Abstract
Venous thromboembolism (VTE) is a common cause of adverse outcomes in patients with cancer. The risk of VTE varies with cancer type, stage and grade, cancer therapy, and supportive care, as well as patient characteristics including age, ethnicity, and inherited and acquired comorbid conditions. VTE prophylaxis should be provided to all hospitalized cancer patients and high-risk outpatients. Low-molecular-weight heparin (LMWH) remains the first-line therapy for VTE in patients with active cancer. Anticoagulation should be continued as long as there is evidence of active disease or patients are receiving cancer treatment. The efficacy of direct oral anticoagulants in the treatment of cancer-associated thrombosis remains incompletely defined. Central venous catheter (CVC)–associated VTE should be treated with anticoagulation alone, unless the CVC is no longer required. Recent studies indicate that anticoagulation may be appropriate for patients with persistent thrombocytopenia or solid tumor brain metastases. Management of recurrent VTE includes the identification of the cause(s) of the recurrence and solutions targeted at addressing the potential precipitants.
Learning Objectives
Discuss risk factors for initial and recurrent VTE in cancer patients
Review the evidence supporting LMWH, warfarin, and direct oral anticoagulants in the management of VTE in cancer patients
Review the management of CVC-associated thrombosis in cancer patients
Discuss the management of recurrent VTE in cancer patients
What is the risk of venous thromboembolism (VTE) in cancer patients?
Thromboembolism is an important cause of morbidity and mortality in patients with cancer. Although both arterial and VTE occur in cancer patients, VTE is significantly more common, so it will be the focus of this paper.1 Cancer patients are at a four- to sevenfold higher risk and constitute ∼20% of all patients with VTE.2 Cancer patients are at a threefold higher risk of recurrent VTE and a twofold higher risk of anticoagulation-associated bleeding.3 Cancer patients with VTE have a 10-fold higher risk of death than patients with VTE alone and a fourfold higher risk of death than cancer patients without VTE.2 VTE is the second leading cause of death among cancer patients.4 Therefore, prevention and treatment of VTE are essential to appropriate management of patients with cancer.
The risk of VTE among cancer patients is influenced by primary site, stage, and histology. The highest risk is associated with cancers of the pancreas, stomach, brain, and lung, and hematologic malignancies, whereas lower risks are associated with breast and prostate cancer (Table 1).5-8 The risk of VTE increases with the extent of disease. In a Danish population-based study, the adjusted relative risk (RR) of VTE hospitalization for stage 1, 2, 3, and 4 cancer was 2.9, 2.9, 7.5, and 17.1, respectively.6 High-grade tumors (grade 3 and 4) are associated with a twofold higher risk of VTE than low-grade tumors (G1 and 2).7 The risk of VTE varies during the course of disease in cancer patients. In the first 6 months after diagnosis of colorectal cancer, the risk of VTE is 5.0/100 person-years compared with 1.4/100 person-years 7 to 12 months post-diagnosis and 0.6/100 person-years 13 to 24 months after presentation.9
Cancer treatment also influences patients’ risk for VTE. Cancer surgery is associated with a RR of 1.7 for VTE compared with non-cancer surgery.10 In a population-based observational study of breast cancer patients, surgery was associated with a 2.2-fold increased risk of VTE in the first month after discharge, which declined in subsequent months. In the Rochester Epidemiology Project, chemotherapy was associated with a 1.8-fold risk of VTE.11 In breast cancer patients, chemotherapy was associated with a 10.8-fold increased risk of VTE during therapy, which declined to baseline 3 months after completion. Hormonal therapy was associated with a 2.4-fold increased risk of VTE during the first 3 months of therapy, which varied by agent. Tamoxifen was associated with a 5.5-fold increased