Abstract
Survival among adolescents and young adults (AYAs) ages 15 to 39 with cancer has not improved to the same extent as that of pediatric and older adult cancer patients, which is thought to relate, in part, to the lower participation of AYAs in clinical trials. Because significant efforts have been made to improve clinical trial enrollment for AYAs, we (1) present contemporary clinical trial enrollment rates by cancer type, sociodemographic characteristics, and treatment setting and (2) discuss provider-, patient-, and system-level barriers to clinical trial participation. Contemporary studies examining clinical trial enrollment among AYAs have continued to find low overall participation relative to pediatric populations, with most studies observing no significant improvements in enrollment over time. In addition to age and cancer type, enrollment varies by treatment setting, health insurance, and race/ethnicity. Access to available clinical trials may be increased by appropriate referral of AYAs to pediatric and adult specialty cancer centers with studies relevant to the AYA population because most AYAs are treated in the community setting. Even with similar access to trials, however, AYAs may be less likely to participate, and therefore, future efforts should focus on better understanding and addressing barriers to enrollment as well as improving education and outreach regarding clinical trials.
Learning Objectives
Understand contemporary clinical trial enrollment rates among adolescents and young adult (AYA) cancer patients and how rates vary by cancer type, patient demographics, and treatment setting
Identify provider-, patient-, and system-level barriers to clinical trial participation in AYA cancer patients
Introduction
Over the past 30 years, survival among adolescents and young adults (AYAs) ages 15 to 39 years old with cancer has not improved to the same extent as that of pediatric and older adult cancer patients.1,2 Although the evidence is limited to explain these national trends, poorer outcomes are postulated to be related to lower participation in clinical trials, poorer access to care, receipt of treatment in facilities without AYA experience, patient and tumor biology, and developmental characteristics.3,4 In 2006, the National Cancer Institute (NCI) Progress Review Group (PRG) examined the state of the science of cancer in AYAs and identified areas of research with the potential to improve the survival disparities,4,5 including expanding clinical trial access and enrollment and increasing treatment choices to accelerate treatment advances.5 At the time that the report was published, we showed low overall clinical trial enrollment in AYAs (14%) relative to published pediatric enrollment rates, with lower enrollment among uninsured older patients and those treated by nonpediatric oncologists.6
Among pediatric cancer patients under 15 years of age, dramatic improvements in survival have been attributed to enrollment in clinical trials.7,8 The majority of pediatric cancer patients are treated at institutions that participate in NCI-sponsored clinical trials (∼90%), which results in as many as two-thirds being enrolled in clinical trials.5 In contrast, older AYAs are more likely to be treated in adult settings (academic and community), with significantly lower rates of clinical trial enrollment.9-11 Other than treatment setting and provider factors (community settings with limited access to trials; knowledge of available trials), underlying reasons for low clinical trial enrollment are not well understood but likely include a combination of patient- (underinsurance; patient concerns) and system-level factors (age restrictions; trial availability).4,8,12
Significant efforts have been made to improve both access to trials and trial enrollment for AYAs with cancer over the past decade,8,13,14 including improved insurance rates under the Affordable Care Act15 and expanded access to community-based clinical trials through the NCI Community Oncology Research Program.16 In addition, initiatives have focused on AYAs specifically by expanding age eligibility, as appropriate, to provide more clinical trial options for AYAs.8,13 Therefore, to further understand recent trends in AYA clinical trial enrollment over the past decade, we present contemporary AYA clinical trial enrollment rates; consider variations in enrollment across cancer types, sociodemographic, and treatment setting characteristics; and discuss barriers to clinical trial participation.
