Abstract
Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) has been regarded for decades as the ALL subgroup with the worse outcome. It represents the most frequent genetic subtype of adult ALL, and increases progressively with age. The introduction of tyrosine kinase inhibitors (TKIs) has enabled to obtain complete hematologic remissions (CHRs) in virtually all patients, including the elderly, to improve disease-free survival and overall survival, as well as to increase the percentage of patients who can undergo an allogeneic stem cell transplant (allo-SCT).
The current management of adult Ph+ ALL patients relies on the use of a TKI with or without chemotherapy followed by an allo-SCT, which still remains the only curative option. Minimal residual disease screening is permitting not only a better stratification of patients, but has also allowed to reconsider the role of autologous stem cell transplant for a set of patients who do not have a donor or are not eligible for an allo-SCT. At present, clinical challenges are represented by the emergence of resistant mutations, particularly the gatekeeper T315I, for which alternative approaches, comprising novel TKIs or therapies based on the combination of TKI with immunotherapeutic strategies, are being considered in order to overcome resistance.
Learning Objectives
To establish the gold-standard management of adult Ph+ ALL that includes a timely diagnosis, the use of a tyrosine kinase inhibitor (TKI) during induction and allogeneic transplant procedures
To describe the new emerging clinical issues represented by the identification of resistant and compound mutations, for which at present there is not a compelling strategy
To describe novel approaches, including those combining TKIs with immunotherapeutic strategies currently under development
The BCR/ABL1 rearrangement, derived from the t(9;22) translocation, also called the Philadelphia (Ph) chromosome, can be detected in ∼20%-30% of adult cases with acute lymphoblastic leukemia (ALL); its incidence increases with age.1 Historically, Ph+ ALL was considered the ALL subgroup with the worse outcome, since the rate of complete hematologic remissions (CHRs) with chemotherapy regimens was lower than that observed in other subsets and the 5 year event-free survival (EFS) was <20%.2
The management and outcome of Ph+ ALL have dramatically changed since the introduction of tyrosine kinase inhibitors (TKI)s, which induce higher remission rates and enable much better survival rates, also in elderly patients. Currently, the gold standard first-line approach is based on the use of a TKI with or without chemotherapy, followed by consolidation procedures; allogeneic stem cell transplant (allo-SCT) still represents the only curative option, with the caveat of transplant-related mortality, long- and short-term toxicities, particularly in less young patients, where Ph+ ALL is more frequent. It must also be considered that a subgroup of patients may be spared this procedure and further toxicity: these cases might be recognized by the dynamics of minimal residual disease (MRD) clearance, the absence of additional genomic lesions (such as IKZF1 and other recurrently mutated genes) and type of fusion protein (p190 rather than p210).
At present, the scientific community is facing other clinical challenges. In fact, also in the TKI era, there are a set of open issues: the emergence of resistant mutant clones represents a major problem, with novel inhibitors currently under investigation; furthermore, the goal of a chemo-free approach based on a combination of TKI and monoclonal antibodies seems today an option to be pursued, at least for some patients. This overview will focus, among others, on these aspects.
Diagnosis
At present, the diagnostic work-up of ALL must include a rapid identification of the Ph+ chromosome and/or BCR/ABL1 rearrangement: this should be performed as soon as possible, during the steroid pre-phase used in many protocols, in order to optimize management of patients. Although in the past the diagnosis of Ph+ ALL was made by conventional cytogenetics, at present these cases are better identified by FISH or, preferably, by reverse transcriptase polymerase chain reaction (RT-PCR). The latter allows not only to identify the presence of the BCR/ABL1 rearrangement in all cases, but also to define the type of transcript, ie, p190 (e2a2) or p210 (b2a2, b3a2). In addition, a quantitative RT-PCR (Q-PCR) assay also enables to quantify the levels of BCR/ABL1 rearrangement and permits adequate MRD analyses. This framework is essential for an optimal diagnostic work-up and management of ALL patients of all ages and is also crucial for a uniform monitoring of MRD during the course of the disease. In the TKI era, the definition of the presence of the BCR/ABL1 rearrangement should be included in the minimal diagnostic work-up requirement for ALL patients of all ages.
