Abstract
Hemolytic disease of the fetus and newborn (HDFN) affects 3/100 000 to 80/100 000 patients per year. It is due to maternal blood group antibodies that cause fetal red cell destruction and in some cases, marrow suppression. This process leads to fetal anemia, and in severe cases can progress to edema, ascites, heart failure, and death. Infants affected with HDFN can have hyperbilirubinemia in the acute phase and hyporegenerative anemia for weeks to months after birth. The diagnosis and management of pregnant women with HDFN is based on laboratory and radiographic monitoring. Fetuses with marked anemia may require intervention with intrauterine transfusion. HDFN due to RhD can be prevented by RhIg administration. Prevention for other causal blood group specificities is less studied.
Learning Objectives
Explain the fetal and infant clinical findings associated with hemolytic disease of the fetus and newborn (HDFN)
Describe the approach to pregnancy management when a mother has red cell alloimmunization
Discuss the prevention strategies for HDFN
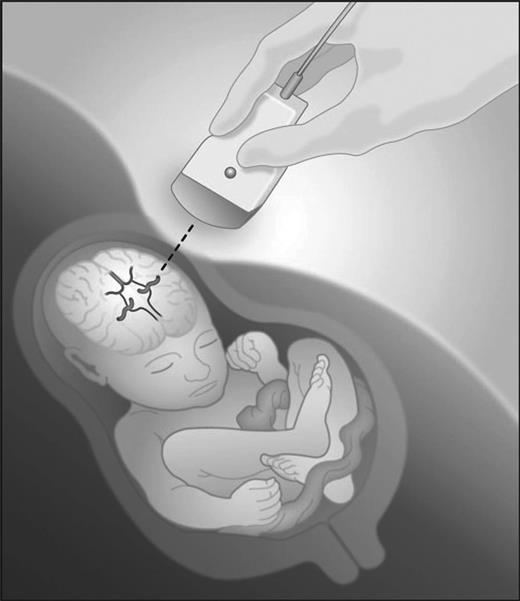
Hemolytic disease of the fetus and newborn (HDFN) is rare condition that occurs when maternal red blood cell (RBC) or blood group antibodies cross the placenta during pregnancy and cause fetal red cell destruction. The fetal physiological consequences of severe anemia in the fetus can also lead to edema, ascites, hydrops, heart failure, and death. In less severe cases, the in utero red cell incompatibility can persist postnatally with neonatal anemia due to hemolysis, along with hyperbilirubinemia and erythropoietic suppression.
Epidemiology and pathophysiology
There are an estimated 3/100 000 to 80/100 000 cases of HDFN per year in the United States.1 The maternal blood group antibodies that cause HDFN can be naturally occurring ABO antibodies (isohemagglutinins), or develop after exposure to foreign RBC; the latter are called blood group alloantibodies. For HDFN to occur, the fetus must be antigen positive (paternally inherited) and the mother must be antigen negative. Several studies have investigated the prevalence of red cell sensitization. In a large series of 22 102 females in the US, 254 (1.15%) of the women were found to have a red cell alloantibodies, of whom 18% had more than one alloantibody.2 In the Netherlands, the prevalence of red cell alloantibodies detected in the first trimester was 1.2%.3
The most common cause of blood group incompatibility results from the ABO blood group system, with incompatibility present in up to 20% of infants.4 However, because anti-ABO antibodies are predominantly IgM class, most are not effectively transported across the placenta. In addition, the A and B antigens are not well developed on fetal red blood cells. Together, this results in a low rate of clinically severe HDFN due to ABO compatibility, although the incidence of more mild disease varies from 1:150 to 1:3000, depending on the parameters used for the case definition, such as bilirubin levels or neonatal anemia.1 Because maternal ABO antibodies are present without previous sensitization, HDFN due to ABO antibodies can occur in the first pregnancy and has a recurrence rate up to 87%.1 It is most commonly seen in group O mothers with group A infants (European ancestry) or group B infants (African ancestry).