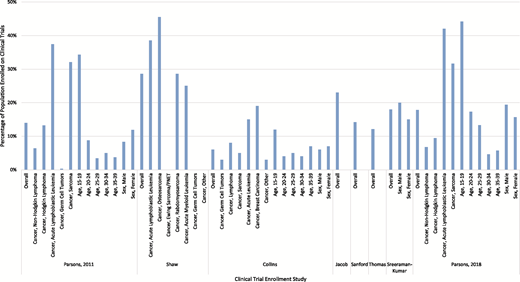
Enrollment by cancer type, age, and sex
Contemporary studies examining clinical trial enrollment among AYAs with cancer have noted continued low overall participation relative to pediatric populations. Since 2010, ∼10 studies have evaluated rates of clinical trial participation among AYAs with cancer across a variety of treatment settings (Table 1), finding highly varied enrollment across patient and cancer characteristics (Figure 1). When the NCI PRG first published their roadmap to address survival disparities among AYAs in 2006,4,5 our population-based analyses of 1358 AYAs in the 2006 Surveillance, Epidemiology and End Results (SEER) Program found that only 14% of 15 to 39 year olds were enrolled in clinical trials, with participation varying by cancer type and age.6 Specifically, clinical trial participation was most common in patients with acute lymphoblastic leukemia (ALL; 37%), sarcoma (32%), Hodgkin lymphoma (13%), and non-Hodgkin lymphoma (6%).6 Participation also dropped dramatically by age, with 34% of those ages 15 to 19 enrolling on a trial compared with just 3% of those ages 35 to 39.6 Decreases in participation by age also were observed for patients with ALL: 49% of those ages 15 to 19 years old enrolled on a trial compared with only 28% of 35 to 39 year olds. Overall, these findings reinforced the recommendations from the NCI PRG that supported the need to improve access to clinical trials and reduce variation across patient populations going forward.
Clinical trial enrollment across patient demographics and cancer type among AYA cancer survivors by study.
Clinical trial enrollment across patient demographics and cancer type among AYA cancer survivors by study.
Since that time, several single-institution and hospital system studies continued to report consistently low participation of younger AYAs on clinical trials. In a single-institution study of 57 15 to 22 year olds treated in a joint pediatric and adult AYA oncology program from 2006 to 2010, Shaw et al17 found 28% overall clinical trial participation, a higher level of enrollment relative to the 3 years before program initiation. Although enrollment was not reported by age or sex, the authors noted significant variation in enrollment by cancer type, with enrollment higher among those with osteosarcoma (45%), ALL (38%), and acute myeloid leukemia (AML; 25%) compared with those with other cancers.17 Jacob and Shaw18 also examined trial enrollment among AYA patients seen at a specialty children’s hospital from 2010 to 2014, finding that 23% of 15 to 32 year olds enrolled compared with 77% of younger patients. Although not evaluated across patient and cancer characteristics, the authors noted no significant improvement in clinical trial enrollment from previous years. Thomas et al19 additionally examined NCI-sponsored clinical trial enrollment among 216 0 to 21 year olds from 2015 to 2016 within a specialty children’s hospital, reporting that fewer AYAs enrolled on either an existing (12% vs 32%) or institutionally available (30% vs 68%) clinical trial than younger patients. Similar to other studies, higher enrollment occurred among young AYAs diagnosed with leukemia or lymphoma compared with solid tumors, with no differences in trial enrollment found by sex.19
From 2015 to 2018, three additional studies were published examining contemporary clinical trial enrollment rates in specialty centers among AYAs across the 15- to 39-year-old age range. Collins et al20 evaluated AYA patients diagnosed from 2008 to 2012 at affiliated pediatric and adult academic hospitals, finding that therapeutic clinical trial enrollment among AYAs (6%) was equivalent to that of older adults (6%) but lower than enrollment for children younger than 15 years old (22%) across the hospitals. Similar to other studies, they found decreasing therapeutic clinical trial enrollment by age within the AYA group, similar enrollment by sex, and a wide variation by cancer type.20 Sanford et al21 additionally evaluated therapeutic and supportive care clinical trial enrollment among AYAs at a pediatric and affiliated NCI-designated cancer center. Among 