Abstract
The myelodysplastic syndromes (MDSs) are a heterogeneous collection of clonal hematopoietic malignancies that compromise a large subgroup of the myeloid neoplasms and collectively are the most common acquired adult bone marrow failure syndromes. Currently, only 3 agents are approved for the treatment of MDS by the US Food and Drug Administration (FDA): azacitidine, decitabine, and lenalidomide. The latter drug, approved in 2006, is the most recent agent approved by the FDA for MDS and there has been mediocre success with novel agents for the past 9 years. The heterogeneity of MDS as a disease group is likely to be a strong contributor to this slow progress but recent developments in molecular characterization of MDS are improving diagnostic accuracy, providing insights into pathogenesis and refining our prognostic ability in the field. With the advent of these developments, appropriately chosen therapeutics or even targeted agents may be able to improve patient outcomes in the future.
Learning Objectives
To describe newer data in somatic mutations for patients with MDS and their implications for diagnosis and prognosis
To explain current use of FDA approved medications for MDS and options in setting of hypomethylating agent failure
To discuss novel agents in clinical trials for the treatment of MDS
The myelodysplastic syndromes (MDSs) are a heterogeneous collection of clonal hematopoietic malignancies that compromise a large subgroup of the myeloid neoplasms and collectively are the most common acquired adult bone marrow failure syndromes.1 They are characterized by poor overall survival (OS) due to ineffective hematopoiesis, progressive cytopenias, and transformation to acute myeloid leukemia (AML). Currently, only 3 agents are approved for the treatment of MDS by the US Food and Drug Administration (FDA): azacitidine, decitabine, and lenalidomide. The latter drug, approved in 2006, is the most recent agent approved by the FDA for MDS. Each was approved based on data showing efficacy in limiting the clinical impact of MDS through better outcomes compared to supportive care. These improvements include decreased transfusion burden, delayed progression to AML, improvements in quality-of-life, and extended survival. All are currently used as monotherapies with modest results. Currently, the only treatment modality capable of curing MDS is allogeneic hematopoietic stem cell transplantation (HSCT). Unfortunately, the applicability of HSCT has historically been limited by the inherent risks of the procedure and the older age and attendant comorbidities of typical MDS patients. Though the recent advent of reduced intensity preparative regimens and more available alternative donors has begun to expand the use of HSCT, outcomes remain mixed. New approved therapeutic options, which can both prolong survival and enhance quality-of-life in MDS (either to serve as definitive treatment or bridge to HSCT and beyond), are urgently needed for this challenging group of patients. The path to this goal is dependent on an increasing understanding of the molecular pathophysiology of MDS. With this knowledge, novel therapeutic combinations and new targeted therapies can be identified and trialed. Unfortunately, recent clinical trials have had mixed results, and no new regimens or targeted therapies have to date shown either great promise or clear progress on the path to approval. This paper will discuss some of the factors underlying this mediocre track record and will discuss how novel insights into the pathophysiology and biology of MDS may inform future advancements and shifts in MDS treatment paradigms.
Diagnosis: accuracy and risk assessment
The incidence of MDS has increased over time due to both improved diagnosis and increasing disease awareness. Concurrently, systematic capture of MDS incidence in various registries has stabilized estimates of disease burden.2 Recent estimates suggest that the current incidence of MDS is ∼5 per 100 000, sufficient to rank MDS as the most commonly diagnosed myeloid neoplasm in the US and Europe.3 Still, the natural history of MDS is poorly understood, a deficit which may be partly mitigated by the impending National MDS Study, an initiative of the National Institutes of Health to establish a publicly available, well-characterized, clinical dataset linked to a prospectively-collected biospecimen repository. This multicenter prospective cohort study could facilitate the understanding of MDS pathogenesis and progression, enhance MDS diagnosis, classification and prognosis, and facilitate biomarker discovery—all with the goal of identifying new therapeutic targets and defining the optimal use of existing therapies.
Most patients are diagnosed with MDS after initially presenting with cytopenias. A thorough evaluation of cytopenias is necessary to fully characterize the underlying disease. Not infrequently, other bone marrow failure syndromes are misdiagnosed as MDS4 ; this is particularly problematic as many of the potential MDS mimics could alter response rates (both positively and negatively) if these populations are included in MDS clinical trials. This rigorous characterization of disease in all MDS patients at the time of diagnosis, incorporating as much new knowledge as possible, is imperative to improve disease outcomes.
