Abstract
Non–chemotherapy idiosyncratic drug–induced neutropenia (IDIN) is a relatively rare but potentially fatal disorder that occurs in susceptible individuals, with an incidence of 2.4 to 15.4 cases per million population. Affected patients typically experience severe neutropenia within several weeks to several months after first exposure to a drug, and mortality is ∼5%. The drugs most frequently associated with IDIN include metamizole, clozapine, sulfasalazine, thiamazole, carbimazole, amoxicillin, cotrimoxazole, ticlopidine, and valganciclovir. The idiosyncratic nature of IDIN, the lack of mouse models and diagnostic testing, and its low overall incidence make rigorous studies to elucidate possible mechanisms exceptionally difficult. An immune mechanism for IDIN involving neutrophil destruction by hapten (drug)-specific antibodies and drug-induced autoantibodies is frequently suggested, but strong supporting evidence is lacking. Although laboratory testing for neutrophil drug-dependent antibodies is rarely performed because of the complexity and low sensitivity of tests currently in use, these assays could possibly be enhanced by using reactive drug metabolites in place of the parent drug. Patients typically experience acute, severe neutropenia, or agranulocytosis (<0.5 × 109 neutrophils/L) and symptoms of fever, chills, sore throat, and muscle and joint pain. Diagnosis can be difficult, but timely recognition is critical because if left untreated, there is an increase in mortality. Expanded studies of the production and mechanistic role of reactive drug metabolites, genetic associations, and improved animal models of IDIN are essential to further our understanding of this important disorder.
Learning Objectives
Describe the clinical findings, incidence, and mortality associated with idiosyncratic drug-induced neutropenia (IDIN)
List the drugs most frequently implicated in IDIN
Discuss the current mechanisms proposed to explain IDIN and methods for drug-dependent neutrophil antibody testing
Granulocytes/polymorphonuclear leukocytes are a group of white blood cells (WBCs) that includes basophils, eosinophils, and neutrophils. Neutrophils are both the most abundant granulocyte and WBC in blood. A healthy human adult has 4.5 to 10 billion WBC/L, and ∼60% are neutrophils. Neutrophils are critical to host defense against infectious agents such as bacteria and fungi. They phagocytize, kill, and digest these foreign invaders before they are allowed to multiply and cause disease. A reduction of neutrophils (segmented and band cells) in the blood to an absolute neutrophil count (ANC) of 1.5 × 109 cells/L is considered neutropenia. An ANC <0.5 × 109 cells/L is considered severe neutropenia (often termed agranulocytosis), and individuals with an ANC <0.1 × 109 cells/L are at severe risk of morbidity and mortality from infections.
Because there are numerous causes of neutropenia, making the determination of the possible cause in a patient is very difficult for clinicians. Some of these many possible causes are listed in Table 1. Although it is not the most common cause of all neutropenias (ANC <1.5 × 109 neutrophils/L), drug-induced neutropenia must be considered in patients with unexplained neutropenia. Drug-induced neutropenia is also a serious concern for the development of new drugs1 because it can be missed in clinical trials if the incidence is low; in severe cases, it can even lead to withdrawal of a drug from the market.2
Drug-induced neutropenia
Drug-induced neutropenia is caused by decreased production or increased destruction of neutrophils. Decreased production is frequently a consequence of chemotherapeutic drugs that cause suppression of bone marrow myeloid progenitor cells. Chemotherapy drug–induced neutropenia has a very high incidence in oncology patients.3 Idiosyncratic drug-induced neutropenia (IDIN) resulting from increased neutrophil destruction is commonly caused by adverse idiosyncratic reactions to nonchemotherapy drugs and is the focus of the present article. The topic is important because IDIN is a major source of morbidity and mortality, which adds significantly to the cost of health care.
IDIN incidence and mortality
Although IDIN is not the major cause of all neutropenias (ANC <1.5 × 109 neutrophils/L), it is reportedly the cause in as many as two-thirds to three-quarters of severe cases of neutropenia/agranulocytosis (ANC <0.5 × 109 neutrophils/L), and because most IDIN incidence data have been calculated for agranulocytosis cases in adults, IDIN is the focus of the present article.4-6 Recent data from the large, 10-year FAKOS (Berlin Case–Control Surveillance Study) of drug-induced agranulocytosis in adult patients (age range, 19-88 years) involving 51 hospitals in Berlin, Germany, showed that 72% of 88 cases of confirmed agranulocytosis were probably drug related.7 The actual annual incidence of IDIN varies across different reports and ranges from 2.4 to 15.4 cases per year per million population.4,8,9 The annual incidence of IDIN also increases with age and polypharmacy.6,10,11 Reports of the incidence of IDIN in pediatric patients are scarce, but a recent report showed an annual incidence of 3.92 cases per 10 000 pediatric patients.12
Mortality associated with IDIN is currently estimated at 5%,11 which is significantly lower than estimates 20 years ago, which were as high as 20%.10 This finding is probably a result of improved education of both patients and physicians regarding the risk of IDIN, especially for commonly implicated drugs (eg, clozapine), which allows for early recognition and early initiation of optimum therapy.
