Abstract
The majority of patients with Hodgkin lymphoma are cured with frontline therapy; however, 10% to 15% with early-stage disease and 20% to 30% with advanced stage require second-line therapy that includes a potentially curative transplant, of which an additional 50% to 55% are cured. Those with multiply relapsed disease traditionally would receive novel agents on a clinical trial or combination chemotherapy as a potential bridge to an allogeneic stem cell transplant. This treatment paradigm has changed with the availability of brentuximab vedotin, an antibody drug conjugate used pre- and post-ASCT, as well as for palliation. With the availability of the checkpoint inhibitors, nivolumab and pembrolizumab, there will be another shift in treatment, with these agents being used for palliation and potentially replacing allogeneic stem cell transplantation in certain patient populations. Finally, up-front management is also changing and this will have an impact on how patients in the relapsed and refractory setting will be treated.
Learning Objectives
Understanding risk factors that predict outcome in relapsed and refractory Hodgkin lymphoma
Understanding how to incorporate brentuximab vedotin and the checkpoint inhibitors into the treatment paradigm
This chapter will focus on 5 aspects that are critical in the treatment of patients with relapsed and refractory transplant-eligible Hodgkin lymphoma (HL): the role of pre–allogeneic stem cell transplant (ASCT) positron emission tomography (PET) imaging, incorporation of brentuximab vedotin (BV) into salvage therapy, patients eligible for post-ASCT consolidation, use of checkpoint inhibitors (CPI), and the future role of allogeneic hematopoietic cell transplantation (allo-HCT) in the management of this disease.
The treatment algorithm for the primary management of HL has evolved over the past 3 decades, leading to a change in the patient populations requiring aggressive second-line therapy. The optimal chemotherapy for advanced stage HL (ASHL) can be endlessly debated, but progression-free survival (PFS) has improved by 15% in the past 2 decades.1,2 For early-stage HL (ESHL), PFS is unchanged at close to 90% despite decreasing the number of cycles of doxorubicin (Adriamycin)-Bleomycin-Vinblastine-Dacarbazine administered and reducing the dose and size of radiation therapy fields.3 From 1990 to 2010, relapsed/refractory (rel/ref) HL patients that underwent consultation for autologous stem cell transplantation (ASCT) had received either full-course chemotherapy or combined modality therapy (CMT) as frontline treatment; currently there exists a substantial proportion of patients who received short-course chemotherapy alone or were enrolled in PET-adapted advanced-stage studies, of which the results were not successful. The presumed response that all rel/ref HL patients must receive salvage therapy (ST) followed by high-dose chemo-radiotherapy (HDT)/ASCT, and if chemosensitive disease is achieved, may need to be addressed. In addition, when this second-line approach fails, do all patients require a consultation for a reduced-intensity conditioning (RIC) allo-HCT in the era of excellent new agents, where palliation for prolonged periods of time is now standard and expected? These issues will be discussed in this chapter as well as at the ASH meeting; there are many historical reviews cataloging various salvage regimens and transplant conditioning regimens to which the reader can be referred and these will not be discussed further.4,5
What are the current patient populations requiring second-line therapy?
Currently any patient with adequate end-organ function and performance status, and who are <75 years old, are treated aggressively with second-line therapy with the intent for high-dose therapy (HDT)/ASCT to follow. These patient populations include: early or advanced stage, relapsed and primary refractory HL treated with full chemotherapy; and early-stage HL treated with combined modality therapy with disease either insid,e the radiation field or advanced-stage disease at the time of ST. As therapy for ESHL evolves it is currently unclear whether patients treated with short-course chemotherapy alone (3-4 cycles of doxorubicin [Adriamycin]-Bleomycin-Vinblastine-Dacarbazine), with early stage disease at the time of ST, require HDT/ASCT. For example, in the RAPID study,6 many patients with relapsed disease received radiation therapy alone for salvage. Because so few patients with ESHL relapse, it will be difficult to assess the need for HDT in this specific patient population in a randomized controlled clinical trial.