2154 15 to 39 year olds treated from 2010 to 2014, the authors found clinical trial enrollment rates of ∼14% overall. There were no differences in enrollment by age or sex, but there were variations by cancer type. In the pediatric setting, a high proportion of leukemia patients participated in a therapeutic or supportive care study (65%) followed by lymphoma (45%), brain/central nervous system cancer (38%), and sarcoma (33%) patients.21 In the adult setting, leukemia/myeloma patients (19%) had the largest percentage enrolled on a study.21 Finally, among a cohort of 1740 AYAs with invasive melanoma diagnosed between 1986 and 2015 in an NCI-designated cancer, Sreeraman Kumar et al22 found overall therapeutic clinical trial enrollment rates of 18%. In contrast to other studies, the authors found significantly higher enrollment among males (20%) than females (15%); however, they found only a borderline improvement in enrollment over time (1986-2006: 16%; 2007-2015: 20%).22
Recently, there have been new national and population-based studies evaluating trends in clinical trial enrollment since the introduction of dedicated efforts to improve access to and enrollment on clinical trials for AYAs. Roth et al10 examined the enrollment of 90 985 AYAs from 2004 to 2013 on Children’s Oncology Group (COG) therapeutic and supportive care trials, finding that the proportional enrollment of AYAs (number of AYA enrollments divided by total enrollments) relative to pediatric populations declined significantly from 34% of enrollments from 2004 to 2008 to 31% of enrollments from 2009 to 2013. Specifically, proportional enrollment of AYAs declined significantly from 34% in 2004 to 2008 to 24% in 2009 to 2013 for those with ALL at Community Clinical Oncology Program (CCOP) sites but not for other cancers, which may relate to a shift from COG trials to adult cooperative group trials that were not considered in this study.10 At non-CCOP sites, no change in estimated proportional enrollment was observed for ALL (27%), but significant increases were seen in proportional enrollment of AYAs with AML (26%-32%) and rhabdomyosarcoma (24%-34%). Finally, in a recent update to our 2011 population-based study, we examined patterns of AYA clinical trial participation over time.23 Among 3135 AYAs diagnosed in the 2006 and 2012/2013 SEER Program, we found that clinical trial participation increased from 15% to 18% among patients diagnosed with non-Hodgkin lymphoma, Hodgkin lymphoma, ALL, and sarcoma.23 Consistent with other studies, clinical trial participation in 2012/2013 varied by cancer type and was the highest in patients with ALL (42%) and sarcoma (31%) compared with patients with Hodgkin (9%) and non-Hodgkin (7%) lymphoma.23 In addition, we continued to find lower clinical trial enrollment among those who were older at diagnosis: 65% of ALL patients 15 to 19 years old participated in a clinical trial vs 30% of ALL patients 35 to 39 years olds. Across all cancers, our analyses indicated improved clinical trial enrollment among young adults, such that we no longer observed significant differences in enrollment between 20 to 29 year olds and 15 to 19 year olds in 2012/2013.23 Consistent with improved enrollment among young adults, Siegel et al24 reported on the estimated accrual proportion from 2000 to 2009 and from 2010 to 2015 onto NCI-sponsored national ALL treatment and found that the steep drop in clinical trial accrual after 15 years of age was less dramatic after 2010 for AYAs younger than 30 years old but not those 30 to 39 years old. Unlike ALL, however, Bleyer et al25 reported steady declines in NCI-sponsored treatment trials from 2010 to 2015 for all cancers except Kaposi sarcoma.
Variations in enrollment
Treatment setting