risk, whereas aromatase inhibitors were not associated with VTE. The risk of VTE was particularly high when surgery was performed in the 2 months after chemotherapy.8 Immunomodulatory regimens containing thalidomide or lenalidomide with high-dose dexamethasone with or without multi-agent chemotherapy have been associated with an increased risk of VTE, whereas regimens containing low-dose dexamethasone or bortezomib are associated with a lower risk.12 Although initial studies suggested vascular endothelial growth factor receptor antagonist bevacizumab was associated with an increased risk of VTE, a more recent meta-analysis taking into account the time on therapy did not identify an increased risk (RR, 0.91; 95% confidence interval [CI], 0.77-1.06).13 No increase in VTE has been noted with other vascular endothelial growth factor inhibitors.14 In contrast, monoclonal antibodies targeting the epidermal growth factor receptor have been associated with a twofold increased risk of VTE, whereas small molecule epidermal growth factor receptor tyrosine kinase inhibitors have not.15 Use of erythropoiesis stimulatory agents increases the risk of VTE by 1.7-fold.16
VTE risk is also influenced by individual patient characteristics. Age ≥65 years (odds ratio [OR], 1.1) and female sex (OR, 1.1) have been associated with a slightly increased risk of VTE in hospitalized cancer patients. Black patients have a 1.2-fold increased risk, whereas Asian patients have decreased risk (OR, 0.74).17 Ashrani et al noted obesity (body mass index >35 kg/M2) was associated with a fourfold increased risk.11 In the MEGA case-control study, factor V Leiden heterozygosity was associated with a twofold higher risk of VTE.18 Comorbid conditions such as infection (OR, 1.8), renal disease (OR, 1.5), arterial thromboembolism (OR, 1.5), pulmonary disease (OR, 1.4), and anemia (OR, 1.4) have all been associated with VTE in cancer patients.17
Which cancer patients should receive VTE prophylaxis?
Systematic reviews of hospitalized medical and surgical patients have demonstrated that VTE prophylaxis significantly reduces the risk of VTE at the cost of increased major bleeding.19,20 However, the majority of these studies were conducted more than a decade ago and relied almost exclusively upon radiologic rather than clinical end points. These limitations have prompted a reappraisal of universal thromboprophylaxis in hospitalized patients. This reassessment is particularly relevant for hospitalized medical oncology patients because no primary VTE prophylaxis trials have been conducted in medically ill cancer patients and only a minority of participants in the landmark medical prophylaxis trials had cancer.21 Consequently, limited evidence supports VTE prophylaxis in hospitalized medical oncology patients.
A number of expert opinion and evidence-based VTE risk assessment models (RAMs) have been developed for hospitalized medically ill and surgical patients to identify those who should receive thromboprophylaxis.22 Although several of these RAMs have been validated in independent patient populations, it is premature to use these models for risk assessment in cancer patients. Each of the RAMs consider all cancers to be associated with a similar risk of VTE. In addition, none of the models include other known cancer-specific VTE risk factors. Finally, none of the RAMs were developed or validated in cancer patient populations. Therefore, at the present time, the author recommends that all hospitalized medical and surgical oncology patients receive thromboprophylaxis consistent with the National Comprehensive Cancer Network (NCCN) guidelines.23 The American Society of Clinical Oncology (ASCO) guidelines recommend routine thromboprophylaxis in all hospitalized patients except for those admitted solely for a minor procedure or chemotherapy.24 Future research initiatives should focus on demonstrating the efficacy of VTE prophylaxis in hospitalized medical oncology patients, and developing and validating cancer-specific VTE RAMs.
Which ambulatory cancer patients should receive VTE prophylaxis?