Induction therapy
The inclusion of a TKI in the induction phase represents the gold-standard management of Ph+ ALL patients, because it leads to much higher CR rates and improved long-term outcome; it is now generally accepted that the use of TKIs has also the relevant advantage of increasing the likelihood of carrying out an allo-SCT in a greater number of patients compared with historical controls. The first studies used imatinib as TKI, together with or following induction treatment. This approach led to a significant improvement in the management of all Ph+ ALL patients, including the elderly, because remission rates outreached 90% in the majority of studies and improvements in disease-free survival (DFS) and overall survival (OS) rates were also recorded.3-12 A similar experience has been obtained also with the second generation inhibitor dasatinib, a more potent oral inhibitor of the BCR/ABL1, c-KIT, and SRC kinase families.13 In all these combination studies, toxic deaths were invariably recorded during induction in ∼5% of cases.
The GIMEMA group14-17 has, over the years, adopted a different induction strategy based on the administration of a TKI, either imatinib or dasatinib, plus steroids alone in induction, without systemic chemotherapy, in addition to intrathecal central nervous system (CNS) prophylaxis. These regimens have been used in adult and elderly populations (∼200 cases have been treated so far), and have led to CHR rates in 96%-100% of patients without toxic deaths in induction, thus indicating that this strategy is effective, feasible and safe, and partly doable at home. In fact, in the GIMEMA LAL 0201B study,14 designed for elderly Ph+ ALL (median age 69 years, range 61-83), all patients achieved a CHR with imatinib plus steroids alone and intrathecal CNS prophylaxis; in the subsequent LAL 1205 trial for patients >18 years (with no upper age limit), based on dasatinib (plus steroids) as induction treatment, a CHR was achieved in all 53 evaluable patients15 ; in the LAL 0904 protocol, that enrolled 51 (49 evaluable) patients and was based on the sequential use of imatinib followed by chemotherapy, CHR rates reached 100%, with DFS and OS at 36 months of 50% and 69%, respectively16 ; finally, in the Total Therapy LAL 1509 protocol (dasatinib followed by chemotherapy), 58/60 (97%) patients achieved a CHR at the end of induction, and DFS and OS are of 50% and 72% at 2 years, respectively.17 Similarly, the PETHEMA group18 has shown in the Ph-08 trial that reducing the intensity of chemotherapy while increasing the dose of imatinib led to a 100% CHR in the 29 patients treated and increased EFS. Along the same line, Chalandon et al19 recently compared the results obtained in 268 patients treated either with a reduced intensity chemotherapy and imatinib or with the standard imatinib/HyperCVAD treatment, showing that CHR rates were significantly better in patients receiving a de-intensified treatment; a slight superiority, though not significant, was observed also in terms of OS and EFS at 5 years, thus reinforcing the concept that a less aggressive regimen during induction is capable of inducing the same or better long-term outcomes as intensive treatments. Figure 1 depicts the improvements witnessed over the years by introducing imatinib in the induction backbone and the advantages achieved by de-escalating chemotherapy.