Pre-ASCT FDG-PET
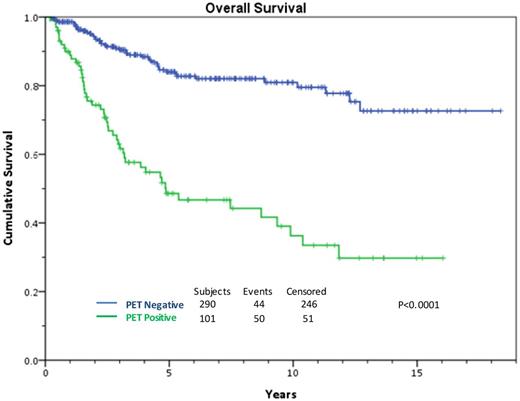
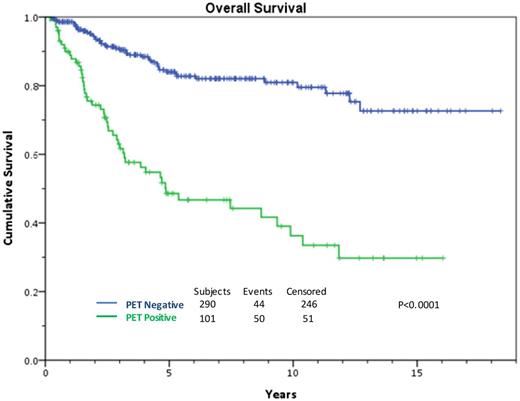
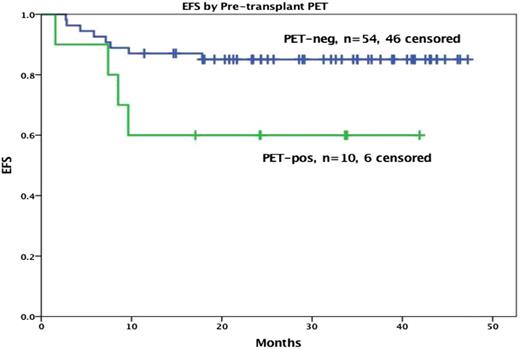
HDT/ASCT is standard of care for relapsed or primary refractory HL if chemosensitive disease to ST is confirmed, leading to a cure in approximately 50% of the transplanted patients.7 It is very clear that the cure rate ranges from 25% to 75% depending on prognostic factors.8-10 Response to ST, whether complete or partial, is by far the most important predictor of outcome in this setting. In 2010, our group determined that chemosensitive disease should be defined by the pre-transplant 18F-fluorodeoxyglucose (FDG)-PET result.11 These results have been expanded and updated. A negative scan after platinum-based therapy leads to a 10-year overall survival (OS) rate of 75%. For patients with documented improvement on computed tomography (CT), but with a persistent PET-avidity, only 30% are cured (Figure 1).
All transplanted patients, functional imaging negative vs positive pretransplant treated MSKCC, 1994-2014.
All transplanted patients, functional imaging negative vs positive pretransplant treated MSKCC, 1994-2014.
There are now many clinical trials suggesting that a negative pretransplant FDG-PET has prognostic value,12,13 and this has been confirmed in a recently published meta-analysis review.14,15
I define the post–ST PET scan as an interim evaluation; remission is defined 90 to 100 days post-ASCT. Based on this premise, nuclear medicine reports need to be interpreted using the 5-point (Deauville) criteria, with scores 4 and 5 defined as a positive scan.16 How many attempts should be made to achieve the goal of a negative scan? There is no correct answer, but it is reasonable to attempt 2 distinct ST programs for advance-stage disease. For stage I/II disease that can be encompassed into a reasonable radiation therapy field, this treatment should also be attempted to achieve a complete response (CR) before ASCT. There is evidence that patients requiring more than one regimen to achieve PET negativity have a nearly similar outcome compared with those who receive only one ST program.17
Complicating that matter further, not all patients with a post–ST PET negative, complete response are cured and the contrary is also true; as much as 40% of patients transplanted with PET-avid disease achieve long-term PFS rate. Patients with nodal-only disease have a superior outcome to that of patients with stage IV disease. For example, patients with nodal-only disease in remission at the time of ASCT have an 80% to 90% cure rate compared with 55% to 65% for patients with extranodal disease.18-20
Summary/Recommendation: A negative pre-ASCT PET scan is critical if a favorable outcome with HDT/ASCT is to be achieved and should be required for this treatment to continue to be the standard of care; however, since as much as 40% of patients with a positive scan are cured, ASCT should not be withheld, especially for patients without extranodal involvement.