The @RISTOS study, a prospective multicenter observational study of 2373 cancer surgery patients, found that the incidence of symptomatic VTE was 2.1% at 30 ± 5 days. The mean time to VTE was 17 days. Over 40% of events occurred more than 21 days after surgery. More than 80% of patients received in-hospital thromboprophylaxis, 31% post-discharge prophylaxis, and 23% continued prophylaxis beyond day 21. Risk factors for VTE included age >60 years (OR, 2.6), previous VTE (OR, 6.0), anesthesia ≥2 hours (OR, 4.5), advanced stage disease (OR, 2.7), and bed rest ≥4 days (OR, 4.4). Postoperative deaths occurred in 41 patients of which 19 were due to VTE (46%) and 4 were due to bleeding (10%). Three of these 4 patients had already discontinued VTE prophylaxis.25
These data have supported an aggressive approach to VTE prophylaxis in cancer surgery patients. The Enoxacan II study demonstrated that extended-duration enoxaparin (ENOX) (40 mg daily for 21 days beyond the usual 6 to 10 days of postoperative prophylaxis) was associated with a 60% relative risk reduction of VTE (4.8% vs 12%; relative risk reduction, 60% [95% CI, 10-82]; P = .02).26 However, only 4 of the 20 events in the placebo group were proximal DVT (3 events) or pulmonary embolism (PE) (1 event). A recent meta-analysis of 7 prospective studies of extended-duration prophylaxis after cancer surgery found that all VTE was reduced by 56% (2.6% vs 5.6%; RR, 0.44 [95% CI, 0.28-0.70]) and proximal DVT was reduced by 54% (1.4% vs 2.8%; RR, 0.46 [95% CI, 0.23-0.91]). A nonsignificant reduction in PE was noted (0.8% vs 1.3%; RR, 0.56 [95% CI, 0.23-1.40]). Major bleeding (1.8% vs 1.0%; RR, 1.19 [95% CI, 0.47-2.97]) and all-cause mortality (4.2% vs 3.6%; RR, 0.79 [95% CI, 0.47-1.33]) were similar.27 Based upon these data, the current NCCN and ASCO guidelines recommend extended-duration prophylaxis for high-risk abdominal-pelvic cancer surgery patients.23,24
In a prospective multicenter observational study of ambulatory cancer patients receiving chemotherapy, Khorana et al noted that 1.9% of patients developed VTE during a median follow up of 2.4 months.28 Subsequent randomized thromboprophylaxis trials have demonstrated significant reductions in VTE, although the absolute reductions were modest (Table 2).29-32 Clinically significant reductions in VTE were seen in the FRAGEM and CONKO-004 studies of advanced pancreatic cancer patients.31,32 Patients with multiple myeloma have a ninefold increased risk of VTE due to the underlying disease and its treatment.33 In an open randomized thromboprophylaxis trial in low-risk myeloma patients, aspirin (ASA) 100 mg daily was as effective as ENOX 40 mg daily for VTE prevention (VTE ASA 2.27% vs ENOX 1.20%; P = .452).34 To target prophylaxis to the cancer patients most likely to benefit, Khorana et al developed an evidence-based risk stratification tool that has been validated in multiple independent studies.35 Several prospective studies evaluating direct oral anticoagulants (DOACs) in the prevention of VTE in high-risk cancer patients are currently underway (Table 3). Until the results of these studies are available, the NCCN and ASCO guidelines recommend thromboprophylaxis for patients with multiple myeloma receiving anti-angiogenesis agent-based chemotherapy regimens. Anticoagulant thromboprophylaxis is recommended for high-risk myeloma patients, whereas low-dose ASA is recommended for lower risk patients. The ASCO guideline recommends that thromboprophylaxis may be considered in other highly selected high-risk patients. The NCCN guideline does not recommend thromboprophylaxis in other ambulatory cancer patients outside of a clinical trial.23,24
What is the recommended initial, short-term, and long-term treatment of VTE in cancer patients?