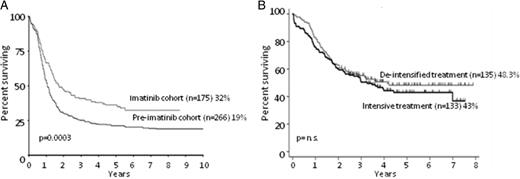
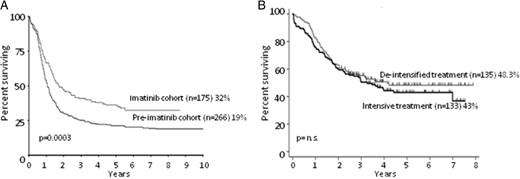
OS of Ph+ ALL patients receiving TKIs. (A) OS at 10 years of patients enrolled in the UKALLXII/ECOG2993 trial, comparing cases receiving imatininb (imatinib cohort, n = 266) versus those not receiving it (pre-imatinib cohort, n = 175). Adapted from Fielding et al.11 (B) OS at 5 years of patients receiving imatinib combined with de-intensified treatment (n = 135) versus those receiving imatinib plus intensive treatment (n = 133). Adapted from Chalandon et al.19
OS of Ph+ ALL patients receiving TKIs. (A) OS at 10 years of patients enrolled in the UKALLXII/ECOG2993 trial, comparing cases receiving imatininb (imatinib cohort, n = 266) versus those not receiving it (pre-imatinib cohort, n = 175). Adapted from Fielding et al.11 (B) OS at 5 years of patients receiving imatinib combined with de-intensified treatment (n = 135) versus those receiving imatinib plus intensive treatment (n = 133). Adapted from Chalandon et al.19
As for the other TKIs, for which clinical trials are ongoing, few data are available. With regard to nilotinib, a Korean phase II study20 (presented in an abstract form) tested nilotinib together with chemotherapy in 50 newly diagnosed patients: 45 (90%) obtained a CHR and 5 (10%) died during induction. Overall, the relapse-free survival (RFS), EFS, and OS at 2 years were 71.1%, 49.4%, and 66.2%, respectively; no updates have been provided by the investigators. More recently, Ottmann et al21 reported the results of an EWALL study for 47 elderly patients (>55 years of age, 12 patients older than 70 years) in which standard chemotherapy was combined with nilotinib: a CHR was obtained in 97% of cases (36 evaluable for response), with 30% achieving also a complete molecular remission; toxicity was represented by 34 SAE (serious adverse events). However, the median follow-up of this study is of 211 days and it is thus difficult to draw definitive conclusions.
Finally, the MDACC group22 (again in an abstract form) reported the results of a phase II study combining the pan-TKI ponatinib with the HyperCVAD regimen. Thirty-seven patients were included and the short-term results appear very promising: in fact, a CHR was achieved in all cases and a major molecular response in 75% of patients. With a median follow-up of 18 months, 31 CHR patients are alive and 6 have died in CHR, 3 due to cardiac-related toxicities (1 occurring after ponatinib was interrupted), 1 from multi-organ failure, 1 from head injury after a fall, and 1 from sepsis post-transplant. The 1 year progression-free survival and OS rates are 96% and 86%.
The results of the different studies are summarized in Table 1. Taken together, they indicate that the optimal induction strategy should be therefore based on the administration of a TKI, with few or no chemotherapy at all, to avoid early deaths. Virtually all elderly patients (with no age limit) can obtain a CHR with a TKI alone plus steroids. One open issue remains the choice of the best TKI. Although results are comparable in terms of CHR achievement, dasatinib induces a more sustained MRD, but is also aggravated by more toxicity, such as pleural and cardiac effusion; ponatinib is a very attractive compound, given its role on the T315I mutation, but warnings were provided because of possible thrombotic effects,23 vascular adverse events (VAEs), and hypertension. As a result, ponatinib was transiently removed from the market in the United States in 2013 and later reintroduced. Thus, caution and careful evaluation of potential risk factors must be applied when using this compound.
Following CHR achievement, consolidation/intensification treatment, which varies according to the different study groups but generally includes high-dose chemotherapy (particularly for younger patients), is aimed at further reducing/eradicating MRD levels. Consolidation/intensification treatment should be performed in all cases with persistent MRD positivity, and even more importantly in individuals who do not have a donor or are not eligible for an allo-SCT.
MRD monitoring and other biologic features
Q-PCR BCR/ABL1 quantification represents the best approach for MRD monitoring in Ph+ ALL. The levels of BCR/ABL1 reduction achieved early during therapy are a good indicator of subsequent response and outcome, because high levels of residual BCR/ABL1 transcripts at different treatment stages, or a consistent and reproducible increase of BCR/ABL1 levels, indicate poor responsiveness to chemotherapy and to TKI.
With few exceptions,5,10 there is a general consensus on the prognostic role of MRD. Yanada et al5 observed no association between rapid achievement of BCR/ABL1 negativity and long-term outcome; also, the GRAAL group10 showed that early MRD evaluation did not influence significantly patients' outcome, both in terms of OS and DFS. At variance, Lee et al24 documented that an early 3-log reduction in BCR/ABL1 transcripts strongly predicted a reduced relapse risk. Similarly, Ravandi et al25 reported on the impact of MRD, by multiparametric flow-cytometry (MFC) and/or by Q-PCR, in patients treated with imatinib or dasatinib and showed that MRD positivity by MFC at 3 and 12 months, and by Q-PCR at 3, 6, 9 and 12 months is predictive of OS. The GIMEMA14-17 group has shown that a rapid and profound MRD reduction upon induction is associated with a significantly better DFS in patients treated both with imatinib14,16 and dasatinib.15,17 (Figure 2).