A risk-adapted treatment strategy is currently used in MDS and the majority of clinical conversations with patients center on assessment of risk and accordingly appropriate therapeutic choices. Patients are placed into risk-stratified categories to help patients and physicians choose suitably aggressive or conservative therapies. Historically, various prognostic scoring systems have been used to place patients in risk categories. The current prognostic scoring systems and risk assessment tools (IPSS or the IPSS-R5,6 and the WHO 2008 guidelines1 ) incorporate cytopenias, blast percentage, cytogenetic risk groups, and at times, age or transfusion requirements. It is important to emphasize that these systems only guide choice of therapy and do not predict response. The most recent of these scoring systems was the IPSS-R, which was an evolution of the IPSS to weigh the various factors with respect to their impact on disease risk. The IPSS-R gives greater weight to cytogenetic lesions than to blasts and cytopenias are treated individually as opposed to grouped by number only. Worse cytopenias have greater severity. Finally, a more explicit method for considering patient age is included in the IPSS-R. The IPSS-R is slightly more complex to calculate but the increased granularity of the requisite clinical data has improved risk stratification for MDS patients. Notably, however, the IPSS-R does not include molecular abnormalities. Even with these scoring systems, most clinicians still divide MDS at prognosis into higher-risk (IPSS ≤1.0 or IPSS-R ≤4.0) and lower-risk disease (IPSS ≥1.5 or IPSS-R ≥4.5).
Somatic mutations in MDS driver genes (especially those associated with poorer outcomes) have been shown to add independent prognostic information.7 Changes to these classifications systems will be forthcoming as the availability to detect somatic molecular mutations increases and we are better able to define the genetic and molecular basis of MDS.8 Clinical application of this knowledge should allow not only more refined diagnoses, but may provide an ability to predict therapeutic response, to currently approved and future agents. Future therapeutic choices may be predicated on a fuller characterization of risk that incorporates this additional diagnostic information. Even more exciting is the possibility that a more advanced molecular understanding of MDS pathophysiology may facilitate the design of targeted therapies.
Disease characterization: role of molecular genetics
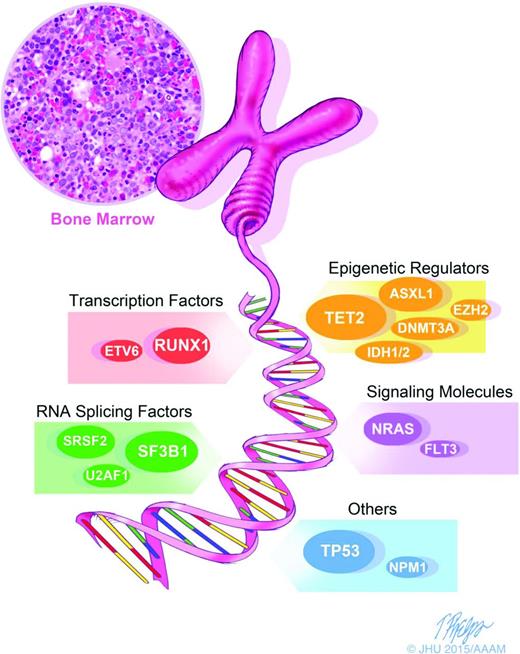
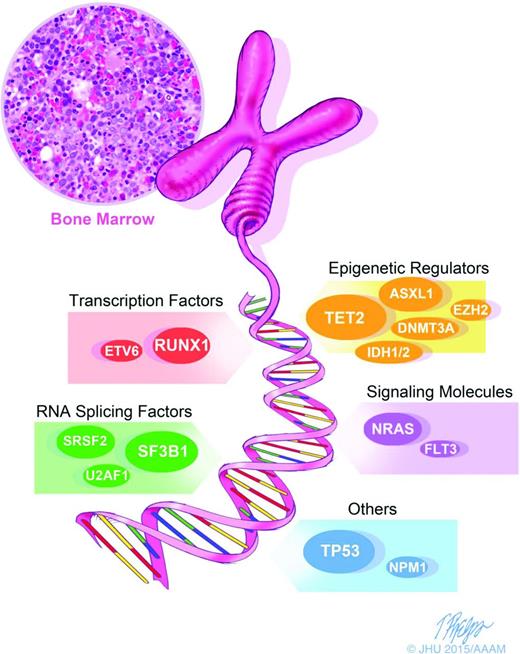