Therapy of VTE can be divided into 3 phases: initial (first 5 to 10 days of therapy), short term (months 1 to 6), and long term (>6 months). Meta-analyses have shown no difference in recurrent thrombosis or bleeding with low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH).36 UFH may be preferable in hospitalized patients requiring invasive procedures or patients at high risk for bleeding. For initial and short-term therapy of VTE in cancer patients, LMWH remains the standard. In the Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) trial, dalteparin was associated with a 50% reduction in recurrent VTE compared with warfarin (dalteparin 9% vs warfarin 17%; HR, 0.48 [95% CI, 0.30-0.77]) and a similar risk of major bleeding (dalteparin 6% vs warfarin 4%; P = .09). Mortality was similar in both groups (dalteparin 39% vs warfarin 41%; P = .53).37 In the recently completed Comparison of Acute Treatments in Cancer Hemostasis (CATCH) trial, 900 patients with active cancer were randomized to tinzaparin 175 units/kg subcutaneously daily vs tinzaparin transitioned to warfarin (international normalized ratio, 2-3). The risk of recurrent VTE was numerically lower with tinzaparin compared with warfarin (tinzaparin 7.2% vs warfarin 10.5%; HR, 0.65 [95% CI, 0.41-1.03]; P = .07). The risk of major bleeding (tinzaparin 2.7% vs warfarin 2.4%; HR, 0.89 [95% CI, 0.40-1.99]), and mortality (tinzaparin 34.7% vs warfarin 32.2%; HR, 1.08 [95% CI, 0.85-1.36]) were similar.38
These studies were limited to 6 months, but cancer patients often remain on anticoagulation longer. To confirm the efficacy of long-term LMWH, the DALTECAN trial enrolled patients with active cancer and a new VTE to dalteparin for 12 months.39 Of 334 enrolled patients, 185 (54.7%) completed 6 months of treatment and 109 (32.2%) completed 12 months. The most common reasons for withdrawal were death (73, 33.2%), an adverse event (60, 26.2%), and withdrawal of consent (42, 18.3%). Recurrent VTE occurred in 37 patients (11.1%). The incidence of recurrent VTE was 8.7% during months 1 to 6 and 4.1% during months 7 to 12. Major bleeding occurred in 34 patients (10.2%). The incidence of major bleeding was 3.6% in the first month, and 0.8% to 1.8% per month during months 2 to 6 and 0% to 1.4% per month during months 7 to 12. A total of 116 patients (33.8%) died during the 12-month study, 105 from cancer, 4 from PE, and 2 from bleeding.39 The DALTECAN study establishes the outcomes associated with long-term LMWH treatment of VTE in cancer patients.
Although systematic reviews and guidelines recommend LMWH for treatment of VTE in cancer patients, a recent analysis of the Registro Informatizado de Enfermedad TromboEmbólica suggests that vitamin K antagonists (VKAs) may be effective in cancer patients after 6 months of LMWH therapy. Among 1502 cancer patients who received 6 months of LMWH, 763 continued on LMWH, whereas 739 switched to warfarin. The risk of recurrent VTE (HR, 0.67 [95% CI, 0.44-1.02]) and major bleeding (HR, 1.05 [95% CI, 0.79-1.55]) were similar.40 A small retrospective study of cancer patients with VTE also noted favorable results with warfarin therapy compared with LMWH.41 These studies suggest that VKAs may be as effective as LMWH in some cancer patients with VTE. Further research is warranted to confirm these findings.