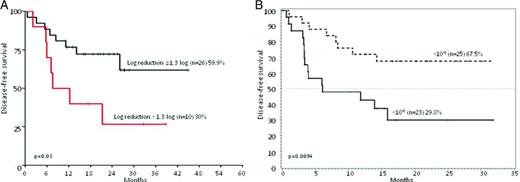
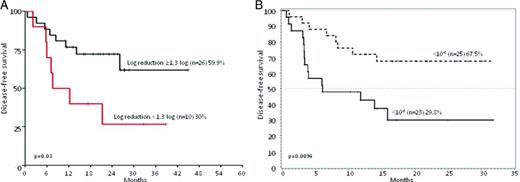
DFS of Ph+ ALL patients stratified according to MRD levels. (A) DFS at 36 months of patients enrolled in the GIMEMA 0904 trial (imatinib plus steroids) stratified on the basis of a MRD reduction cut-point of 1.3 log at the end of induction (day +50): 26 patients achieved a MRD reduction ≥1.3, whereas 10 did not. Adapted from Chiaretti et al.16 (B) DFS at 20 months of patients enrolled in the GIMEMA 1205 trial (dasatinib plus steroids) stratified based on a MRD reduction cut-point level of <10−3 at the end of induction (day +85): 25 patients achieved BCR-ABL levels <10−3 and 23 did not. Adapted from Foà et al.15
DFS of Ph+ ALL patients stratified according to MRD levels. (A) DFS at 36 months of patients enrolled in the GIMEMA 0904 trial (imatinib plus steroids) stratified on the basis of a MRD reduction cut-point of 1.3 log at the end of induction (day +50): 26 patients achieved a MRD reduction ≥1.3, whereas 10 did not. Adapted from Chiaretti et al.16 (B) DFS at 20 months of patients enrolled in the GIMEMA 1205 trial (dasatinib plus steroids) stratified based on a MRD reduction cut-point level of <10−3 at the end of induction (day +85): 25 patients achieved BCR-ABL levels <10−3 and 23 did not. Adapted from Foà et al.15
Thus, the role of MRD emerges as a clear prognostic factor and could indeed be pivotal in driving further personalized strategies. In fact, a persistent MRD positivity and/or increase may be indicative of the emergence of resistant mutations and mutational screening should therefore be performed in these patients to drive therapeutic decisions and the possible introduction of novel TKIs.
An open issue in this respect remains the definition of precise cutoff levels for the quantification of the disease; they are usually very variable among studies and highlight the need for standardization and harmonization of the methodologies used for BCR/ABL1 quantification in Ph+ ALL. From a more clinical standpoint, attention should be also paid to patients' compliance to drug ingestion, as well as to clinical conditions that might interfere with adsorption. In case of MRD increase, clinicians should carefully inquire patients' compliance.
Beyond MRD, other biologic factors appear to play a prognostic role: IKZF1 deletions, encoding for the transcription factor Ikaros, are frequently (≈80%) detected in Ph+ ALL, both in children and adults.26,27 IKZF1 deletions are predictors of poor outcome in Ph+ ALL, regardless of age. It must be noted that, although there is no evidence of their prognostic role in terms of CHR achievement, IKZF1 deleted cases have a shorter DFS and higher incidence of relapse.26-28
With few exceptions,12 there is also increasing evidence that patients carrying the p210 fusion transcript, prevalently found in chronic myeloid leukemia (CML), and also defined as major breakpoint cluster region (Mbcr) or b3a2 and/or a b2a2, have a worse prognosis, both in terms of response to therapy and long-term outcome,17,19,28 than cases harboring the p190 fusion transcript [minor breakpoint cluster region (mbcr) or e2a2] of the BCR/ABL1 protein.
Taken together, a hypothetical model for patients' stratification, that will need to be validated in larger cohorts of patients, should include MRD levels, presence/absence of IKZF1 deletions and type of fusion transcript (the role of mutations will be discussed separately).