Morphologic and karyotypic analyses (eg, ring sideroblasts, deletions in the long arm of chromosome 5), have been the backbone of MDS diagnosis and classification. The recent introduction of novel sequencing techniques has extended diagnostic analysis beyond morphology and karyotyping and allowed for the detection of somatic mutations in MDS. These advances are leading to a new descriptive approach to MDS (Figure 1) and should allow the more precise definition of distinct MDS categories., Hopefully, this will help penetrate the heterogeneity of MDS and allow more disease-specific therapy. Several large and elegant series have demonstrated the promise of genomic techniques in MDS cohorts. Bejar et al9 showed that >51% of MDS patients have molecular mutations. They specifically identified several somatic point mutations with adverse prognostic significance: TP53, EZH2, ETV6, RUNX1, and ASXL1. Papaemmanuil et al10 and Haferlach et al11 detected and confirmed additional molecular mutations in 74% and 89.5% of patients, respectively. The most common 12 mutated/deleted genes were TET2, SF3B1, and ASXL1 in >20% of patients; SRSF2, DNMT3A, and RUNX1 in >10%; and U2AF1, ZRSR2, STAG2, TP53, EZH2, and CBL in >5% of cases.9-11 (Figure 1) These molecular markers add an additional descriptive layer at the time of disease evaluation that may elucidate both pathogenesis and prognosis.
Diagnostics in MDS: molecular diagnostics are an important corollary to morphology and karyotyping and represent the next step in advancing our characterization of MDS. We are moving toward more precisely defined and distinct categories of MDS, allowing us to see through the heterogeneity and potentially allow drug and trial design for specific populations.
Diagnostics in MDS: molecular diagnostics are an important corollary to morphology and karyotyping and represent the next step in advancing our characterization of MDS. We are moving toward more precisely defined and distinct categories of MDS, allowing us to see through the heterogeneity and potentially allow drug and trial design for specific populations.
A particularly interesting set of mutations are those which affect the spliceosome machinery, including SF3B1, SRSF2, ZRSR2, and U2AF1. These mutations, which occur in >50% of MDS patients, are mutually exclusive and are highly specific to MDS. One splicing factor mutation, SF3B1, has been closely associated with the specific MDS subtype of refractory anemia with ring sideroblasts (RARS).10,12,13 The association between SF3B1 mutation and the presence of ring sideroblasts appears to be causal, and is the first gene mutation to be strongly associated with a specific morphological feature.13 Ring sideroblasts are characterized by an excess accumulation of iron in erythroblast mitochondria, and SF3B1 mutant RARS cases show a specific pattern of abnormal iron distribution characterized by coarse iron deposits, which markedly differs from wild-type RARS cases.14 SF3B1 mutations are more prevalent in lower-risk MDS and are independent predictors of favorable clinical outcome.15 It is not yet feasible to choose therapy based on these mutations, but the thorough phenotypic characterization of these mutations makes them attractive for targeted therapies.