The availability of clinical trials depends on whether AYA patients are treated in pediatric, adult cancer specialty, or community settings. We previously reported that clinical trial enrollment was higher among AYAs treated by pediatric oncologists, a finding that we continued to observe in more recent 2012/2013 data.6,23 This is supported by two recent studies that examined clinical trial enrollment in affiliated pediatric and adult NCI-designated cancer center hospitals.20,21 From 2010 to 2014, Sanford et al21 found that 11% of AYAs were enrolled in therapeutic and supportive care studies in the adult setting compared with 42% of AYAs in the pediatric setting. From 2008 to 2012, Collins et al20 found that an affiliated pediatric hospital enrolled a higher percentage of AYAs onto therapeutic studies (15%) than the adult cancer center (3%) and public hospital (5%). Recent data from the CCOP observed a lower proportional enrollment of AYAs on applicable COG studies at CCOP vs non-CCOP COG group member sites from the period 2004 to 2008 to the period 2009 to 2013 (24% vs 28%, respectively; P < .001), with enrollment declining during this period.10 These findings suggest that CCOPs, which are located in the community setting and have a clinical research infrastructure involving both medical and pediatric oncologists, did not result in higher AYA enrollment than traditional COG member treatment settings.10
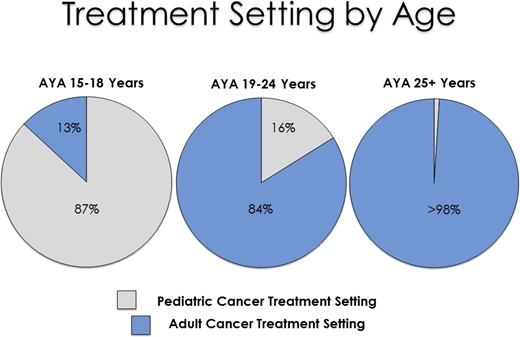
Recent population-based data in California suggest that most (68%) of the 1473 AYA ALL patients diagnosed from 2000 to 2014 were treated in an adult cancer setting.26 As expected, treatment setting differed significantly by age (Figure 2).26 Although 56% of AYA ALL patients received their initial ALL treatment at either an NCI-designated cancer center or COG center, this differed substantially based on adult or pediatric setting of care, with 89% (pediatric setting) and 41% (adult setting) of AYAs receiving care at these centers, impacting the availability of clinical trials as well as outcomes.26 In particular, treatment in the pediatric setting or at an NCI-designated cancer center or COG center (for patients treated in the pediatric setting) was associated with superior survival.26 Although not differentiated by adult and pediatric setting, we also found that 30% of hospitalized AYA AML patients (n = 1059; 1999-2012)27 and 36% of hospitalized AYA ALL patients 19 to 39 years old (n = 1779; 1991-2014)28 received their care at NCI-designated cancer centers, a factor associated with lower early mortality.
Initial treatment setting among AYAs with acute lymphoblastic leukemia from 2004 to 2014 in California.26
Initial treatment setting among AYAs with acute lymphoblastic leukemia from 2004 to 2014 in California.26
Health insurance
We previously reported that uninsured AYAs diagnosed in 2006 were 25% as likely to enroll onto clinical trials compared with AYAs with private health insurance, with no differences in enrollment between private and other types of insurance.6 More recent data (2010-2014) at a pediatric and affiliated NCI-designated cancer center found that AYAs with private insurance were more likely than those with Medicaid (pediatric setting) or receiving financial assistance (adult setting) to be enrolled on therapeutic and supportive care protocols.21 Furthermore, AYAs with insurance compared with those with unknown insurance were more likely to enroll on a clinical trial at an NCI-designated cancer center,22 suggesting that inadequate health insurance is a barrier to clinical trial enrollment. However, in follow-up data at the population level, we saw a doubling in clinical trial participation from 5.7% in 2006 to 12.8% in 2012/2013 among the uninsured, such that health insurance was no longer associated with clinical trial enrollment.23 During this time, we also showed significant reductions in the overall uninsured population as a result of early provisions from the Affordable Care Act, which should have improved access to cancer care and clinical trials for the newly insured.15,29 Indeed, 15 to 25 year olds were found to have significantly smaller reductions in NCI-sponsored clinical trial accrual than older cancer patients who were not impacted by these provisions.25 Although the Affordable Care Act policies should not have influenced access to or use of medical care among those who remained uninsured, perhaps reductions in the overall uninsured population increased the availability of resources for the remaining uninsured individuals to support their nonreimbursed, trial-related expenses.