The most clinically significant forms of HDFN are caused by maternal blood group alloantibodies are of IgG1 and IgG subclasses, which cause hemolysis more effectively than other IgG subclasses. IgG1 and IgG3 are transported across the placenta by the Fc receptor from the second trimester onward.5 Once in the fetal circulation, the antibody binds antigen-positive fetal red cells that are then cleared by the fetal spleen. Free hemoglobin is metabolized into bilirubin that is conjugated by the maternal liver. As anemia worsens, fetal hematopoiesis increases, termed “erythroblastosis fetalis” and organs involved in red blood cell synthesis (liver, spleen) may enlarge. In the most severe cases, portal hypertension and reduced hepatic synthesis of albumin leads to low plasma oncotic pressure, edema and ascites. “Hydrops fetalis” refers to the state of widespread effusions and associated high-output cardiac failure and death.6 A large population-based study in Sweden found that the presence of maternal red cell antibodies was significantly associated with adverse outcomes, with a 1.4-2.4 relative risk of preterm delivery and a 1.5-2.6 relative risk of stillbirth in mothers with red cell allosensitization as compared to those without.7
After delivery, the passive blood group antibody can continue to affect neonatal red cells causing ongoing anemia until the maternal antibody is no longer present, which can be weeks to months after birth. In early neonatal anemia, bilirubin from red cell destruction can rise quickly because the fetal liver's metabolic machinery is not well developed. Very high levels of unconjugated bilirubin can lead to bilirubin encephalopathy, which clinically presents acutely as lethargy, and can include neurological and muscular manifestations, such as hypotonia, hypertonia, a weak suck, seizures, and/or coma. Chronic and permanent effects of kernicterus, which is permanent neuronal damage from hyperbilirubinemia, includes cerebral palsy, auditory dysfunction, intellectual, or other handicaps.6 Infants may also enter a hypoproliferative phase of anemia due to erythropoietic marrow suppression from maternal antibody, as well as intrauterine transfusion, and simple transfusion (Table 1).8 Low erythropoietin production by the infant may also be contributory to low hemoglobin levels.9 Late hemolysis can continue to cause low blood counts and elevated bilirubin.
Neonatal manifestations of HDFN anemia by time of onset
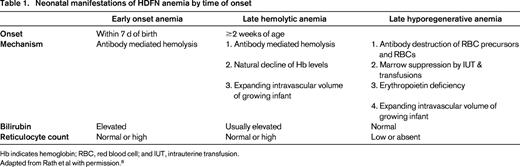
Hb indicates hemoglobin; RBC, red blood cell; and IUT, intrauterine transfusion.
Adapted from Rath et al with permission.8
Maternal alloimmunization results from exposure to foreign red blood cells through previous or current pregnancy, previous transfusions, or organ transplant. During pregnancy, there is spontaneous mixing between fetal and maternal circulation (fetal–maternal hemorrhage; FMH). The mixing increases throughout the pregnancy; 3%, 12%, and 45% in trimesters I, II, and III, respectively, although the amounts of fetal blood in the maternal circulation is generally very small. Hemolytic disease of the fetus and newborn due to red cell alloantibodies rarely occurs in first pregnancies because the highest risk for FMH is later in the pregnancy, especially at delivery, and new alloantibodies are more likely to be formed after delivery. Any physical perturbation of a fetus or placenta in utero also increases the risk of FMH, such as trauma, abortion, ectopic pregnancy, amniocentesis, or multiple pregnancy.10 Once exposed, the maternal immune system may or may not respond to foreign red cell antigens.11 The immune response to red cell antigens is complex and not fully understood. It is clear that the RhD antigen is the most potent immunogen of all of the red cell antigens; 85% of RhD-negative individuals will sensitize (form anti-D) after challenge with a 200 mL transfusion of red cells, although more recent data suggests this is far lower.12 Although as little as 0.1 to 1 mL of RhD-positive red cells can stimulate antibody production, the volumes of FMH are generally small, which contributes to relatively low alloimmunization rates in pregnancy. Before Rh(D) immunoprophylaxis was implemented in 1968, 16% of ABO compatible D-negative mothers with D-positive infants developed anti-D antibody. However, a much lower amount (≤2%) developed anti-D in mother/fetus pairs that were ABO incompatible because of the ABO antibody mediated clearance of fetal red cells from the maternal circulation.13
Although RhD remains the most prevalent cause for HDFN due to allosensitization, other red cell antigens are known to be commonly etiologic (Table 2). Other antibodies that have been less commonly reported include E, k, Kpa, Kpb, Ku, Ge, M, Jsa, Jsb, Jka, Fya, Fyb, S, s, and U.4 A Dutch case-controlled study utilizing a national database of 900 pregnant women with RBC sensitization to non-RhD red cell antibodies enumerated the risk factors for maternal allosensitization. Factors found to be associated with red cell allosensitization in the women were previous major surgery, red cell or platelet transfusion, multiparity, having had a previous male child, and operative removal of the placenta.14
Red blood cell antibody specificity in female population studies
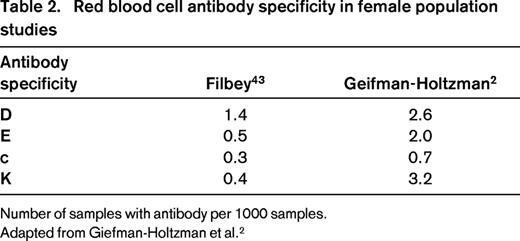
Number of samples with antibody per 1000 samples.