Other mutations have also been associated with outcomes in MDS. The presence of DNMT3A mutations is associated with a more rapid progression from MDS to secondary AML,16 and mutations of ASXL1,17 RUNX1, IDH1,18 and EZH219 also portend a poor prognosis. TP53 mutations have been identified at an early disease state in ∼20% of lower-risk MDS patients with 5q deletion, and suggest an increased risk of transformation to AML.20 The increasingly clear pattern of acquired somatic mutations in MDS has important implications in both prognostic and treatment paradigms. As our molecular knowledge of MDS increases, a novel MDS prognostic score based on molecular mutations has been suggested.11 Though not yet validated in larger samples of patients, this molecular approach to prognosis has exciting potential. The presence of a specific mutation may change prognosis independent of IPSS score.7,9 Furthermore, the more mutations that are present in an individual patient, the worse their prognosis is likely to be. Mutations may also have implications for specific therapeutics: mutations in TP53, TET2, or DNMT3A identify patients with MDS with shorter overall survival after HSCT.21 Thus, in the near future, mutational analysis may significantly inform treatment decisions.10,11
At present, clinicians should consider initiating molecular mutation analysis at diagnosis in patients with clear MDS or clinical suspicion of MDS, in whom the results of morphology and cytogenetics are noninformative (eg, in cases without increase of blasts or ring sideroblasts and/or with a normal karyotype). These molecular analyses can also be performed in patients with a confirmed diagnosis of MDS after therapy failure or prior to allogeneic HSCT, to improve risk stratification and prognostication. However, this information should be used judiciously, with acknowledgement of the inherent limitations of the data currently supporting molecular diagnostics in MDS. It is important to remember that in the absence of any other evidence of MDS, these mutations are not diagnostic. Similarly, it has recently been demonstrated that with age, an increasing number of patients will have DNA evidence of clonal hematopoiesis in the absence of any other evidence of myeloid malignancy.22 Although such clonal populations are associated with an increased risk of developing a subsequent hematologic malignancy (including MDS) their presence cannot be used in isolation to make a diagnosis23 As molecular diagnostic techniques become increasingly used in the evaluation of MDS, it will be essential to closely study their performance to ensure that the reported associations with disease phenotypes and outcomes are real. More importantly, the role of molecular genetic testing in MDS needs to move beyond the current utility for diagnosis and prognosis. If we are to improve clinical outcomes in MDS, tailored therapies based on specific molecular characteristics need to become a reality.
Standard therapeutic options
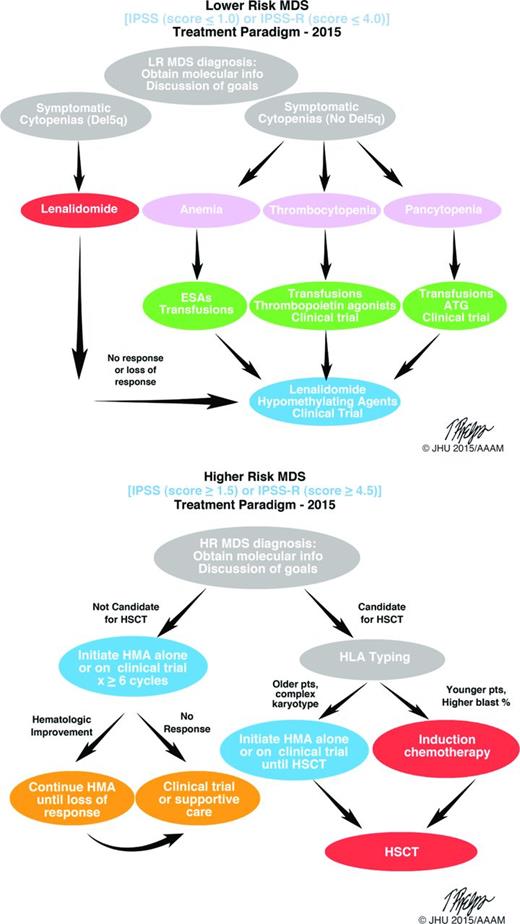
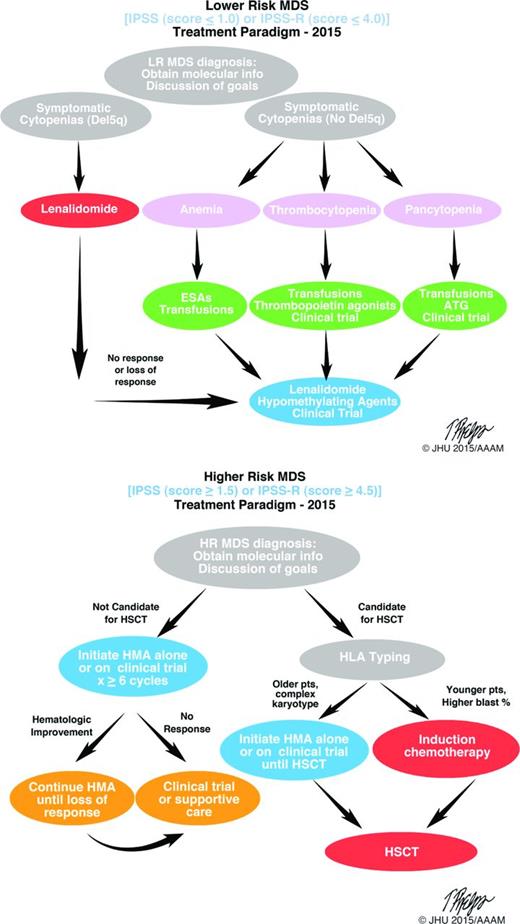
The current (2015) standard treatment paradigms for the treatment of lower and higher risk MDS are shown in Figure 2. Early disease characterization for both higher- and lower-risk disease (as defined by IPSS and IPSS-R) are important to guide therapy; similarly, open discussion with patients regarding goals of therapy will also help guide treatment decisions. Lenalidomide, a thalidomide analog, is an immunomodulatory agent that has demonstrated clinical efficacy in MDS patients with lower IPSS scores and a deletion in the long arm of chromosome 5 (del5q). This was the most recent drug approved and demonstrates the success of studying a drug in the context of a well-defined subset (del5q) of patients. Targeting specific patient populations, as defined by molecular markers, has great promise for the future of successful therapeutics in MDS.