Large randomized clinical trials have demonstrated that DOACs are at least as effective as conventional therapy with LMWH transitioned to VKA. However, only a small number of cancer patients were enrolled in these studies. In a meta-analysis, Vedovati et al noted recurrent VTE in 23 DOAC patients (3.9%) and 32 VKA patients (6%) (OR, 0.63; 95% CI, 0.37-1.10), and major bleeding in 19 DOAC patients (3.2%) and 22 VKA patients (4.2%) (OR, 0.77; 95% CI, 0.41-1.44).42 These outcomes are significantly better than those noted in previous cancer thrombosis trials suggesting that the cancer patients enrolled in the DOAC studies are not comparable to those in the CLOT and CATCH studies (Table 4).37,38,43-45 In the RECOVER studies, only 13% of the patients with active cancer at baseline had metastatic disease and all-cause mortality was only 15%.43 In the EINSTEIN studies, only 22% of patients with cancer at baseline had metastatic disease and 30% underwent treatment with chemotherapy. All-cause mortality was only 16%.44 In the AMPLIFY trial, approximately one-third had metastatic disease, but the overall mortality rate was 6% and 7.7% in the cancer patients taking apixaban and warfarin, respectively.45 Furthermore, the inclusion/exclusion criteria differed significantly between the DOAC studies, and the CLOT and CATCH trials. A single center study of 118 cancer-associated thrombosis patients treated with rivaroxaban noted recurrent VTE in 4 cancer patients (3.3%) and 5 patients without cancer (2.8%) during mean follow up of 1.3 years. Ninety cancer patients were treated with chemotherapy (76%). Twenty-six cancer patients (22%) and 0 patients without cancer died.46 Similar findings were reported in a single center study from the Memorial Sloan Kettering Cancer Center.47 Although these results are encouraging, the author believes it is premature to use DOACs as first-line agents for cancer-associated VTE. Ongoing trials such as the Hokusai VTE Cancer Study will be essential to confirm these earlier trials (Table 3). Consequently, the NCCN and ASCO guidelines do not recommend the use of DOACs until we have additional information about their efficacy in cancer patients.
What is the appropriate duration of therapy for cancer-associated VTE?
The current NCCN, ASCO, and the American College of Chest Physicians guidelines recommend that anticoagulation for cancer-associated VTE be continued as long as there is evidence of disease or ongoing cancer therapy.23,24,48 No randomized trials have examined the duration of anticoagulation in cancer patients. Consequently, only expert opinion and common sense are available to provide guidance. Therefore, clinicians should make these decisions on a case-by-case basis after reviewing objective imaging for persistent cancer, tumor markers, and persistent risk factors for VTE, as well as the patient’s preferences, risk of anticoagulation-associated bleeding, and their adherence to therapy. A number of risk factors for recurrent VTE have been identified in cancer patients (Table 5), including an evidence-based RAM (The Ottawa Score).3,49-53 End-of-therapy duplex studies may provide useful prognostic information because a negative duplex ultrasound at the conclusion of therapy was associated with a significantly lower risk of recurrent VTE in the Cancer Duration of Anticoagulation based on Compression UltraSonography (DACUS) study (2.8% vs 21.9%; P < .001).54 In addition, end-of-therapy duplex studies documents residual disease in the event the patient develops symptoms that may represent a new thrombotic event. Although validation studies of the Ottawa Score have had mixed results thus far, the author does think it is worthwhile to consider known evidence-based risk factors when making decisions on anticoagulation therapy in cancer patients.55,56
What is the appropriate approach to treatment of central venous catheter (CVC)–associated DVT?
CVCs are widely used in cancer patients for administration of medications and blood products, and blood sampling for laboratory testing. Cancer patients are at a twofold greater risk for CVC-associated VTE than patients without cancer.57 In a prospective cohort of 444 cancer patients, symptomatic CVC-associated VTE developed in 19 patients (4.3%, incidence 0.3 per 1000 CVC days).58 In a prospective study of 558 patients from the Registry of Patients with Venous Thromboembolism, Baumann-Kreuziger et al noted an incidence of recurrent VTE of 7 per 100 patient-years and major bleeding of 8.9 per 100 patient-years. The risk of recurrent thrombosis was decreased by 77% in patients who received anticoagulation for more than 90 days (OR, 0.23; 90% CI, 0.1-0.56).59 A systematic review of upper extremity DVT found that cancer patients had a two- to threefold higher risk of recurrent thrombosis, and a fourfold higher risk of bleeding than patients without cancer.60 The NCCN guideline recommends anticoagulation alone, without CVC removal, for treatment of CVC-associated VTE. Thrombolysis is reserved for limb-threatening thrombotic events or massive PE. Patients should be treated for at least 3 months or for the duration of the CVC, whichever is longer. High quality prospective studies are needed to identify the best approach to treatment of CVC-associated VTE.
When should vena caval filters be used in cancer patients?