Adapted from Giefman-Holtzman et al.2
Diagnosis
All pregnant women should have testing performed, including a blood type (ABO, RhD) and antibody detection test (indirect antiglobulin test) that detects IgG antibodies.15 For patients with red cell sensitization, the antibody specificity is determined and initial risk stratification occurs. Certain blood group antibodies such as anti-I, -P1, -Lea, and -Leb, may be ignored because the corresponding (cognate) antigens are incompletely developed at birth, the antibodies are typically not IgG, and clinical experience has established the rarity of their causing HDFN.4 Women with red blood cell sensitization to clinically significant red cell antigens (such as D, E, c, K, etc) are transitioned into a pathway of more intensive diagnostic testing and monitoring. If paternity is assured, the paternal blood type is usually determined to predict fetal risk of inheriting the antigen that the maternal antibody is directed against. For most blood group systems, serological testing of the father's blood type is sufficient to predict homozygosity or heterozygosity of the antigen. For instance, anti-K and anti-k antisera can detect K and k antigens, respectively, and provide accurate prediction of the risk that the fetus has inherited the blood group antigen. For RhD, serological testing alone cannot predict the number of RhD genes that the father carries because there is no antithetical allele for the RhD gene. Thus, in the case of maternal anti-D sensitization, paternal genotyping to detect copy number of the RhD gene is recommended.16 Fetuses may bear a 50% risk of antigen inheritance when testing identifies paternal heterozygosity for the antigen in question.. Direct fetal genotyping can determine fetal blood group expression in these settings and provide accurate prediction of fetal risk for HDFN in sensitized mothers. Red cell genotyping can be accomplished using fetal amniocyte genetic testing (obtained via amniocentesis or chorionic villus sampling), or using fetal DNA obtained from maternal serum.17 The latter methodology has found broad appeal due to its noninvasive approach.18
For pregnancies at risk of HDFN due to maternal alloimmunization and possible fetal RBC expression of the cognate antigen, prenatal care by maternal–fetal medicine physicians is recommended. A detailed maternal history is useful to determine previous pregnancy outcomes, particularly for past stillbirths or hydropic fetal losses, and potential etiology of the offending red cell antibody. In addition, fetal ultrasound to determine gestational age and absence of ascites is indicated.19
The red cell antibody titer, or strength, helps with further stratification, although the relatively subjective nature of these assays should always be kept in mind.20,21 Traditionally, serial antibody titers are used to detect ongoing sensitization, with arbitrary thresholds of increasing antibody strength used to indicate ongoing and increasing immune stimulation, presumably due to the presence of fetal red cell antigen. If there was a previously affected pregnancy, trending of the titer will not be a reliable measure of increasing sensitization. In addition, transfusion laboratories establish critical antibody titers at which the antibody strength has reached a level that may lead to significant fetal anemia (titers of 1:16-32 are commonly used).4 However, because the Kell blood group antigens are present on early red cell precursors, a maternal anti-K of relatively low titer, such as 8, may lead to severe hypoproliferative anemia.22 Using other techniques for antibody strength determination, such as flow cytometry, may be more precise than antibody titers.23
Fetal management
For pregnancies that have reached 16-24 weeks, or when a critical antibody titer is reached (depending on maternal history of previously affected pregnancies), fetal anemia is monitored using cerebral MCA Doppler velocity measurements every 2 weeks for risk stratification (Figure 1).19,24 Correlative studies support that the use of the noninvasive MCA Doppler technique as a surrogate measurement for assessing fetal anemia.19 Doppler readings that are >1.5 multiples of the mean (MoM) are very sensitive, with a 12% false-positive rate, thus trending is important. Weekly fetal monitoring, such as ultrasound and fetal heart rate monitoring, is also often performed).
Diagram of fetal middle cerebral artery Doppler velocimetry testing. Adapted from Moise42 with permission.
Diagram of fetal middle cerebral artery Doppler velocimetry testing. Adapted from Moise42 with permission.