MDS treatment algorithms. Accurate diagnosis, disease classification (lower or higher risk) and goals of treatment are important at initial diagnosis. Once therapy is appropriate, selection of suitably intense therapies versus disease-modifying treatments, including stem cell transplantation, can be pursued. Clinical trial enrolment should be considered at all steps in the process. Molecular phenotyping at diagnosis is valuable. LR indicates lower risk; ESA, erythropoietin stimulating agent; ATG, anti-thymocyte globulin; HR, higher risk; HMA, hypomethylating agent; and HLA, human leukocyte antigen.
MDS treatment algorithms. Accurate diagnosis, disease classification (lower or higher risk) and goals of treatment are important at initial diagnosis. Once therapy is appropriate, selection of suitably intense therapies versus disease-modifying treatments, including stem cell transplantation, can be pursued. Clinical trial enrolment should be considered at all steps in the process. Molecular phenotyping at diagnosis is valuable. LR indicates lower risk; ESA, erythropoietin stimulating agent; ATG, anti-thymocyte globulin; HR, higher risk; HMA, hypomethylating agent; and HLA, human leukocyte antigen.
The hypomethylating agents (HMA), azacitidine and decitabine, are considered standard in higher risk MDS. Several multicenter randomized studies have compared treatment with HMA treatment to supportive care in MDS patients, including the D-0007 (decitabine), AZA-001 (azacitidine), CALGB-9221 (azacitidine), and EORTC-06011 (decitabine) trials. These studies have demonstrated delayed progression to AML following HMA therapy, with an improvement in median progression-free survival over supportive care ranging from 3 to 8 months. Interpretation of these trials has varied. Some construe these outcomes as decidedly positive; others view these data as dramatically demonstrating the need for more effective agents. Regardless, optimal outcomes with HMA therapy demands close attention to the details of treatment. Therapy must be started expeditiously, used with the correct dose and schedule, and continued until progression occurs. Typically, this requires at least 6 cycles comprised of azacitidine for 7 days at 75mg/m2 or decitabine for 5 days at 20mg/m2, both administered every 28 days.24 With retrospective review of larger cohorts of patients treated with these agents and examination of the molecular phenotypes, it may become possible to identify subsets of patients with specific somatic mutations that have higher or lower response rates to these therapies.25,26 This is the first step toward using novel molecular data to guide therapy and improve outcomes for MDS patients and emphasizes why full characterization at diagnosis is so important. Although deviation from these current therapeutic strategies may not be guided at this time by molecular data, as our biologic knowledge of these diseases increases, this information will be increasingly important in in disease classification, prognosis, and therapeutic selection (Table 1). For example, in the del5q subset of lower risk MDS, there is active interest in examination of additional somatic mutations (eg, TP53) to modify risk assessment and identify groups of patients who may benefit from additional therapies in conjunction with lenalidomide.