The only indication for a vena caval filter in cancer patients is the presence of an acute proximal DVT and/or PE, and a contraindication to anticoagulation.61 Because the risk of recurrence is highest in the first month after a thrombotic event, most clinicians would consider a vena caval filter appropriate if a patient must discontinue anticoagulation during this time period. If a patient’s thrombotic event occurred more than 1 month ago, vena caval filters can be considered on a case-by-case basis, taking into account the location of the thrombus, the residual thrombus burden, duration of anticoagulation, anticipated time off anticoagulation, and the patient’s other comorbidities. A retrievable filter should be used because it preserves the option for future removal. A systematic follow-up program should be in place to ensure that filters are retrieved when they are no longer necessary.62
How should thrombocytopenic cancer patients with VTE be treated?
A platelet count of 50 × 109/L is commonly identified as the lowest safe threshold for therapeutic anticoagulation.23 In the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) VTE prophylaxis registry, patients with a platelet count <50 × 109/L were threefold more likely to develop bleeding.63 Small observational studies have suggested that lower doses of anticoagulation could be used for patients with platelet counts <50 × 109/L.64 Based upon these data, the International Society on Thrombosis and Haemostasis has published a guidance document outlining a rational approach to the management of the thrombocytopenic patient with VTE.65 Further investigation is warranted to determine the efficacy and safety of standard and reduced-dose anticoagulation regimens at different platelet thresholds in cancer patients with VTE.
How should patients with VTE who have metastatic or primary brain tumors be managed?
Traditionally, patients with brain tumors often have been considered inappropriate candidates for anticoagulation. However, retrospective observational studies of glioma patients have suggested these patients do better with anticoagulation than with vena caval filters. In contrast, patients with metastatic brain tumors such as melanoma and renal cell carcinoma have been suggested to be at substantially higher risk for poor outcomes. However, recent studies suggest that this orthodoxy may need re-examination. In a retrospective cohort of 74 melanoma patients with brain metastases and VTE, Alvarado et al noted no difference in intracranial hemorrhage between patients who did and did not receive anticoagulation (2 of 57 [4%] vs 0 of 17 [0%]; P = 1.0).66 Donato et al conducted a matched retrospective cohort study of 293 patients with brain metastases of whom 104 received therapeutic ENOX. At 1 year, there was no difference in the cumulative incidence of measurable (19% vs 21%; P = .97), significant (21% vs 22%; P = .87), or total (44% vs 37%; P = .13) intracranial hemorrhage. The risk for intracranial hemorrhage was fourfold higher in patients with metastatic melanoma or renal cell carcinoma than lung cancer, but the risk was not influenced by anticoagulation.67 These studies suggest that anticoagulation might be considered in patients with brain metastases after a careful evaluation of its risks and benefits. Because subtle but important differences between the patients may have been responsible for these results, prospective validation studies are needed.
How should the cancer patient with recurrent VTE be managed?
