ranges.28
Which options exist to preserve fertility?
Women
For women, there are 2 standard of care options for fertility preservation: embryo cryopreservation and oocyte cryopreservation.4,5 Both procedures involve controlled ovarian hyperstimulation using hormonal therapy, followed by surgical retrieval of mature oocytes, typically through a transvaginal needle approach. If embryos are to be cryopreserved, the oocytes are incubated with sperm prior to banking, whereas oocyte cryopreservation does not require a sperm donor. There are now rapid induction protocols that have a random start, do not require synchronization with the patient's menstrual cycle, and are able to be completed in 10-14 days from initiation. Oocyte cryopreservation is no longer considered experimental and is associated with successful pregnancy at rates that are comparable in many centers to embryo cryopreservation (currently ∼35%-40% of transfers resulting in live births, depending on the age of the donor; www.sart.org).29 Ovarian transposition, a surgical procedure which removes the ovary from the radiation field, is an option for early-stage lymphoma patients with disease localized to the pelvic region.
Experimental fertility preservation approaches in women include ovarian tissue cryopreservation and hormonal methods, such as GnRH analogs or combined oral contraceptives. Ovarian tissue cryopreservation entails laparoscopic surgical access to the ovaries, and removal and cryopreservation of either the entire ovary or strips of ovarian cortex.30 When the patient is ready to consider pregnancy, the ovarian tissue is thawed and reimplanted, usually orthotopically onto the remaining ovarian tissue, the opposite ovary, or in the peritoneum,31 although heterotopic placement may also be considered, for example in the forearm.32 Reimplantation of ovarian tissue in a heterotopic location is less invasive, but also requires artificial reproductive techniques (IVF) for uterine implantation. Since 2004,33 24 live births have been reported using this technique; all of these were reimplanted orthotopically and ∼50% of them required assisted reproductive techniques for successful implantation.34 Advantages of ovarian tissue cryopreservation include: (1) gonadal tissue harvest may occur immediately; there is no hormonal therapy, preparation, or waiting period required as there is for oocyte harvesting; (2) like oocyte cryopreservation, a male partner/sperm donor is not required; (3) endocrine function (not just fertility) may be restored in recipients of reimplanted tissue; and (4) this is an (experimental) option for pre-pubertal children. Unfortunately, there is a very significant risk associated with this approach: the threat of malignant contamination of the ovarian tissue, which may result in cancer relapse after reimplantation. This risk appears to be most pronounced in patients with hematologic malignancies, especially leukemia. There are reports supporting35-37 and refuting38,39 the presence of malignant cells in the tissue to be reimplanted.40 Unaffected ovarian tissue was seen primarily in patients who were in remission post-therapy. False-negative flow cytometry of ovarian tissue may occur,38 so the most sensitive testing (ie, molecular studies) should be used when possible. Because of the uncertainty surrounding the safety of reimplantation of ovarian tissue in patients with hematologic malignancies, this approach is not recommended even as an experimental option for patients with leukemia, and possibly with any blood cancer.41
Hormonal therapies are the most controversial consideration. Retrospective studies have suggested a potential benefit to administration of GnRH analogs, summarized by a Cochrane meta-analysis which reported that GnRHa-treated patients had a 1.9-fold increase in resuming menses, a 2.7-fold increase in ovulation, and a markedly reduced risk of amenorrhea (RR 0.08), all statistically significant; however, there was no difference in pregnancy rates or antral follicle counts.42 The German Hodgkin Lymphoma Study Group reported a 12-fold increase in pregnancies reported in GnRHa-treated patients versus controls.43 After autologous HCT, GnRHa showed a protective effect on cyclic ovarian function in patients with lymphoma (67% retained cyclic ovarian function vs 18% in the untreated group) but not leukemia (10% vs 8%).44 Other studies have shown no benefit.45,46 The few large prospective randomized trials of GnRH analogs have focused on breast cancer patients: one was halted for futility after an early interim analysis failed to show a difference in resumption of menses, with lower than expected rates of amenorrhea in the control group,47 whereas another did demonstrate that amenorrhea was less frequent (8% vs 22%, OR 0.3; p = 0.04) and pregnancy more frequent (21% vs 11%; p = 0.03) in the GnRHa-treated group.48
Another hormonal option, combination oral contraceptives (COCs), may be taken continuously with no placebo to minimize menstrual bleeding. COCs have never been studied prospectively for fertility preservation in patients with hematologic malignancies but some reports suggest that they may also be protective. A German study reported a reduced risk of amenorrhea in COC-treated patients compared with controls (44% vs 10%, adjusted OR 0.16; p < 0.0001)49 ; another study demonstrated a 13% risk of premature ovarian failure in COC patients versus 29.8% in controls.50
There are concerns with hormonal approaches to fertility preservation, however. GnRH analogs are associated with menopausal symptoms and accelerated bone loss, a significant concern in patients receiving steroid-intensive therapies. Additionally, it is suggested that GnRH agonists be given 7-10 days prior to initiation of chemotherapy46,48 because there is an initial stimulatory effect (increase in pituitary hormones FSH and LH) leading to a transient “flare” of ovarian activity; this is not required with GnRH antagonists but these must be given more frequently. Worry about thromboembolic risk associated with COC, especially in immobilized, hospitalized cancer patients who may develop other coagulopathies related to disseminated intravascular coagulation or asparaginase-inclusive regimens may make COCs a less-attractive choice.