Primary refractory HL.
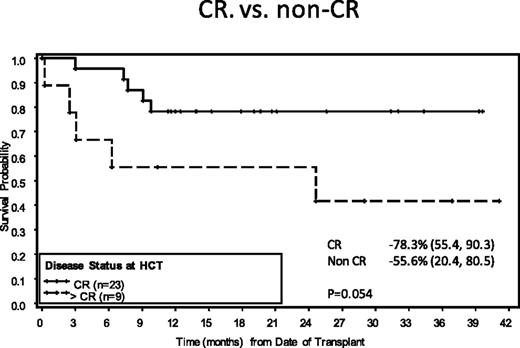
Investigators consider primary refractory HL unfavorable. Most series present data only for the transplanted cohort; there is a lack of intent-to-treat second-line data. Compounding the problem, analyses for primary refractory and relapsed patients are often combined, making it somewhat difficult to tease out the data. If one summarizes the information, <50% of patients with primary refractory HL are progression-free.21 We have recently reported on a large intent-to-treat primary refractory HL dataset compiled from patients treated on prospective ST studies at MSKCC. The median 5-year EFS for these 192 patients was 54%. The same 2 risk factors predicted for outcome: stage IV disease before ST and a positive PET scan after ST. A risk stratification model was created assigning 1 point for each factor. The 5-year EFS was 84% for patients with 0 risk factors, 54% with 1 risk factor, and only 28% for patients with both. Another large relapsed/refractory series was recently reported at ASH in 2015 by Broeckelmann et al. Multivariable analysis once again identified stage IV, primary refractory disease, and inadequate response to salvage chemotherapy as significant independent risk factors for PFS.22
The incorporation of BV into ST
Two to 3 cycles of ST is the standard of care and administered to all patients as part of a curative second-line strategy. Historically, the goal of ST was to achieve chemosensitive disease before curative HDT/ASCT. As stated before, in 2016 the goal of ST is far more significant and a CR or PET-negative response should be the objective. In fact, with the availability of new agents, patients who have a persistently positive scan after >1 ST may be not appropriate candidates for ASCT. Practitioners need to decide whether this patient population should receive novel drugs as part of a chronic or noncurative strategy vs a highly aggressive allo-HCT program.23
Prospective, randomized trials comparing different salvage regimens in HL are not available. If one summarizes the data for platinum and nonplatinum-containing ST regimens, a reasonable benchmark is a 50% to 60% CR or PET-negative rate with standard ST, but can this number be improved?24 BV is an antibody-drug conjugate (ADC) composed of a CD30-directed monoclonal antibody (recombinant chimeric IgG1) that is covalently linked to the antimicrotubule agent monomethyl auristatin E (MMAE). Binding of the ADC to CD30 on the cell surface initiates internalization of the MMAE-CD30 complex, followed by proteolytic cleavage that releases MMAE, leading to selective apoptosis of the Reed-Sternberg cells with minimal off-target side effects, the major one being neuropathy. BV is approved for the treatment of adult patients with relapsed or refractory HL where ASCT failed as well as part of a maintenance or consolidation strategy post-ASCT for high-risk patients, which will also be discussed here.25,26
Given that remission status pre-ASCT is highly predictive of outcome, the incorporation of BV with its favorable toxicity profile into an ST regimen is appealing. As a single agent in heavily pretreated patients, the PET-negative rate after 2 to 4 doses of BV is 30% to 40%. Two strategies have evolved to study this agent as part of ST: sequential administration of BV with chemotherapy and combined chemo-immunotherapy.