Race/ethnicity
Recent data suggest that clinical trial enrollment varies by race/ethnicity in both the pediatric and adult settings. In the pediatric setting, AYAs of black or other races were less likely to enroll on therapeutic and supportive care studies than AYAs of white race, with no differences by Hispanic ethnicity.21 In the adult setting, although AYAs of other/unknown race were less likely than whites to enroll on therapeutic and supportive care studies, Hispanics were more likely to enroll than non-Hispanics.21 No racial/ethnic differences were observed for enrollment on therapeutic trials across affiliated pediatric and adult settings, but Hispanic and Asian AYAs were more likely to enroll on nontherapeutic studies than non-Hispanic white patients.20 Finally, we identified an emerging disparity in our population-based study not seen in our original investigation of clinical trial enrollment—significantly lower enrollment among AYAs of black or other races/ethnicities relative to non-Hispanic white patients.6,23 These findings suggest that some racial/ethnic minorities, particularly black patients, are less likely to enroll on clinical trials than non-Hispanic whites. This was observed in our recent assessment in AYA ALL patients, where the ALL Intergroup C10403 prospective clinical trial was not administered to any AYA black patients in our study population.26 Although the proportion of adult black patients who participated in clinical trials increased from 2000 to 2010, a recent systematic review of cancer clinical trial enrollment in racial/ethnic minorities identified that non-Hispanic whites continue to make up the largest majority of participants.30
Barriers to enrollment
Provider factors
Provider factors influencing access to and referral into a clinical trial may include a combination of treatment setting as well as physician attitudes and knowledge.12 As discussed previously, AYAs with cancer are predominately treated by providers in nonpediatric centers and community hospitals, where access to clinical trials is more limited, reducing opportunities for clinical trial enrollment.11 Although the underlying reasons for this are not well understood, community oncologists may not refer AYAs to pediatric or adult specialty cancer centers due to a lack of awareness of poor AYA outcomes, general nonparticipation in NCI-funded trials, or geographic inconvenience for patients.4 In addition, provider knowledge about available trials for AYAs also remains a significant barrier for enrollment on available trials. Specifically, adult oncologists face barriers in remaining up to date on ongoing pediatric studies in neighboring departments or pediatric hospitals and vice versa.31 However, one approach from Shaw et al17 showed that a partnership between adult medical and pediatric oncologists through an AYA oncology program can lead to improved trial enrollment.
Providers also may feel burdened or overwhelmed by the clinical trials process, both administratively and relationally, creating unfavorable attitudes toward enrolling patients on trials.12 In a survey of 111 oncologists from Texas, Ramirez et al32 showed that the burden of the clinical trial process was associated with a lower likelihood of referring patients to early-phase clinical trials. Specifically, greater participation in the clinical trial process created logistical barriers, such as diverting time and resources away from providers’ clinical practice.32 Additionally, in a qualitative study of 11 AYA providers, Barakat et al33 identified that providers felt challenged to maintain the attention of AYAs, balance information while minimizing coercion, respond appropriately to family structures and decision making, and communicate research with AYAs. To address these concerns, the NCI has developed a series of workshops and trainings to educate providers and encourage more community oncologists to offer clinical trial opportunities.14
Patient factors
Patient-related barriers to clinical trial enrollments are multifactorial in nature and include, although are not limited to, a combination of knowledge, attitudes, personal conflicts, and socioeconomic factors.12 Patient knowledge of clinical trial availability is necessary for clinical trial enrollment. Among 515 AYA cancer patients in the Adolescent and Young Adult Health Outcomes and Patient Experiences (AYA HOPE) study, Shnorhavorian et al34 found that only 17% of AYAs were aware that there were clinical trials available for their stage or type of cancer. Patients who had ALL or were younger at diagnosis (younger than 30 years old) were more likely to know about available clinical trials.34 Among those who knew that a trial was available, 68% enrolled.34 For AYAs who chose not to participate in a trial, the primary reason was concern about involvement in research, including concerns that the experimental treatment was not sufficiently tested and fear about side effects. Additionally, 21% indicated problems accessing trials, including being too sick to enroll on a trial or not being able to find a trial nearby.34
Patient attitudes also drive decision making around clinical trial enrollment. In a study of 100 cancer patients from south Texas, Chalela et al35 found that nonenrolled patients tended to have higher distrust of the medical system and fear/uncertainty of new treatment, considering them important barriers that would keep them from enrolling on early-phase clinical trials. Additionally, they found that Hispanics were more likely than non-Hispanic white patients to identify distrust of researchers due to prior personal/family experience as well as language barriers, factors that keep them from effectively communicating with their doctor and understanding the trial purpose and process.35 In a qualitative study of 13 AYAs, Barakat et al33 further noted that lower levels of developmental and emotional maturity, cognition, and autonomy all created additional attitudinal barriers toward AYA clinical trial enrollment. Furthermore, an overall lack of awareness among AYAs of the importance of research may be a barrier to study recruitment.13