Mortality rate also increases with age and with higher frequency in people aged >65 years; the incidence is also slightly higher in women up to age 65 years.6,9 Other features associated with increased mortality in IDIN are neutrophil count <0.1 × 109 cells/L, concomitant renal disease, septicemia, and shock.4
Most frequently implicated drugs
Numerous drugs have been implicated in IDIN across multiple drug classes. Of 63 cases of probable drug-induced agranulocytosis observed in the FASKO study, the drugs most frequently implicated were metamizole (n = 10), clozapine (n = 6), sulfasalazine (n = 5), and thiamazole (n = 5).7 The 5 drugs most frequently associated with IDIN in a recently reported study of 203 patients were carbimazole (n = 28), amoxicillin (n = 22), cotrimoxazole (n = 19), ticlopidine (n = 10), and valganciclovir (n = 9).9 Table 2 lists the drugs frequently associated with IDIN from 4 different studies, including drugs most frequently suspected in patients tested for drug-dependent neutrophil antibodies at a large diagnostic neutrophil antibody reference laboratory.
Individual drugs such as poly propylthiouracil and other thioamides for the treatment of thyroid disorders and the atypical antipsychotic clozapine are reported to be more frequently associated with IDIN, with incidences of 0.2% to 0.5%13 and 1%,14 respectively. The anthelmintic agent levamisole and the antibiotic vancomycin are also often associated with neutropenia.
Propylthiouracil, carbimazole, and its active metabolite methimazole are used to treat hyperthyroidism (Graves’ disease) by decreasing thyroid hormone levels. In a study of 754 Japanese patients who developed IDIN during treatment with primarily methimazole (96%), 24 patients (3.2%) died.13 IDIN typically develops within 3 months of antithyroid treatment, but cases have been reported that developed within 5 days and after 10 years of treatment.15 Mechanisms proposed for antithyroid IDIN are development of drug-dependent antibodies and antineutrophil cytoplasmic antibodies.
Clozapine, a dibenzodiazepine, is an atypical antipsychotic medication used in the treatment of schizophrenia resistant to conventional neuroleptic agents. Clozapine is perhaps the drug most often associated with IDIN. Clozapine-induced neutropenia occurs in ∼1% of patients, usually during the first 3 months of treatment. The incidence of fatal neutropenia is reduced to 0.03% in patients whose WBC counts are monitored.11 Clozapine carries 5 black box warnings alerting physicians to the risk of severe neutropenia and the requirement to monitor patients’ WBC counts. The mechanisms responsible for severe neutropenia after exposure to clozapine include apoptotic destruction of neutrophils through adenosine triphosphate depletion by an active nitrenium metabolite16 and inflammasome activation by reactive clozapine metabolites.4
IDIN caused by levamisole has been reported for >40 years, originally in patients treated for inflammatory conditions such as rheumatoid arthritis and nephrotic syndrome, and also when used as adjuvant chemotherapy in patients with colorectal cancer.17 By 2003, cases of agranulocytosis were reported in individuals using cocaine adulterated with levamisole,18,19 and the Drug Enforcement Administration reports in 2009 showed that 69% of seized cocaine was contaminated with as much as 10% levamisole.20 Neutropenia reportedly occurs in as many as 61% of people exposed to levamisole-adulterated cocaine.18 Neutrophil autoantibodies have been detected in sera of some patients with levamisole-related IDIN.19
Proposed mechanisms for IDIN
The unpredictability of IDIN, lack of mouse models and diagnostic testing, and low frequency of occurrence makes systematic studies to elucidate possible mechanisms extremely difficult. However, an immune-mediated mechanism is currently most often proposed to explain IDIN. Although an immune mechanism is frequently suggested, strong supporting evidence is lacking.23 An immune mechanism for IDIN is consistent with the clinical presentation in which there is often a delay in neutropenia after exposure to drug in accordance with the time required to develop a primary immune response. The average time to develop IDIN after drug exposure is 1 to 6 months and varies with drug.4 In addition, there is often a rapid onset of neutropenia on reexposure to the same drug in patients who previously developed IDIN, which is consistent with immunologic memory.23 One of the most incredible in vivo demonstrations in humans that IDIN is immune in nature was performed in 1952. Investigators injected serum from a patient who developed agranulocytosis associated with aminopyrine exposure into a normal healthy subject together with aminopyrine; the healthy subject experienced acute, profound neutropenia (0.5 × 109 cells/L) after the serum injection.24 The immune mechanisms for IDIN most frequently described are the hapten hypothesis and the danger signal hypothesis, both of which are discussed in detail in the following sections.