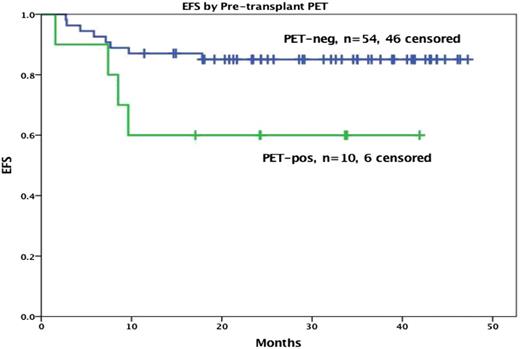
The MSKCC and the City of Hope (COH) lymphoma groups reported on the sequential approach.27,28 The designs of the studies were similar, with the primary difference being the BV dosing schedule. In the MSKCC trial, patients received 6 weekly doses of BV at 1.2 mg/kg; patients at COH were given 2 to 4 doses of an every-three-week dosing at 1.8 mg/kg. Patients in both studies with a PET-negative response went directly to HDT/ASCT, and additional ST was avoided; if PET-avid disease was persistent, then additional ST was given. The CR rate to BV alone was 30% to 40%, but this rate improved to 80% with the sequential programs. Consistent with previous data, the PET-negative patients had >75% 3-year PFS post-ASCT (Figures 2 and 3). There was no difference in outcome for patients only receiving BV compared with those requiring sequential therapy to achieve a CR. This approach is very appealing because some patients were able to avoid additional cytotoxic ST. However, predicting which of these patients will achieve CR is difficult. Our approach is to offer BV as initial therapy for patients with nodal-only disease.29
At ASH in 2015, 2 very different chemo-immunotherapy ST programs were presented. The first, an outpatient regimen, combined BV with bendamustine. LaCasce et al reported preliminary data from a phase 1/2 study evaluating the safety and efficacy of these 2 agents as a ST program.30 Fifty-five patients were treated; unexpectedly, there were severe infusion reactions seen in >50% of cycles, leading to an amendment and subsequent steroid premedications, which reduced this adverse event to become manageable. A PET-negative response was documented in 76% of patients; stem cell collection was adequate despite the concern of the known side effect of bendamustine on the bone marrow. Although the median follow-up is short at 18 months, 75% of patients are progression-free, making this outpatient ST appealing. In contrast, Garcia-Sanz et al reported data on 36 patients treated with an aggressive inpatient regimen: ESHAP+BV.31 As expected, hematologic toxicity was significant. The CR rate in this ongoing clinical trial was reported at 83% with no stem cell mobilization failures. Both of these phase 2 studies are also administering post-ASCT BV as part of a consolidation strategy based on the results of the Aethera study, which will be described in the next section. Currently, the Seattle and HOVON groups are studying ICE+BV and DHAP+BV, respectively.
Summary/Recommendation: The addition of BV to ST will increase the CR rate by 20%. However, the implication that all patients require this treatment is unclear. It seems reasonable for patients with low disease burden that single-agent BV should be initiated if available or chemotherapy alone if it is not. For patients with stage IV disease, chemo-immunotherapy or ST alone should be the initial treatment.
POST-ASCT BV consolidation
Before 2015, there were only 2 phase 3 studies published for patients with relapsed HL.32,33 Each study confirmed that ASCT was superior to ST alone; nearly all patients had relapsed as opposed to primary refractory disease. Little progress has been made in the past 2 decades except for decreased ASCT treatment–related mortality, which is secondary to improved supportive care. Unfortunately, PFS remains at 50% if data are analyzed by intent-to-treat.