Nevertheless, hormonal therapies may be the only options currently available to some patients with blood cancer,4 and a careful discussion of risks, side effects, and the true expected benefit, which may be small or nonexistent, is required between the hematologist and the patient.
Men
The only standard of care option for male fertility preservation is sperm cryopreservation (sperm banking).5 Unfortunately, many male cancer patients face challenges in sperm banking: men with cancer have higher rates of azo- or oligospermia even prior to initiation of therapy,22,51 and face significant stressors which may make it impossible to bank sperm. It is notable that perhaps the greatest barrier to sperm banking is failure to refer these patients: fewer than one-half of oncologists consistently refer male patients for sperm cryopreservation.52,53
Experimental techniques in men include testicular tissue cryopreservation, which is subject to the same concerns and challenges as ovarian tissue banking, with perhaps even greater worry about tissue contamination in patients with ALL, as the testis is a known sanctuary site.54
Children
Pre-pubertal children are candidates only for experimental approaches, such as harvesting gonadal tissue for cryopreservation. There is a significant and urgent need for further research to expand fertility preservation options, particularly, non-invasive approaches, in children.
What is different about patients with hematologic malignancy?
Patients with acute leukemia and some types of lymphoma often require urgent/emergent cytotoxic therapy. Furthermore, the typical treatment regimens for acute leukemia and Hodgkin lymphoma, all delivered with curative intent, do not involve alkylating agents at sufficient doses to induce immediate and irreversible ovarian failure, whereas patients with non-Hodgkin's lymphoma, particularly diffuse large B-cell NHL, do require cyclophosphamide at moderate doses as a component of curative therapy. Hence, some key points:
In general the curative chemotherapeutic regimens used in favorable- and some intermediate-risk AML, ALL, and HL are believed to carry <20% risk of permanent amenorrhea.5 However, these regimens likely result in subfertility and/or diminished ovarian reserve and a shortened “fertility window.”55 This makes counseling patients about threats to their fertility particularly challenging, as the risk is truly undefined. It is possible to harvest oocytes successfully after therapy for acute leukemia or Hodgkin lymphoma, and there have been cases of successful fertilization and reimplantation; some of these patients were treated with GnRH analogs during initial therapy which may have been protective.56,57
Most acute leukemia patients, and many lymphoma patients, require urgent or emergent cytotoxic therapy and may be unable to delay treatment for the ∼10 days needed to successfully harvest oocytes. In addition, they may be unable to safely undergo standard-of-care fertility preservation methods due to cytopenias, active infections, cardiopulmonary issues, or clinical instability. It is essential for the practitioner to be able to identify patients who are not eligible for standard fertility preservation techniques.
Although permanent amenorrhea may be infrequent after initial therapy for leukemia and many lymphomas, relapse in these diseases is common. Treatment of relapse requires additional, often intensive, chemotherapy followed by hematopoietic cell transplantation. Both of these interventions will further diminish, and in the case of myeloablative HCT, likely eradicate, ovarian reserve and cause acute ovarian failure and/or azoospermia. The impact of reduced intensity HCT on fertility is unknown, but appears to be significant.27,58 Thus, even after therapy is complete, it may be appropriate to refer patients to a reproductive specialist to discuss fertility.