Hapten hypothesis
Haptens are low-molecular-weight (usually <5000 Da) molecules that are not capable of eliciting an immune response by themselves but can when coupled to a large carrier molecule, usually a protein. Antibodies formed against the hapten then bind the hapten–carrier complex and, in the case of IDIN, presumably neutrophil glycoprotein–drug complexes on the cell membrane surface leading to neutrophil clearance/destruction. Drugs such as penicillin and some cephalosporin drugs when covalently linked to cell surface proteins can elicit drug-specific/hapten antibodies. This mechanism is well described for drug-dependent antibodies (DDAbs) targeting red blood cells, which cause drug-induced immune hemolytic anemia.25
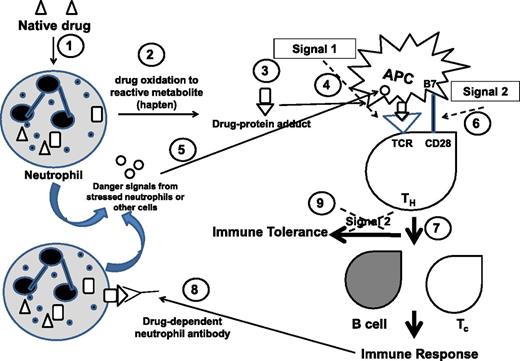
For the hapten mechanism to occur, the drug must bind tightly, usually covalently, to the protein carrier forming protein–drug adducts. Drug metabolism or biotransformation primarily occurs in the liver where the parent drug molecules are converted into water-soluble metabolites that can be eliminated in the urine to prevent cellular accumulation and toxicity. Biotransformation also produces both stable and reactive intermediates and metabolites. Reactive metabolites can readily make covalent linkages with cellular proteins, including neutrophil membrane glycoproteins. Although the majority of drug biotransformation occurs in liver hepatocytes, there is increasing evidence that it can occur in other cells, including neutrophils.4 Neutrophil enzymes such as myeloperoxidase are capable of producing reactive drug metabolites20 that can form drug–protein adducts which attach to the neutrophil cell membrane with the potential to elicit drug-specific antibody production through a hapten mechanism (Figure 1). Antibody production would of course require presentation of small peptides from drug-modified carrier proteins by antigen-presenting cells (APCs) in the context of class II HLA to T helper (TH/CD4+) lymphocytes. The majority of evidence in support of the hapten mechanism comes from studies of idiosyncratic drug reactions that cause liver injury and skin hypersensitivity reactions.2,23,26 However, a large number of drugs associated with a significant incidence of agranulocytosis (including methimazole, propylthiouracil, captopril, levamisole, clozapine, procainamide, dapsone, sulfonamides, vesnarinone, amodiaquine, trimethoprim, diclofenac, carbamazepine, phenytoin, indomethacin, and ticlopidine) are all reported to be oxidized to reactive metabolites,20,27 which can form drug–protein adducts that are believed to cause neutropenia by a hapten-type mechanism.20,28 Hapten antibodies were reported to cause IDIN in patients exposed to flecanide.29
Hapten and danger hypotheses of IDIN immune mechanism. (1) Native drug in the bloodstream enters neutrophils, liver macrophages, and other cells. (2) Drug is oxidized to reactive metabolite(s). (3) Reactive drug (hapten) forms a covalent link with endogenous proteins, forming drug–protein adducts. (4) Drug–protein adducts are taken up by antigen- presenting cells (APCs) and peptide-drug presented in context of class II HLA to T helper (TH) cells that engage through T-cell receptor (TCR): Signal 1. (5) Stressed neutrophils or other cells release danger signals that activate APCs. (6) B7 on activated APCs engages CD28 on TH cell for co-stimulus that is Signal 2. (7) The activated TH cell provides signals to B cells to produce drug-dependent neutrophil antibodies against drug/hapten and to stimulate hapten-specific cytotoxic T cells (Tc): Immune response. (8) Drug-dependent neutrophil antibodies bind drug–protein adducts on neutrophil membrane that cause neutrophil destruction/clearance. (9) In the absence of danger signals, there is no Signal 2, resulting in immune tolerance.