In late 2010, the Aethera study was initiated. It was a phase 3, randomized, placebo-controlled trial that evaluated whether post-ASCT consolidation treatment with BV could prevent disease progression in patients with HL at high risk for relapse.26 A consensus panel determined that primary refractory HL, initial remission duration of <1 year, or extranodal involvement constituted unfavorable risk; patients were required to have one of these factors for study eligibility. Aethera was written before the near-uniform agreement that an abnormal pre–ASCT PET scan is the single most important factor in predicting outcome, and in fact the PET data that are available on study make recommendations for post-ASCT BV consolidation confusing.
Patients were selected to randomly receive BV 1.8 mg/kg every 3 weeks or placebo for 16 cycles (∼12 months), beginning 30 to 45 days after ASCT. Patients with progression of disease after ST were not eligible. Imaging studies were quarterly for the first year and then at 18 and 24 months during long-term follow-up (LTFU). Clinical lymphoma assessments were performed at each cycle of treatment, quarterly during the first year of LTFU, and every 6 months thereafter.
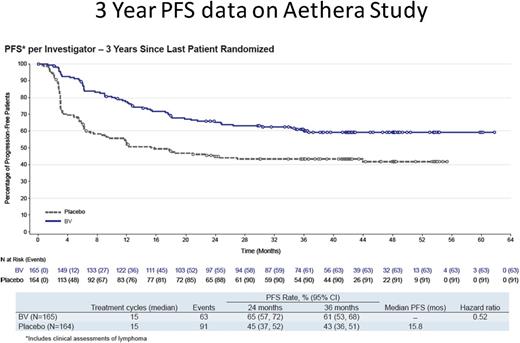
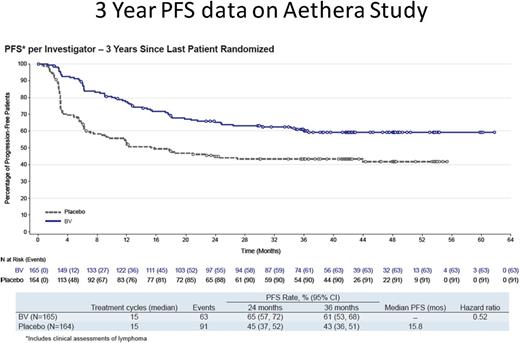
A total of 329 patients were randomized to the BV (n = 165) or placebo (n = 164) treatment arms. Importantly, 60% of patients had primary refractory HL. Median PFS was not reached in the BV arm and was 15.8 months in the placebo arm; 3-year PFS rates were 61% and 43%, respectively (Figure 4). There were only 6 PFS events recorded after the 24-month evaluation, 3 in each cohort, and it is highly likely that patients in either arm at this time point are cured; there is currently no difference in OS.
Administering 16 doses of BV does come with additional toxicity, namely neuropathy. Among the 112 patients in the BV arm with treatment-related peripheral neuropathy, 88% experienced improvement of symptoms at the time of 3-year analysis. Discontinuation of treatment because of any adverse events (AE) occurred in 54 patients (33%) in the BV arm, primarily as a result of neuropathy. Patients who discontinued treatment as a result of an AE received a median of 9.5 cycles (range, 1-15) in the BV arm. Interestingly, the 2-year PFS rate in these patients was 69% vs 82% for patients who completed all 16 treatment cycles.