Allogeneic stem cell transplant (allo-SCT) and the role of TKI post-transplant
Despite the major advancements witnessed following the advent of TKIs and despite its significant toxicities and mortality, allo-SCT still remains the only truly curative option for Ph+ ALL, mostly for younger adult patients,. The NILG group reported that in patients receiving imatinib followed by allo-SCT (n = 45) compared with those not undergoing allo-SCT (n = 29), the DFS and OS at 5 years were 46% versus 8% and 42% versus 29%, respectively.8 In this study, all subjects were scheduled to receive an allo-SCT if a suitable donor was identified. The results of the UKALLXII/ECOG2993 study11 showed a clear benefit in patients receiving an allo-SCT (n = 82) vs those who did not (n = 39) in terms of OS, EFS and RFS (50% vs 19%, 46% vs 14%, and 69 vs 18%, respectively). Finally, in the GIMEMA0904 protocol, a higher OS at 3 years and lower rate of relapse in transplanted patients were observed, which however did not reach significance.16
Recently, the EBMT group has provided a comprehensive update on the role of allo-SCT in the TKI era in the largest cohort of patients so far reported (n = 390). The following conclusions were reached: (1) as expected, TKI administration prior to transplant is associated to a significantly better OS both in univariate (47% vs 38% in the pre-TKI era) and multivariate analysis; similarly, it correlates with a lower relapse rate in univariate (33% vs 50%) and multivariate analysis; (2) MRD at the time of transplant—which, however, was not uniformly evaluated—was not associated with differences in OS, leukemia-free survival (LFS), relapse rate and nonrelapse mortality; and (3) the significant beneficial effect of the prophylactic administration of a TKI following allo-SCT was confirmed, in terms of improvements of LFS, OS, and relapse rate.29
Some caveats must, nevertheless, be taken into account. In the pediatric setting, that however includes patients with different clinical and possibly biologic features, van der Veer et al30 reported that a subgroup of cases not carrying IKZF1 aberrations (n = 20) have an excellent outcome (4 years DFS 78.6%), as opposed to IKZF1-deleted patients (n = 43; 4 years DFS 51.9%), also without undergoing an allo-SCT. In the current EsPhALL protocol for childhood Ph+ ALL, allo-SCT after the consolidation blocks is based on risk stratification (good or poor) and donor availability; in the good risk patients, only a genotype-matched donor is permitted, whereas in the poor risk group any type of donor is allowed. Daver et al12 recently reported on the long-term outcome (13 year follow-up) of patients treated with an imatinib plus hyperCVAD regimen, and showed no clear benefit of transplant.12 In our GIMEMA 1509 trial, based on dasatinib plus steroids administration during induction, followed by a consolidation according to the molecular response, we identified a small set of patients who achieved a sustained complete molecular response upon induction (ie, BCR/ABL1/ABL1 ratio = 0) and who possibly may be spared a transplant procedure.17
Thus, even in the TKI era allo-SCT still remains an indispensable procedure for eligible adult patients; further efforts need to be made to identify patients who may possibly be spared the procedure and its related toxicity.
Another important issue is the use of a TKI after transplant. There is a general consensus that a TKI should be administered following transplant. It must be noted, however, that the administration of TKIs following allo-SCT may be poorly tolerated, as reported by the PETHEMA experience,7 mostly because of concomitant transplant-related toxicities.
Autologous stem cell transplant (auto-SCT): a new era?
Some considerations must be made on the “novel” role of auto-SCT in Ph+ ALL. Several reports have analyzed the outcome of small numbers of patients who underwent an auto-SCT and suggested that results were comparable in terms of OS, DFS, and RFS with those obtained following an allo-SCT.8,10,19,31 A more comprehensive study has been recently published by Giebel et al,32 who evaluated the role of auto-SCT in a cohort of 177 adult Ph+ ALL: patients were subdivided into 3 categories, according to the period in which the procedure was performed: 1996-2001, 2002-2006, where sporadic cases already received TKI during therapy, and 2007 onward, when all patients received imatinib. OS and LFS significantly increased among the 3 categories, being 16% and 11%, 48% and 39%, and 57% and 52%, respectively. Furthermore, a more detailed subanalysis was performed in 29 patients treated with imatinib: OS and LFS were of 65% and 60%, respectively, with a better trend, though not significant, for patients in complete molecular response (n = 22) versus those who were MRD+ at the time of transplant (n = 7; 72% and 65% vs 57% and 57%, respectively). This latter finding will need to be confirmed in larger series of patients. It must be noted that TKI treatment was continued after the transplant. Similar results were recently published by Chalandon et al, who showed comparable results between allo- and auto-SCT.19
These data indicate that an auto-SCT can be effective in Ph+ ALL patients who do not have a donor or are not eligible for an allo-SCT, and should be considered as an intensification treatment followed by TKI administration.