Hapten and danger hypotheses of IDIN immune mechanism. (1) Native drug in the bloodstream enters neutrophils, liver macrophages, and other cells. (2) Drug is oxidized to reactive metabolite(s). (3) Reactive drug (hapten) forms a covalent link with endogenous proteins, forming drug–protein adducts. (4) Drug–protein adducts are taken up by antigen- presenting cells (APCs) and peptide-drug presented in context of class II HLA to T helper (TH) cells that engage through T-cell receptor (TCR): Signal 1. (5) Stressed neutrophils or other cells release danger signals that activate APCs. (6) B7 on activated APCs engages CD28 on TH cell for co-stimulus that is Signal 2. (7) The activated TH cell provides signals to B cells to produce drug-dependent neutrophil antibodies against drug/hapten and to stimulate hapten-specific cytotoxic T cells (Tc): Immune response. (8) Drug-dependent neutrophil antibodies bind drug–protein adducts on neutrophil membrane that cause neutrophil destruction/clearance. (9) In the absence of danger signals, there is no Signal 2, resulting in immune tolerance.
Danger hypothesis
Presentation of hapten-modified granulocyte proteins by APCs via major histocompatibility complex to TH cells is referred to as “signal 1” (Figure 1).4,26,30 For an immune response to proceed, APCs must be activated by signal 2; in the absence of signal 2, immune tolerance results. Signal 2 is the danger signal produced by injured cells that leads to co-stimulation of APC and TH cells through interaction between B7 on APCs and CD28 on TH cells, culminating in production of specific antibodies and cytotoxic T cells. There are numerous endogenous molecules (eg, heat shock proteins, hyaluronan fragments) reported to serve as ligands for pattern recognition receptors such as toll-like receptors on APCs that can serve as danger signals. It is not clear how a drug–protein adduct might serve as a danger signal. One possibility is that other danger signals work synergistically with drug danger signals because increased risk of IDIN has been linked to open-heart surgery and specific viral infections,4 both of which create danger signals. Neutrophils “stressed” by exposure to reactive drug metabolites or reactions with drug-dependent neutrophil antibodies could theoretically release danger signal molecules as well. Another possibility is activation of inflammasomes by reactive drug–protein conjugates.31 Inflammasome activation leads to production of inflammatory cytokines such as interleukin-1β and interleukin-18, resulting in cell death and release of danger signals. It is apparent that both the hapten and the danger hypotheses are required for an immune response to result in IDIN.
Other support for an immune mechanism of IDIN
Both class I and II HLA genes have been associated with several drug-induced adverse effects, which are also supportive of an immune mechanism.23 Recently, 2 groups found a strong association between HLA genes and IDIN caused by thioamides. A Japanese study found an association between HLA class II DRB1*08032 and methimazole-induced agranulocytosis.32 A separate study in Taiwan performed both direct HLA sequencing and a genome-wide association study of DNA from 42 thionamide-induced agranulocytosis patients and 1208 Graves’ disease control subjects.33 The study reported increased independent susceptibility for developing agranulocytosis and having HLA-B*38:02 and/or HLA-DRB1*08:03.