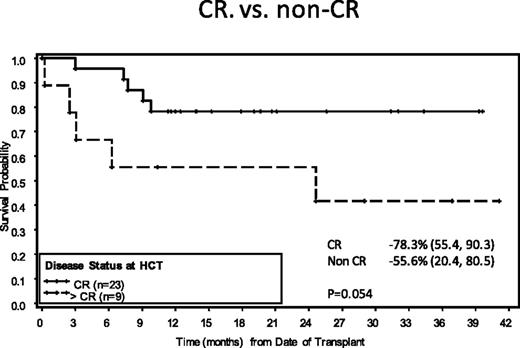
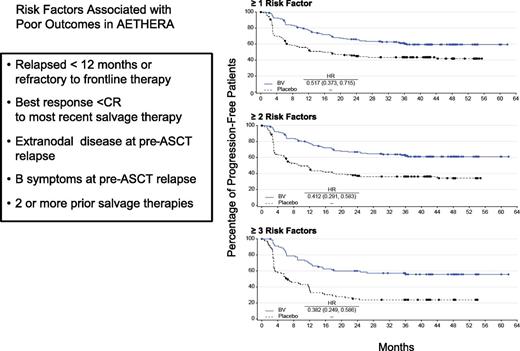
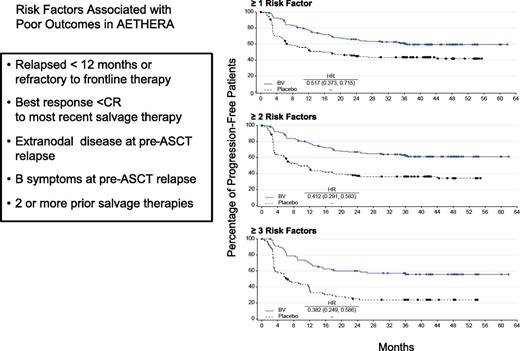
Five risk factors predicted for PFS on the Aethera study: initial remission duration <1 year, <CR to most recent salvage therapy, extranodal disease at the time of ST, B symptoms at the time of ST, and >1 ST required to achieve chemosensitive disease. It is clear from the data that patients who have at least 2 of these risk factors achieve benefit from consolidation (Figure 5). If one looks critically at the pre–ASCT PET results, recommendations are fairly straightforward. Because a CR is a favorable risk factor, patients need at least 2 of 3 additional unfavorable risk factors (remission duration <1 year, extranodal disease or requiring >1 ST) for benefit to be achieved with BV consolidation.
Summary/recommendations: Consolidation therapy improves PFS and it is reasonable to give a BV-naïve patient 1 year of post-ASCT therapy if he/she has at least 2 adverse risk factors. In patients who have previously received BV pre-ASCT and meet the risk factor requirements to be enrolled in Aethera, a patient should have achieved at least a partial response to BV-based treatment. In general, in this situation, which I suspect will become more frequent in the United States, I reduce the number of doses of BV post-ASCT to 10 or fewer.
Treatment when ASCT fails: chronic HL vs reduced-intensity conditioning (RIC) allo-HCT
In 2009, when the Aethera study was written, the median OS of patients where ASCT failed was 30 months; in 2016, that number has doubled and will likely continue to increase. The reason is the robust drug development program in HL, specifically BV and the CPI. BV has been extensively discussed, but when given for palliation, OS improves by about 1 year. Other agents are also active, and results are summarized in Table 1. Historically, for patients where a good response was documented, a consult for an allo-HCT ensued. These patients were given the option of transplant or palliation. The 1-year mortality from an allo-HCT was 30%, with an equal number of patients being cured. This paradigm is clearly changing with the availability of the CPI. The role of allo-HCT in HL is an area of great disagreement among lymphoma physicians.
The case for chronic administration of CPI
Targeting the PD-1/PDL1 pathway has revolutionized the way some of the common solid tumors are being treated. Studies are behind in the hematologic malignancies but are rapidly catching up. Recently the CPI, nivolumab, been approved for HL palliation and it is likely that pembrolizumab will also be approved. PDL1/2 is nearly uniformly expressed on Reed-Sternberg cells through chromosomal translocation, gene amplification, and EBV-related mechanisms. In HL, as well as primary mediastinal B-cell lymphoma, amplification of chromosome 9p23-4 is the most common genetic abnormality reported and the genes encoding PDL1 and 2 reside there; therefore, these 2 diseases are primed to respond to CPI.34
Both nivolumab and pembrolizumab are highly active, and phase 1 data were presented at ASH in 2014 and 2015.29,35 Results are quite similar with both agents, and treatment was administered for up to 2 years provided that the HL remained stable and/or the patient continued to have clinical benefit. These are unusual response criteria, and much different compared with traditional agents, when any time progression is documented, the study drug is discontinued. Because of this “rule,” PFS in these phase 1 studies are inflated. However, because clinical benefit included complete resolution of B symptoms, maintenance of palliative treatment was the correct choice, in my opinion.