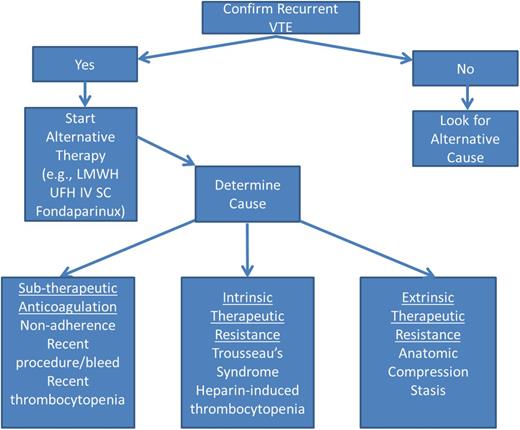
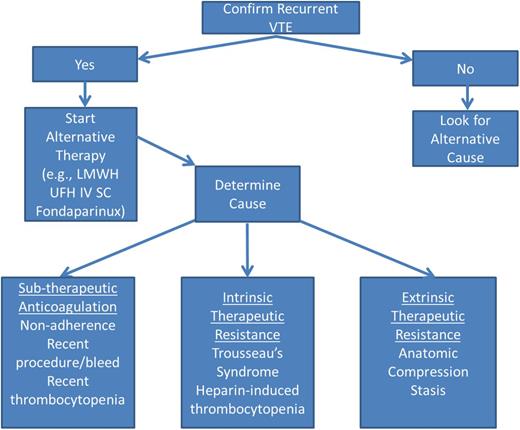
When managing a cancer patient with recurrent VTE, it is important to objectively confirm that a recurrence has occurred because post-thrombotic syndrome, infections (eg, pneumonia), cardiopulmonary failure, or hepatic or renal disease can result in similar symptoms (Figure 1). If a recurrent VTE is confirmed, the cause(s) of recurrent VTE should be identified and alternative therapy should be initiated. Options for alternative therapy include switching to a LMWH or UFH, or changing the dose or administration schedule, or switching to fondaparinux, which has a longer elimination half-life. A common cause of recurrent VTE is subtherapeutic anticoagulation due to poor adherence to therapy or recent interruptions for procedures, bleeding, or thrombocytopenia. In these instances, strategies to improve adherence (closer monitoring, alternative anticoagulant regimens, perioperative bridging anticoagulation, etc) are important. Intrinsic or endogenous therapeutic resistance can result from Trousseau syndrome related to progressive cancer or suboptimally treated neoplastic disorders (eg, failure to control erythrocytosis in polycythemia vera). In the case of Trousseau syndrome, heparin therapy and control of the underlying malignancy are essential to prevent recurrent thromboembolism. For patients with polycythemia vera, adequate control of erythrocytosis is critical for the success of anticoagulant regimens. Heparin-induced thrombocytopenia can occasionally cause recurrent VTE in cancer patients. In these instances, standard management of heparin-induced thrombocytopenia is appropriate. Extrinsic causes of therapeutic resistance include nodal or tumor masses causing vascular compression and stasis, or CVCs or vena caval filters that create turbulence and obstruct blood flow, which precipitates thrombosis. In these cases, eliminating the cause of stasis is essential. In regard to anticoagulation management, LMWH dose escalation has been demonstrated to be a successful strategy.68 An international registry of 212 cancer patients with recurrent VTE found that 73% had metastatic cancer at the time of their event and 70% were on LMWH. At the time of breakthrough thrombosis, 70% were on a therapeutic or supra-therapeutic dose. Subsequently, 33% continued on the same therapeutic dose, 31% were prescribed an increased dose, and 24% switched to another medication. During 3-months of follow up, 11% suffered another VTE, 8% developed major bleeding, and 27% died. The risk of recurrent VTE was less common with LMWH than VKA (HR, 0.28; 95% CI, 0.11-0.70).69 This study supports the use of LMWH for cancer patients with recurrent VTE and underscores the challenges of managing these patients.
Conclusion
VTE is a frequent complication in patients with active cancer. Observational studies and randomized clinical trials conducted over the last 2 decades have contributed immensely to our understanding of the pathogenesis of VTE and its management. However, many unresolved questions remain, including the optimal approach to primary prophylaxis in hospitalized and ambulatory medical oncology patients, treatment of cancer-associated VTE in standard bleeding risk (eg, appropriate duration of therapy, anticoagulant regimen, etc) and high bleeding risk populations (eg, thrombocytopenia, central nervous system metastases), and the role of direct oral anticoagulants. Fortunately, cancer-associated thrombosis is an active area of clinical investigation, therefore we should have answers to some of these important questions in the near future.
Correspondence
Michael B. Streiff, Division of Hematology, Department of Medicine, Johns Hopkins University School of Medicine, 1830 East Monument St, Suite 7300, Baltimore, MD 21205; e-mail: mstreif@jhmi.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author has received research funding from Janssen, Portola, Roche, and the Patient-Centered Outcomes Research Institute, and has consulted for Bio2 Medical, Janssen, CSL Behring, and Merck.
Author notes
Off-label drug use: None disclosed.