When fetal anemia becomes moderate to severe as indicated by Doppler MoM measurements exceeding 1.5, invasive testing via cordocentesis is done to determine fetal hematocrit. If the fetus has not reached an acceptable gestational age for delivery, and the hematocrit level is <30%, intrauterine transfusion is usually indicated. Blood products for fetal transfusion should be ready at the start of the procedure for immediate use. Intrauterine transfusion (IUT) is performed by inserting a needle into the umbilical vein using ultrasound guidance and infusion of red cells at a predetermined hematocrit level. The selection of red cell products for intrauterine transfusion is typically Group O, Rh D-negative (or -positive, depending on maternal blood group antibody), leukocyte reduced, hemoglobin S-negative, CMV-safe (CMV seronegative or leukocyte reduced), irradiated, and antigen-negative for maternal red cell antibody/antibodies. Because authors have reported that there is a risk of additional maternal red cell antibody formation after IUT, some centers have adopted providing prospectively matched Rh C, c, E, e, and K-matched transfusions.25 Despite this, there is evidence that women undergoing Rh- and K-matched IUTs still form additional alloantibodies [to Duffy (FY), Kidd (JK) and (MNS) S blood group antigens].26 Following one or more IUT procedures, the fetal circulation is comprised primarily of donor red cells, as fetal marrow production is suppressed and the remaining circulating red cells are destroyed.27 The procedure of intrauterine transfusion carries a 1%-3% risk of fetal adverse events such as infection or rupture of membranes; procedure outcomes in the early second trimester are poor.28-30
Small patient series have explored using maternal treatment with plasma exchange and/or intravenous immunoglobulin (IVIG) to blunt the effect of the maternal antibody on the fetal red cells with some success.31 The American Society for Apheresis considers plasma exchange in this setting a Category II (second line therapy), with a weak grade of evidence.32 The ultimate decision for delivery is based on the treating physician's judgement, and it is standard to maintain pregnancy until the fetus has reached a safe gestational age. For severely affected fetuses, the risk of continued monitoring and intrauterine transfusion is balanced against this, and most suggest delivery at 37-38weeks, although earlier delivery may be warranted in severe HDFN.24
Neonatal management
At birth, the connection to the maternal circulation is severed, and the risk of neonatal hyperbilirubinemia increases significantly because of the immature development of the metabolic pathway to break down bilirubin in the neonatal liver. Although most jaundice in newborns is benign, management of hyperbilirubinemia is critical in the neonatal period because of the risk for bilirubin-induced encephalopathy.33 Affected infants may need phototherapy to oxidize unconjugated bilirubin to allow for urinary excretion. For patients with known HDFN, close observation of bilirubin levels and hemoglobin is warranted to determine whether neonatal exchange transfusion is needed to wash out bilirubin and maternal antibody, and/or if transfusions are indicated to support oxygen carrying capacity to the tissues.8 Administration of IVIG to the newborn has been used to reduce the need for exchange transfusions and phototherapy, but it does not affect the need for top off transfusions, and a Cochrane review suggests that more study is needed to determine the best use of IVIG in this setting.8 When levels of bilirubin reach critical levels, exchange transfusion is indicated (Table 3). Blood product selection is similar to that of IUT, however, because infant whole blood is also being removed, the RBC unit is usually mixed with a plasma unit to create reconstituted whole blood. After a two-volume exchange transfusion, ∼90% of the red cells have been replaced and 50% of the bilirubin has been removed. After exchange transfusion, a platelet count should be performed to monitor for iatrogenic thrombocytopenia.
Guidelines for exchange transfusion in infants 35 or more weeks gestation
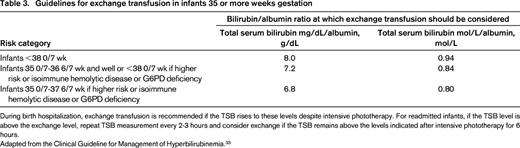
During birth hospitalization, exchange transfusion is recommended if the TSB rises to these levels despite intensive phototherapy. For readmitted infants, if the TSB level is above the exchange level, repeat TSB measurement every 2-3 hours and consider exchange if the TSB remains above the levels indicated after intensive phototherapy for 6 hours.