Beginning with Nivolumab, the dose is 3 mg/kg every 2 weeks and 23 patients with HL were treated. The patients were heavily pretreated with a median of 5 prior regimens received, but some were BV-naïve. The objective response rate was 87%, but only 5 patients achieved a CR based on PET criteria. Amazingly, 13 patients in CR or PR are no longer receiving treatment and the median PFS has not been reached. The 18-month OS was 83% and the majority of deaths were secondary to transplant-related morality in patients referred for an allo-HCT.
Patients enrolled in the study with pembrolizumab were eligible only if BV treatment failed. Patients received pembrolizumab at 10 mg/kg every 2 weeks. Thirty-one patients were enrolled. The CR and PR rates were 16% and 48%, respectively; 70% of the responses lasted longer than 24 weeks, with a median follow-up of 17 months.
These phase 1 studies have led to large phase 2 clinical trials in which response rates were presented at ASCO and EHA this year. Eighty patients were included in the most recent published data for nivolumab; the CR and PR rates were 27% and 45%, respectively. Sixty patients were included in the most recent published data for pembrolizumab; 30 patients failed ASCT and BV, and 30 progressed on BV but were ASCT-ineligible. In the first cohort, the CR and PR rates were 20% and 50%, respectively, and 27% and 53%, respectively, in the second cohort. In both studies, most of the patients with demonstrable response continue to receive treatment.
The side effect profiles of the CPI are different from traditional therapy and are usually immune-related. These include endocrinopathies such as thyroiditis, adrenal and pituitary dysfunction, hepatitis, colitis, and pneumonitis, which is of particular concern in patients with HL. Most of the patients treated with CPI with HL had previously received agents known to cause pulmonary dysfunction such as radiotherapy, bleomycin, gemcitabine, carmustine, and BV. I would caution administering a CPI when a patient has a history of pneumonitis requiring steroid support for symptom control.
Summary/Recommendation: The CPI have been administered to patients with HL for the past 2 to 3 years and the OS of a patient with HL where ASCT failed has increased nearly the same amount. I will continue to use CPI liberally for palliation and keep patients on treatment until obvious disease progression ensues; however, a number of appealing clinical trials are underway combining CPI with other agents including BV and histone deacetylase (HDAC) inhibitors and, if available, I would recommend these options for my patients.
The case for RIC allo-HCT
The early studies of allo-HCT in patients with HL were reported before the RIC era; OS was poor because of treatment-related mortality.36 Therefore, most transplant physicians would agree that an ablative allo-HCT is no longer a viable treatment option in HL. There is clear evidence of the graft-versus-lymphoma effect in HL based on 2 lines of evidence: graft-versus-host disease after allo-HCT is associated with improved PFS and, second, donor lymphocyte infusions (DLI) as part of a T cell–depleted strategy reduces the relapse rate. In 2016, with the availability of umbilical cord and haplo-identical transplants, nearly all patients will have a donor.37 Therefore, if a patient is referred for RIC allo-HCT, this treatment will likely commence provided that the patient meets the standard criteria for an allo-HCT, including some evidence of chemosensitive disease. However, it raises a critical question: which patients, and at what time should a patient be referred?