BCR/ABL1 mutations
Approximately 80%-90% of patients with Ph+ ALL who relapse while on imatinib have BCR/ABL1 mutations, with a predominance of P-loop and T315I mutations; with dasatinib, relapse is most often associated with the T315I mutation, whereas P-loop mutations are less common. Mutations, including the gatekeeper T315I, may already be present at diagnosis, but this does not seem to correlate with a subsequent relapse or persistence of remission.33 Next generation sequencing analyses are unraveling the genomic complexity of BCR/ABL1 mutations and highlighting the presence of multiple mutations.34
Beyond T315I mutations, an emergent clinical problem is also the occurrence of the so-called compound mutations,35 defined as the presence of 2 or more mutations in the same molecule, whose emergence might be also sustained by selective pressure induced by TKI treatment. Compound mutations, together with T315I mutations, currently represent the greatest challenge to deal with: the most clinically relevant ones are located on 12 key positions (ie, including key residues) and, importantly, confer resistance, as expected, to imatinib, nilotinib, dasatinib, ponatinib, rebastinib, and bosutinib. Even more importantly from a clinical standpoint, the T315I-inclusive compound mutants confer resistance also to ponatinib. Therefore, for such cases, the use of alternative approaches is urgently required.
Novel drugs and strategies
As a direct consequence of the emergence of resistant mutations, further research is ongoing to identify novel compounds or alternative combined approaches. Among others, the activity of the pan-aurora kinase inhibitor danusertib, which displays a potent activity also against the gatekeeper T315I mutant, has been tested in a phase I study, in patients with CML and Ph+ ALL.36
PF-114 is a selective inhibitor that is capable in vitro and in mice models to: (1) inhibit the autophosphorylation of BCR/ABL1 and BCR/ABL1 T315I mutants, (2) reduce tumor growth, and (3) prolong survival in mouse models.37 Its biochemical profile indicates activity also against the T315I mutation. The vascular endothelial growth factor (VEGF) receptor inhibitor axitinib, a TKI already approved for patients with refractory renal cell carcinoma, has been shown to be particularly active in vitro on primary cells from CML patients harboring the T315I mutation and partly effective also in T315I wild-type CML cases.38 In vivo activity has also been observed in a CML case carrying the T315I mutation.38
Other possible compounds are based on different mechanisms of action. Imozide derivatives, which are potent STAT5 inhibitors, have activity in BCR/ABL1-positive cells, also those harboring T315I mutations.39 Similarly, augmenting PP2A (protein phosphatase 2A) activity by dissociating it from its endogenous inhibitor SET, is an appealing approach to overcome TKI-based resistance, because it augments BCR/ABL1 degradation.40
Furthermore, interesting in vitro studies have recently documented the possibility of combining synergistic compounds. Applemann et al41 reported the results of the combination of dasatinib together with ruxolitinib and dexamethasone in mouse models and nicely showed the additive efficacy of ruxotilinib in inhibiting downstream STAT-dependent pathways, which resulted also in prolonged survival. The addition of dexamethasone had further beneficial effects, because it prevented the occurrence of the mutations otherwise observed when combining dasatinib and ruxolitinib, ie, T315I or the F317L and P-loop mutations.
The combined in vivo activity of bosutinib and the Chk1 inhibitor PF-00477736 against imatinib-resistant cells was investigated in a mouse model; a synergistic effect between the two drugs in reducing tumor growth compared to each drug alone was observed.42 The results are summarized in Table 2.