Somatic mutations as predictors of response to standard therapy
As discussed above, specific somatic mutations are being retrospectively analyzed for associations with response to therapy. For example, scoring systems based on the presence of TET2 and DNMT3 mutations have been suggested to predict response and survival after HMA therapy.25 Each of these mutations, if present, predicts a positive response to HMA therapy. These molecular features combined with other clinical parameters, such as degree of thrombocytopenia and leukopenia, may also independently predict better response and progression-free survival. Additionally, the absence of ASXL1 mutations and presence of SF3B1 mutations are also positive predictors of response to HMA therapy; associations again strengthened with known data on cytogenetic risk, age and hemoglobin levels.25 Molecular features can also predict lack of response to HMAs: the association of TET2 mutations with a higher probability of response to HMAs is negated if ASXL1 is coexpressed.26 Similarly, MDS patients with a complex karyotype, especially with chromosomes 5 and 7 anomalies, do not enjoy any significant survival benefit from treatment with decitabine or azacitidine.25 The same holds true with mutations of TP53, which not only predict lack of HMA response, but have been associated with shorter OS.27,28 Despite these data, it should be noted that no current molecular evidence would truly preclude the use of HMAs, as these are still the only effective treatment for the majority of MDS. Nonetheless, based on these data, one could argue that mutational assessment is imperative prior to initiation of therapy. The information obtained from mutational assessment could guide treatment discussion and decisions; more importantly, however, these data can be used proactively to anticipate resultant progression after therapy. If markers predict inefficacy of HMAs, this would allow consideration or anticipation and planning of alternate therapies, such as HSCT. Finally, molecular data can also assist in prognosis: the presence of a single mutation may worsen prognosis, regardless of IPSS score, and dramatically change a patient's life expectancy.7 The presence of multiple mutations in a single patient is also associated with poor outcomes and may have bearing on prognosis.11
Beyond the hypomethylating agents: is this the impasse?
In spite of demonstrable improvements in MDS treatment with HMAs and the fact that HMAs remain the only effective therapy for higher-risk MDS, the disease is still incurable. Furthermore, in cases of absence or loss of response to either azacitidine or decitabine, outcomes are extremely poor.29,30 Primary HMA failure is defined as either no response to or progression during HMA therapy; median OS in this situation is 8.6 months. Secondary HMA failure is defined as relapse after an initial response, and has a median OS of 6.4 months.30 Therapeutic options for either primary or secondary HMA failure are limited (Table 2). HMA failure is usually a class effect; there are few data to suggest a benefit from switching between decitabine and azacitidine when one or the other has failed. Intolerance to one of these medications remains a viable reason to switch agents.
In hopes of improving both response rates and durability of response, combination therapy has been explored in depth as both first-line therapy and in the setting of HMA failure. Combination therapy is based on the theory that efficacy is increased by combining drugs with different mechanisms of action. Unfortunately, the bulk of available evidence seems to oppose any such synergy, and it appears that the addition of other agents to HMAs does not provide marked benefit over single HMA therapy. The most promising combination has been of the 2 approved agents azacitidine and lenalidomide.31 Among 36 patients enrolled (18 phase 1, 18 phase 2), the overall response rate was 72%: 16 patients achieved a complete response (CR), and 10 had hematologic improvement. Median CR duration was 17 months (range, 3-39). In reviewing the patient population, it was noted that TET2/DNMT3A/IDH1/2 mutational status was associated with response in some patients,31 arguing that modest response rates in trials could be increased within select populations. Other combinations have shown less promise and increased treatment toxicities have been seen. For example, azacitidine has been combined with various histone deacetylase (HDAC) inhibitors without a significant increase in efficacy but with increased toxicity. The recently published HDAC/HMA cooperative group study, E1905, combined azacitidine and etinostat; this combination did not improve outcomes compared to azacitidine alone; Rates of overall hematologic response were 46% and 44%, respectively, but with increased adverse events in the combination arm.32 Similarly, the combination of decitabine and valproic acid yielded reasonable response rates (55%), but was not better than decitabine alone. Again, neurologic and other toxicities were higher in the combination arm.33 The most recent cooperative group effort, the S1117 trial, compared AZA monotherapy to either AZA and vorinostat or AZA and lenalidomide. This study was stopped earlier due to lack of efficacy of either combination arm beyond single agent AZA. Enrollment criteria were broad in this upfront trial, and patients with CMML and treatment-related MDS were enrolled in this trial. This disease heterogeneity could have contributed to the low response rates. Future reviews of these data may suggest populations (defined molecularly or otherwise) that could benefit from further investigation of combination regimens.