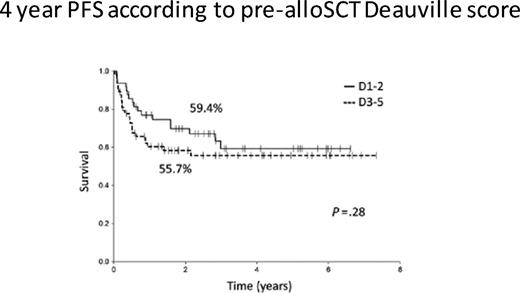
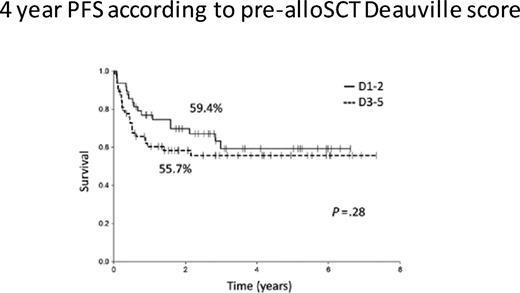
To answer this question, one must consider the likelihood of cure after RIC. In 2016, registry data from the CIBMTR reported PFS and OS rates of only 20% and 37%, respectively, after a RIC for HL. If one evaluates RIC in the context of chemosensitive disease and performance status before transplant, the EBMT reported that 3-year PFS improved to 42% in favorable patients. We have data from MSKCC on 71 patients who underwent RIC for HL and reported that extranodal disease, less than CR at the time of RIC, and alemtuzumab-containing regimens were all associated with a poor outcome.38 However, patients without any of these risk factors have a 3-year PFS approaching 60%. Confusing the matter further, data from the University of London where all the patients (n = 116) were transplanted with a T cell–depleted, alemtuzumab-based RIC, there was no difference in outcome in patients who had chemosensitive disease pretransplant based on PET status at the time of conditioning, with 3-year PFS rates of 59.4% and 55.7%, respectively, for PET-positive vs PET-negative response (Figure 6).39 These data are helpful in the context of BV treatment as a bridge to RIC. In the pivotal phase 2 clinical trial leading to US Food and Drug Administration approval of BV, 34 of 102 patients achieved a PET-negative response.40 In addition, approximately one third of these CR patients have never relapsed. Therefore, patients achieving a CR to BV after a failed ASCT may benefit from BV treatment of up to 16 cycles and then can be monitored.
Are the results better for RIC in patients who have not received a previous ASCT? There are no modern studies comparing ASCT vs RIC allo-HCT. However, it is clear that there are subgroups of patients that have been described here that have <30% chance of being cured with ASCT.
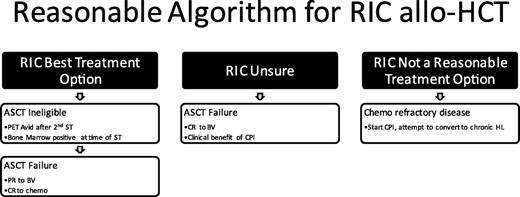
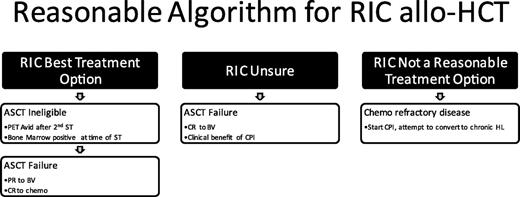
Summary/recommendation: A reasonable treatment paradigm is depicted in Figure 7.
Conclusion
A negative pre-ASCT PET scan is critical in the curative outcome of rel/ref HL and the incorporation of BV into ST can improve this metric. Post-ASCT consolidation with BV should be reserved for patients with multiple risk factors because the AEs are significant (namely, neuropathy). The checkpoint inhibitors are now the standard treatment of palliation and the question of how long these agents should be given before allo-HCT demands prospective analysis.
Correspondence
Craig Moskowitz, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: moskowic@mskcc.org.
References
Competing Interests
Conflict-of-interest disclosure: The author has received research funding from Seattle Genetics and Merck, and has consulted for Seattle Genetics, Merck, and BMS.
Author notes
Off-label drug use: None disclosed.