Other promising approaches are based on the combination of TKI with immunotherapy strategies. Blinatumomab, a bispecific T cell–engaging antibody binding CD19 and CD3, is currently being investigated in a randomized phase III study for relapsed/refractory B-precursor ALL adult patients and was previously tested also for patients with detectable MRD. This compound has been associated with very encouraging responses, mostly in molecularly refractory/relapsed cases. Blinatumomab appears effective also in Ph+ ALL patients: in an earlier study published by Topp et al43 5 patients with Ph+ ALL were included and 3 of them achieved a molecular response. The updated results of this study (median follow-up: 33 months)44 showed that 4 of the 5 cases did not receive an allo-SCT; 2 of them, both receiving TKIs as consolidation treatment, are still in CHR, whereas the other 2 patients who did not receive further consolidation, relapsed after 4.2 and 5.1 months. These data indicate that Blinatumomab is capable of inducing a molecular response also in this poor prognostic group and that further treatment, represented by either an allo-SCT or a TKI as consolidation therapy, is necessary to prevent relapses. Following these results, the Alcantara study (EudraCT Number 2013-000706-36) has been designed specifically for relapsed/refractory Ph+ adult patients and analyses are ongoing.
In line with this, the authors of this review are currently activating a phase II chemo-free protocol for adult Ph+ ALL patients (no upper age limit), meant to evaluate the feasibility and efficacy of an induction treatment based on dasatinib, steroids, and intrathecal therapy, followed (in patients with MRD persistence or reappearance) by the administration of Blinatumomab.
Along the same line, Inotuzumab ozogamicin, a CD22 monoclonal antibody bound to calicheamicin, might be used in combination with TKIs to control the disease.
Finally, CAR (chimeric antigen receptor) T cells targeting CD19 represent a novel appealing approach that has been tested in a variety of high-risk lymphoproliferative disorders, including adult Ph+ ALL. Results are currently preliminary and a longer follow-up and larger cohorts of patients are required to establish the feasibility and efficacy of this strategy.
In the near future, it is conceivable that the management of Ph+ ALL might benefit from the introduction of the compounds mentioned above or by the use of combined strategies. However, it must be underlined that, at present, the T315I mutation and/or compound mutations represent a clinical emergence, for which the only possible strategy is to try to abrogate the mutated clone and promptly carry out a transplant procedure.
Concluding remarks
In conclusion, whereas “old” issues, such as achievement of CHR and dismal survival rates, have been partly solved in both adult and elderly patients, we are currently facing other open issues: (1) the achievement of sustained and durable molecular responses should be the next major goal to further improve the outcome of Ph+ ALL patients, possibly obtainable through the combination of a TKI plus immunotherapeutic approaches, followed or not by a transplant; (2) the upfront, or at least early, recognition of cases that might be spared an allo-SCT procedure will eventually reduce the nonrelapse mortality rates; and (3) the occurrence of resistant clones and compound mutations is still a clinical challenge, that might benefit by combination approaches, based on the use of a TKI together with other molecules interfering with downstream signaling pathways.
Finally, these improvements could be translated also to the so called “BCR/ABL1-like” cases. This subgroup, identified in both children and adults, and that accounts for about 15%-20% of ALLs, has, from a biologic standpoint, a specific transcriptional profile, concomitant IKZF1 deletions and/or CRLF2 rearrangements, and deregulation of several TKs. From a clinical standpoint, an association with a more unfavorable prognosis has been reported45 ; the latter has been recently reconsidered in childhood cases, because it has been shown that MRD-based risk-directed therapy, including transplant procedures, might overcome the dismal outcome.46 This has still to be confirmed in adult patients, often not eligible to intensive treatments. The role and impact of TKI-based treatment in BCR/ABL1-like ALL patients needs to be further explored.
Acknowledgments
The work on Ph+ ALL was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC), Special Program Molecular Clinical Oncology 5x1000 (N 10007), Milan, Italy; Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR), Rome, Italy; Fondo per gli Investimenti della Ricerca di Base (FIRB): codice RBAP11TFZ_003; and Progetto Giovani Ricercatori 2010: Policlinico di Modena, Codice: GR-2010-2313609.
Correspondence
Robin Foà, Division of Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Via Benevento 6, Rome 00161, Italy; Phone: +39-06-85795753; Fax: +39-06-85795792; e-mail: rfoa@bce.uniroma1.it.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.