Due to the lack of effective alternative strategies, off-label use of agents typically used in AML treatment paradigms have been employed at the time of HMA failure. Clofarabine, low-dose cytarabine, or even induction chemotherapy have been used. This may be reasonable in some patients, depending on individual patient goals, but the risks are not insignificant, and there are few data to support this approach. The development of HMA failure, and the associated grim prognosis, often leads to revisiting discussions of HSCT, even in patients who have previously declined this option. However, HSCT has limited applicability in this population, especially in the setting of physical deconditioning. Supportive care at the time of HMA failure may be the only reasonable option for elderly or deconditioned patients or those who are otherwise ineligible (because of geographic, donor, social, or financial reasons) for either HSCT or enrollment in a clinical trial. To prevent this dire situation, we need to be able to intervene more effectively from the onset and along the entire continuum of disease. More effective first line agents are urgently needed for MDS, but almost as important is the identification of optimum treatment strategies in the setting of HMA failure. Novel therapies, especially in higher-risk disease, may be the way to approach this goal.
Novel approaches in MDS therapy: promise unfulfilled?
Multiple trials in both lower risk and higher risk MDS have explored alternative agents in hopes of achieving hematologic improvements, reducing or eliminating transfusions, and sustaining more durable response beyond the currently approved agents. In lower-risk disease subtypes, many therapeutic strategies have been aimed at relieving the morbidity of problematic cytopenias. Multiple agents have been investigated, all with rather disappointing response rates (Table 3). The marked morbidity of profound thrombocytopenia has led to increasing off-label use of thrombopoietin agonists (romiplostim and eltrombopag) in MDS patients with thrombocytopenia. The potential risk of proliferation and expansion of blasts cautions against the routine use of these agonists, though it is reassuring that use of thrombopoietin mimetics has not definitively correlated with transformation to frank AML.46 A recent placebo-controlled trial in lower-risk MDS showed that both platelet transfusion dependence and clinically significant bleeding events were reduced with romiplostim therapy.47 Further analysis of the data from this study demonstrated a relationship between endogenous thrombopoietin levels and platelet response to romiplostim; lower baseline thrombopoietin levels (<500 pg/mL) and limited platelet transfusion history predicted a greater likelihood of a platelet response to romiplostim.48 This strategy of identifying patient characteristics which may predict response is being retrospectively applied to the data from several of the trials listed in Table 3. For example, post hoc analysis of the Luspatercept study by Platzbecker et al40 suggested higher response rates in patients with SF3B1 mutations (Table 3). It may be that several of these agents are much more effective in specific variants of MDS than in “all-comers.” Identification of appropriate study populations will be of critical importance in future MDS trials.
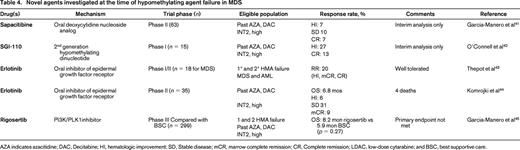
Given the lack of available therapies in the setting of HMA failure, patients should be encouraged to participate in clinical trials when geographically feasible and performance status allows. Multiple clinical trials are ongoing for patients who have failed HMA agents (Table 4). Results so far in this clinical setting have been modest but this remains an area of active investigation. Interim results from a Phase 2 trial of the oral deoxycytidine nucleoside analog sapacitabine in HMA failure patients (both primary and secondary) demonstrated an overall response rate of 14%-19% and median OS of ∼8 months. The final results of this study are eagerly anticipated. Another agent, rigosertib, a dual PLK1/PI3K inhibitor, has been studied in a Phase I/II trial after HMA failure; 40% of evaluable patients demonstrated either a BM or peripheral blood response. Median OS was ∼10 months.49 Unfortunately, the results of the Phase 3 trial of rigosertib (compared to best supportive care) do not show a statistically significant improvement in the primary outcome of OS, but the trend is encouraging: OS for rigosertib arm was 8.2 months compared with 5.9 months in the control arm. Other agents, including tosedostat (aminopeptidase inhibitor), ipilimumab (anti-CTLA4 inhibitor), and anti-PD1 therapies are all being actively investigated in patients who have failed HMAs. As discussed above, many of these trials include molecular phenotyping of diagnostic samples, and analyses are planned to see if a certain molecular signature predicts either response or nonresponse, hopefully allowing improved trial design in the future.