Adapted from the Clinical Guideline for Management of Hyperbilirubinemia.33
Hyporegenerative anemia can last for many weeks after birth (Table 1). Infants must be carefully monitored for clinical signs of ongoing anemia, whichis most likely manifested by poor feeding, the most aerobic activity for neonates. They may also have increased sleep as anemia worsens. In ongoing anemia, the reticulocyte production from the fetal bone marrow may be decreased, and other cells lines, such as neutrophils can be affected. Weekly monitoring of reticulocytes and hematocrit will help to guide decision making about transfusion, and also provide reassurance when the marrow is recovering.
Prevention (transfusion, RhIG)
Prevention of HDFN can be divided into primary and secondary measures; there are no international standards, thus, nations differ on preventative measures including dosing and dosing schedules of RhIg and approach to transfusion. Primary prevention focuses on prevention of maternal alloimmunization in the first pregnancy. This is encompassed by the policy employed by some transfusion services, or blood banks, to provide red cell transfusions to females of childbearing potential that are more highly matched than standard transfusions to prevent transfusion-induced red cell sensitization. For instance, in some European countries, K-negative RBC units are provided to women younger than 45-50 years of age. Other nations provide additional matching for antigens, such as c and E.34 In a retrospective review in Croatia, 48% of 214 pregnancies with maternal sensitization to E, K, or c had a history of transfusion, suggesting that matching for these antigens may be protective, however, the paternal antigen status was not reported.35 A study in the Netherlands found that a proportion of maternal sensitization to non-RhD blood groups with clinically affected offspring was due to intrauterine transfusion itself.26 Therefore, these measures may be effective in reducing the incidence of HDFN; however, further comparative studies are needed.
Secondary prevention of HDFN focuses on the RhD RBC antigen given that RhD immune globulin (RhIg) is available to prevent naïve RhD-negative immune systems from synthesizing anti-D antibody after exposure to small amounts of RhD antigen. The risk of an RhD-negative mother becoming allosensitized can be reduced to from 16% to <0.1% by the appropriate administration of RhIg.13 At this time, there are no other pharmaceutical therapies that can prevent blood group sensitization for other blood group antigens. The transfusion service laboratory plays a critical role in guiding therapy with RhIg. For pregnant women who are RhD-negative, RhIg is typically administered at 2 time points over the course of the pregnancy. The first dose is provided at 28 weeks gestation, as recommended by the American College of Obstetricians and Gynecologists (ACOG) because the majority of allosensitization appears to occur after this time point, and the second after delivery of an RhD-positive infant.36 The 28 week dose reduces the rate of allosensitization to RhD from 1.5% (antenatal administration only) to 0.1%. As noted above, any procedure or trauma that increases the rate of fetomaternal hemorrhage should elicit the administration of an additional RhIg dose. The antenatal RhIg dose is increased when the volume of fetomaternal hemorrhage is found to be ≥10 mL using the Kleihauer–Betke test. Although used for many years, the Kleihauer–Betke test has been found to be imprecise, and recent attention has focused on newer technologies, such as flow cytometry to provide more accurate quantification of the FMH volume37
As molecular testing advances throughout the field of medicine, so does the application of blood typing using molecular techniques. In certain patients, serological reagents do not accurately detect the RhD type. The most common genetic backgrounds that account for this serological typing problem are called weak D phenotypes. Recently, authors have encouraged the use of RhD genetic testing for patients with a weak D phenotype to provide accurate and actionable results for RhD blood typing and RhIg administration.38,39 Further scientific study is needed to elucidate the clinical significance of different RhD genotypes in various ethnic backgrounds and the risk factors for RhIg failure.40 Precise determination of fetal RhD typing has been widely accepted in Europe and improved the ability to guide RhIg therapy.18
In conclusion, HDFN is a multifaceted disease that has distinct technical considerations over several critical time periods of fetal and neonatal development. Advances in maternal–fetal medicine, such as the inventions of RhIg, IUT and noninvasive fetal genetic testing, have led to dramatic improvements in the outcomes of HDFN and prevention of maternal allosensitization.41 Future advances in blood typing and noninvasive testing will continue to improve the care of mothers and their offspring affected by blood group incompatibility.
Correspondence
Meghan Delaney, Bloodworks NW, 921 Terry Ave, Seattle, WA 98104; Phone: 206-689-6500; e-mail: meghand@bloodworksnw.org.
References
Competing Interests
Conflict-of-interest disclosures: M.D. has consulted for Williams Kastner and has received honoraria from Grifols/Novartis and Bioarray/Immucor; and D.C.M. declares no competing financial interests.
Author notes
Off-label drug use: IVIG is included as a therapy for mothers with HDFN. This will be discussed in brief.