Special consideration: CML
Management of fertility in patients with chronic myelogenous leukemia represents a very specific challenge. Patients under the age of 50 represent 25% of all patients with CML.6 Patients with chronic phase CML require life-long therapy with a tyrosine kinase inhibitor (TKI); all approved TKIs also inhibit c-kit, Src, platelet-derived growth factor receptor (PDGFR), and other tyrosine kinases, which are likely to impact fetal development and may also impair fertility. All TKIs are labeled “Category D” by FDA (evidence of human fetal risk, but potential benefits may warrant use of the drug in pregnant women despite potential risks). Emerging case series data in male CML patients suggests that it is likely safe for men to father children while taking imatinib59-61 ; significantly fewer data exist for the second- and third-generation TKIs. Men should be counseled appropriately and encouraged to consider cryopreservation of sperm prior to initiating therapy. For women with CML, there is strong evidence to suggest that TKIs during pregnancy confer a small increased risk of spontaneous abortions and a significantly increased risk of congenital malformations including bony and renal deformities, and others.62-64 Hence, it is recommended that TKIs be discontinued for pregnancy. Women who become pregnant and discontinue imatinib without achieving a durable major molecular response (MMR) experience poor outcomes, with loss of responsiveness to imatinib upon resumption of drug.65 It is therefore recommended that women defer pregnancy until a major molecular response (MMR) of >2 years duration is achieved, with reliable contraception practiced until this milestone is reached. Once the patient has attained a MMR >2 years, the TKI may be discontinued to attempt pregnancy. Given the unpredictability of conception, patients may be off therapy for a significant period of time, increasing the risk of relapse; discussion of this risk must be part of the counseling process. In vitro fertilization and embryo reimplantation may lend structure and predictability to the timing of pregnancy. Alternatively, young women with CML may consider cryopreservation of oocytes/embryos prior to initiating therapy (or after durable MMR is achieved) and a gestational surrogate could be considered, which would allow the patient to initiate (or resume) TKI therapy promptly after oocyte harvest. An outstanding review of pregnancy in CML was recently published.66
Moving forward
Physicians and other care providers of patients with hematologic malignancies face many uncertainties in counseling patients about fertility. There is an urgent need for research in this population. Unanswered questions abound: should patients, particularly female patients, who were unable to undergo fertility preservation prior to treatment be referred after completing therapy for oocyte or embryo cryopreservation in case of future relapse or future premature ovarian failure? If so, what is the appropriate waiting time after completion of therapy to minimize the risk of chromosomal damage to these oocytes? What is the true risk of cancer contamination of gonadal tissue? Should this tissue be harvested before or after cancer treatment? How much reassurance can or should we provide to these patients about their risk of infertility, particularly when that risk may change over time? Female fertility may recover, but is likely to diminish in the future, whereas it is possible that male fertility will improve. What is the role of less-established but less-invasive methods of fertility preservation such as hormonal approaches? Can or should these interventions be offered? Are the risks worth the possible benefits in an unstudied population? What are the long-term outcomes of children born with assisted reproductive technologies implemented before treatment? After treatment?
Discussing the potential threat to fertility with our hematologic malignancy patients is especially challenging because of the undefined risk and variable timing of gonadal failure, the undetermined likelihood and timing of gonadal recovery, and the unknown risks and efficacy of the nonstandard but immediately available fertility preservation options, such as hormonal therapies. The diagnosis of a blood cancer is often precipitous. Patients must digest the enormity of their diagnosis and the substantial risks to their health, learn of and cope with potential threats to fertility, and then make extremely difficult decisions about fertility preservation that have lifelong impact with minimal data to support them. In cases where it is not possible to offer fertility preservation before treatment, there may be future opportunities to address fertility, to circumvent a potentially shortened “fertility window” or to take advantage of the hiatus in therapy between initial chemotherapy and HCT.
Hematology and oncology providers are frequently unfamiliar with fertility preservation options; reproductive endocrinologists may not be comfortable with the rare and serious blood cancers; it can be difficult to coordinate a multidisciplinary team approach to these patients. Building bridges between hematologists and reproductive specialists, and facilitating referrals across departments and institutions, is an essential step; educating and empowering patients is another.67-69 The most important step is additional research to support evidence-based, high-quality decisions that will help our patients transition to survivors with full and satisfying lives.
Key points
There is a low but definite risk of gonadal failure associated with initial cytotoxic treatment of most hematologic malignancies.
Even if gonadal failure does not occur immediately, there will likely be a shortened fertility window after treatment in women, and conversely, possible long-term recovery of spermatogenesis in men.
Contraception immediately upon starting cytotoxic therapy is essential, especially in men.
Interventions to preserve fertility are best undertaken directly after a cancer diagnosis and before initiating therapy in eligible patients; standard of care options include oocyte/embryo cryopreservation in women and sperm banking in men. Other modalities (gonadal tissue harvest, hormonal therapies) are experimental; they may be associated with significant risks and efficacy is not well established. All options for pre-pubertal children are experimental.
Providers must be able to identify which patients can safely undergo an intervention for fertility preservation; key considerations include the urgency of initiation of treatment, presence of cytopenias, infections, cardiopulmonary status, and the risk of thromboembolism.
Providers should offer fertility counseling immediately upon diagnosis. Patients who do not receive an established intervention for fertility preservation should also be counseled after treatment when remission is achieved, acknowledging that there is limited data regarding the safety and optimal timing of gonadal tissue or gamete cryopreservation after cancer treatment.
Patients undergoing HCT of any kind—autologous or allogeneic, myeloablative, or reduced intensity—are at substantial risk for permanent gonadal failure, and should see a reproductive specialist during the transplant planning process, if they have not previously been referred.
Oncofertility program building and facilitated referrals from hematology to reproductive specialists should be prioritized. In centers that lack these resources, access to reproductive care may be facilitated through the Oncofertility Consortium (http://oncofertility.northwestern.edu/fertline).
Correspondence
Alison W. Loren, Perelman School of Medicine of the University of Pennsylvania, 3400 Civic Center Blvd, Philadelphia, PA 19104; Phone: 215-615-3138; Fax: 215-615-5887; e-mail: alison.loren@uphs.upenn.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author has received research funding from Merck.
Author notes
Off-label drug use: Use of gonadotropin releasing hormone (GnRH) analogs and combination oral contraceptives will be discussed as possible methods of fertility preservation.