At this time, HSCT is the only therapy with the potential to cure MDS. There is active investigation targeted at making this treatment more tolerable to both patients and physicians and to extend HSCT availability to a broader group of patients. Greater use of alternative graft sources (eg, haplo-identical donors), use of nonmyeloablative or reduced-intensity conditioning regimens in older or sicker patients may all increase the applicability of HSCT. Post-transplant maintenance strategies with azacitidine or immunotherapies (eg, anti-PD1 agents) may increase the durability of response to HSCT, just as post-transplant tyrosine kinase inhibitors have been used in Philadelphia chromosome positive disease, and improve long-term survival. Additionally, education of patients and physicians about the timing, indications, and potential curative result of HSCT is warranted. A recent survey demonstrated that “knowledge gap” regarding the safety and efficacy of HSCT may limit potential candidates for HSCT due to lack of referral to a HSCT center and inaccurate concerns about adverse events and quality-of-life.50
Targeted therapies: putting it all together
As discussed above, detailed mutational analysis will allow more specific characterization of MDS phenotypes. It may become possible to retrospectively analyze data from previous therapeutic trials for specific markers of response. This will allow the generation of targeted strategies in specific patient groups. We will likely be able to design clinical trials for specific populations to optimize the chance of meaningful response rates. A template for this hypothetical approach can be found in the recent targeting of IDH1/IDH2 mutations in acute leukemia, which is showing tremendous promise. In MDS, a possible corresponding example lies in spliceosomal mutations.13 As mentioned above, spliceosome mutations, occur with extreme frequency in MDS and are mutually exclusive, which simplifies their use to identify specific patient phenotypes. A correlation with clinical outcomes has been established with these mutations; the effect on prognosis varies depending on the specific mutation. For instance, MDS with SF3B1 mutations have a good prognosis,15 whereas MDS with SRSF2 or U2AF35 mutations have a poorer prognosis.51 Therapies targeting these mutations are in development, and a phase I study in solid tumors has evaluated safety of E7107, a pladienolide derivative blocking SF3B1.52 Evaluation of this and other agents targeting spliceosome mutation products represents an outstanding example of a novel approach to MDS therapeutics
Conclusions
Treatment of MDS depends on individualized approach based on an individual patient's specific risk stratification based on disease and patient factors. In the last few years, exciting data have accumulated on somatic mutations in MDS and their prognostic impact and correlation with response to therapy with currently available agents. In the very near future, somatic mutation analysis may become an important factor in determining therapeutic strategy. More exciting, however, is the possibility for specific targeted therapies based on knowledge of the functional role of these and other mutations. For the moment, targeted therapy in MDS is an aspirational goal, but the principals we have learned thus far from MDS trials will inform those future efforts. Even as we search for new drugs, we need to ask why previous agents, which have had strong pathophysiologic rationale, are failing. Perhaps we have targeted the wrong pathways. It is also possible that previously unappreciated disease heterogeneity may have led to combining wrong populations in trials, skewing response rates. Despite the seeming lack of progress, as measured by approval of new agents, this is an exciting time for clinical research in MDS. Strategies that involve not only the optimization of hypomethylation, but also the inhibition of mutated proteins, modulation of immune response, and inhibition of signal transduction pathways are all under active investigation at the basic and translational levels. As our understanding disease pathology and the inherent biological complexity of MDS increases, diagnostic accuracy and prognostic assessment will improve, leading to new approaches to therapy. We are not at an impasse in MDS; rather, we are finally developing a roadmap to guide the management of MDS. As we better characterize MDS, and fill in this map, approval and application of novel and effective therapies will surely follow.
Correspondence
Amy E. DeZern, Division of Hematologic Malignancies, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans St, CRBI Rm 3M87, Baltimore, MD 21287-0013; Phone: 443-287-2935; Fax: 410-614-7279; e-mail: adezern1@jhmi.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: Lenalidomide for non-del5q MDS; and vorinostat and valproic acid for higher risk MDS.