Testing for drug-dependent neutrophil antibodies
As discussed, the consensus is that the majority of IDIN is immune mediated. In this scenario, drug-dependent antibodies and/or cytotoxic T cells that target neutrophils or their precursors should be present in the patient’s serum. One way to investigate an immune mechanism is to test the patient’s serum for the presence of drug-dependent neutrophil antibodies. Laboratory tests for drug-dependent platelet antibodies34 and red blood cell antibodies35 show good sensitivity, but the same is not true when using similar assays for detection of drug-dependent neutrophil antibodies. The reasons for this finding are unknown. Testing for drug-dependent neutrophil antibodies is rarely performed; the tests involving neutrophils are technically complex, primarily due to the daily requirement to use freshly isolated cells because neutrophils cannot be stored for long periods. This requirement demands the regular availability of large numbers of blood donors. When testing for neutrophil DDAbs is performed, the methods used are very similar to those used for detection of platelet DDAbs34,36 with substitution of freshly isolated neutrophils for platelets. A typical assay consists of incubation of isolated neutrophils with the patient’s serum in the presence of the implicated drug and also in the absence of drug; this is followed by washing of the cells incubated with drug with wash containing drug and those not incubated with drug just with buffer. Washed neutrophils are then incubated with fluorescent or enzyme-labeled anti-human immunoglobulin G and/or anti-human immunoglobulin M for detection of bound DDAbs. Increased neutrophil antibody reactivity in the presence of drug compared with reactivity without added drug is considered a positive result for drug-dependent neutrophil antibodies. Flow cytometry for immunofluorescent detection of DDAbs (Figure 2) and monoclonal antibody immobilization of granulocyte antigens assay for enzyme-linked immunosorbent assay detection and determination of the neutrophil glycoproteins targeted by DDAbs are methods that have been used.19,37-40 Other methods include immunoblotting, the granulocyte agglutination test, and granulocytotoxicity.41 Despite the availability of these various methods, neutrophil DDAbs, with the exception of those induced by quinine,38,39,41-43 are rarely detected.40,44-46 In the majority of cases, the antibody reactivity against neutrophils is not drug dependent, but the antibodies instead react against neutrophils isolated from all donors tested; this pattern of reactivity is suggestive of autoantibodies, perhaps induced by the drug. In a few cases when neutrophil DDAbs were detected, the glycoproteins targeted by DDAbs included CD177,42 CD11b, CD35, and CD16.40
Fluorescence histograms generated from immunofluorescence detection of immunoglobulin G (IgG) drug-dependent neutrophil antibodies by flow cytometry. Isolated normal donor neutrophils were incubated with patient’s serum and washed; neutrophil-bound antibodies were detected with fluorescent anti-human IgG. Results shown are for serum from a patient exposed to quinine with drug-induced neutropenia incubated with neutrophils showing higher IgG fluorescence (median fluorescence intensity [MdFI] = 315) with quinine (dark histogram) compared with patient’s serum incubated with neutrophils and buffer/no drug (light histogram, MdFI = 59), indicating the presence of quinine drug-dependent neutrophil antibodies. MdFI values are shown for each histogram.
Fluorescence histograms generated from immunofluorescence detection of immunoglobulin G (IgG) drug-dependent neutrophil antibodies by flow cytometry. Isolated normal donor neutrophils were incubated with patient’s serum and washed; neutrophil-bound antibodies were detected with fluorescent anti-human IgG. Results shown are for serum from a patient exposed to quinine with drug-induced neutropenia incubated with neutrophils showing higher IgG fluorescence (median fluorescence intensity [MdFI] = 315) with quinine (dark histogram) compared with patient’s serum incubated with neutrophils and buffer/no drug (light histogram, MdFI = 59), indicating the presence of quinine drug-dependent neutrophil antibodies. MdFI values are shown for each histogram.
One possible reason for the low sensitivity observed for detection of drug-dependent neutrophil antibodies could be the need for reactive drug metabolites in the assay. Native drug is almost always used, and the assay conditions are not optimal to allow for the drug to be metabolized during incubation with neutrophils and patient’s serum; it is therefore likely that if drug-dependent neutrophil antibodies are present, their specific antigen is not. This improvement alone to the test could be crucial to its accurate performance. Platelet and red blood cell drug-dependent antibodies that can only be detected in assays using drug metabolites have been described.47-49 It could be difficult to obtain or produce reactive drug metabolites, but a good start might be to simply incubate the neutrophils to be used in the assay with native drug under conditions that promote oxidation by neutrophil myeloperoxidase; this method could include addition of supernatants from activated neutrophils as an additional source of enzyme.
Diagnosis and clinical management of patients
Because patients affected with IDIN are often asymptomatic, IDIN can be difficult to detect, and therefore, the early detection and timely correction of neutrophils to normal levels are critical to prevent severe infections. If infections are not treated aggressively, 60% of IDIN patients with severe neutropenia will develop septicemia.11 IDIN frequently presents as an infection with symptoms of a sore throat and/or fever. In general, the diagnosis of IDIN should be made by following expert standards of care,50,51 taking a complete medical history, performing a careful examination, and collecting laboratory results (including a complete blood count and review of a peripheral blood film). A lack of immature cells (eg, bands) often results in longer time to recovery of neutrophil levels.11 A complete blood count for patients affected with IDIN will reveal a granulocyte count < 1.5 × 109/L (more often, 0.5 × 109/L), but other cell lines (platelets and red blood cells) are usually at normal levels.
The most important treatment of IDIN is discontinuation of the offending drug. Which drug can be difficult to determine if the patient is taking multiple agents, but initial consideration should be given to those medications frequently associated with IDIN (Table 2). After drug removal, most cases of neutropenia resolve over time, and only symptomatic therapy such as antibiotics for treatment and prophylaxis of infections and good hygiene practices are necessary. The average time for full recovery of the neutrophil count is 9 days (range, 9-24 days).11 Patients experiencing prolonged neutropenia may require additional treatment with hematopoietic growth factors such as granulocyte-colony stimulating factor (G-CSF). Treatment of patients with severe neutropenia using G-CSF is controversial. Some reports show shortened duration of neutropenia,9 antibiotic therapy, and hospital length of stay11 with the use of G-CSF. Use of growth factors for treatment of IDIN was reported to reduce neutrophil recovery to normal levels in 8 days versus 9 days in control subjects.52 A commonly reported dosage for treatment of adult patients is subcutaneous injection of 300 µg/d. A dose of 5 µg/kg per day until neutrophil counts are >0.5 × 109 neutrophils/L has also been recommended.53 The only prospective, randomized trial to date did not confirm the benefit of G-CSF therapy.54 However, the patient numbers studied were small (N = 24), and the G-CSF dosage used was less (100–200 µg/d) than the 300 µg/d recommended by some experts. Patients with a neutrophil count <0.1 × 109/L have been reported to have more infections and fatal complications than those with a higher nadir, and patients with a neutrophil nadir ≤0.1 × 109/L should therefore receive G-CSF 300 µg/d regardless of the presence of infection.11
Conclusions
Numerous reviews of IDIN have been published, several recently,1,4,8,10,11,23,55 but reports of systematic studies of this relatively rare disorder are limited because of the very fact that such drug reactions are idiosyncratic; that is, they pertain to something peculiar to an individual. Therefore, it is difficult to fully understand the incidence, mortality, implicated drugs, and mechanisms responsible for IDIN. Antibiotics, antithyroid drugs, clozapine, and ticlopidine are frequently reported in IDIN cases. Accumulative evidence to date suggests that IDIN frequently occurs through an immune mechanism. Development of improved animal models and diagnostic testing for drug-dependent neutrophil antibodies, additional studies of drug metabolism, and further evaluation of genetic associations in IDIN are all important areas for the focus of future research efforts.
Correspondence
Brian R. Curtis, Diagnostic Labs and Blood Research Institute, BloodCenter of Wisconsin, PO Box 2178, Milwaukee, WI 53201-2178; e-mail: brian.curtis@bcw.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author is on the Board of Directors or an advisory committee and has consulted for Ionis Pharmaceuticals.
Author notes
Off-label drug use: Recombinant granulocyte colony-stimulating factor in treatment of drug-induced neutropenia.

![Figure 2. Fluorescence histograms generated from immunofluorescence detection of immunoglobulin G (IgG) drug-dependent neutrophil antibodies by flow cytometry. Isolated normal donor neutrophils were incubated with patient’s serum and washed; neutrophil-bound antibodies were detected with fluorescent anti-human IgG. Results shown are for serum from a patient exposed to quinine with drug-induced neutropenia incubated with neutrophils showing higher IgG fluorescence (median fluorescence intensity [MdFI] = 315) with quinine (dark histogram) compared with patient’s serum incubated with neutrophils and buffer/no drug (light histogram, MdFI = 59), indicating the presence of quinine drug-dependent neutrophil antibodies. MdFI values are shown for each histogram.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2017/1/10.1182_asheducation-2017.1.187/4/m_hem00026f2.jpeg?Expires=1768044467&Signature=Wi~Bt6K5GU8tBWN7ztyXfY7GQVM3bCKUOt6sPHjbK2bt~pR7MLQmDSq-EhD3-mIqmAxAP4uA7JQ1l64ONNiy0XxxXpt89NgwfSmiE3bNS0UiblqpEd5mTzc0YwEVFvXWNkEe2-JYi5Tm~E-rajuEHV4aYdBnOm61nv-kMZJh21bxjAelaCQoSgBqZTWW~~~hyzvQSSYbpcABPD5NqjllcmtuOXGd6TLxXPVsBFUfc2CfUpvJI~r1ZGlWtRmMyFumHrvkf6RmXBjAH58g2Vz7-JkJVpLy3wwGoRrEyWdFARQ0adM~nKvRddMmEEytgKIw5fIXFqd5GHvu1yJEnWBn0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)