AYAs with cancer are also encountering the medical system at a time of intense change and development, introducing potential conflicts from personal, professional, and family obligations that may hinder participation in clinical trials. Barakat et al33 found that AYAs identify a combination of both physical (eg, acute distress, lower health-related quality of life) and procedural (eg, additional processes, increased length of treatment, quick decision making in times of crisis) barriers that may directly conflict with their ability and willingness to participate in a clinical trial. In general, AYAs struggle with their adherence to cancer treatment relative to both older and younger patients.36 Therapy for many common AYA cancers, including ALL, is long and intense, putting pressure on patients and their support networks to adhere to frequent clinic visits and complex medication regimens.37 Participation in a clinical trial complicates this further with additional appointments, testing, regulatory requirements, and medications.37 Patients must then balance these additional requirements from participating in a trial with educational and employment expectations, family responsibilities, and romantic relationships.31 Finally, socioeconomic barriers, including lacking health insurance or needing financial assistance, as discussed above can hinder patient’s ability to participate.6,12,21
System factors
System-related factors represent an additional set of barriers that include age restrictions, limited trial availability, and extensive regulatory barriers for AYA patients. At the health care facility level, there may be age limits for admission or a lack of developmentally appropriate facilities, impacting where patients receive their care.4 Historically, the age eligibility criteria of clinical trials have been set to a lower age range of 18 to 21 years old for studies designed by adult investigators and an upper age range of 16 to 22 years old for pediatric studies, impacting the availability of studies for the AYA cancer population.8,13 In a study of all patients seen at a specialty pediatric hospital from 2010 to 2014, Jacob and Shaw18 showed that lack of trial availability was cited as the reason for nonrecruitment in 42% of new pediatric cancer patients younger than 15 years old compared with 62% of those in older age groups (15-22 year olds), findings similar to those reported from 2001 to 2006 at the same institution.38 In 2015 to 2016 data from another specialty pediatric hospital, Thomas et al19 observed that children younger than 15 years old were more likely than 15 to 21 year olds to enroll on a clinical trial despite that there was no significant difference in clinical trial availability between these age groups, suggesting that factors other than availability serve as barriers to enrollment. However, in a prior study at this pediatric and two affiliated adult academic hospitals, Collins et al20 reported that clinical trials were available for 8 of the 10 most common cancers in AYAs compared with all 10 of the most common cancers in children. Furthermore, COG trials were accessible to AYAs at the children’s hospital but were not accessible to those cared for at the adult institutions.20 These findings highlight the importance of AYAs being referred to institutions with access to available clinical trials.
Regulatory barriers are extensive and can create significant burden to system-wide participation in clinical trials. Regulatory guidelines and funding requirements to protect patients may have the unintended consequence of added system barriers to opening new trials. Recent interpretation of the guidelines by the Office for Human Resource Protections has restricted where clinical trial participants can receive their chemotherapy, even if the medication is considered noninvestigational (ie, standard of care).39 Furthermore, lengthy regulatory procedures and unfamiliarity with age-specific (eg, pediatric vs adult) protocols may prohibit opening of pediatric protocols in adult centers and vice versa.31 This results in common adult-type cancers predominating the available trials in adult facilities, where the majority of AYAs are treated,31 resulting in fewer trial offerings for AYAs.
Conclusion
Contemporary studies examining clinical trial enrollment among AYAs have continued to find low overall participation relative to pediatric populations, with a number of provider-, patient-, and system-level factors impacting these rates. Most studies have not observed significant improvements in enrollment over time, despite the substantial efforts that have been made to increase participation of AYAs in clinical trials, including the creation of AYA-focused committees within the cooperative oncology groups, formation of the National Clinical Trials Network and National Clinical Trials Network AYA Working Group, and development of collaborative studies and other initiatives to expand clinical trial availability and participation for AYAs.4,8,13,25 Among proposed recommendations to improve clinical trial enrollment among AYAs,8,12,25 access to available clinical trials may be increased by appropriate referral of AYAs to pediatric and adult specialty care centers with studies relevant to the AYA population13 because most AYAs are treated in the community setting. For ALL and AML, where most AYAs have been found to be treated in the community setting, this has implications for improving survival.26-28 Even with similar access to trials, however, AYAs may be less likely to participate,19 and therefore, efforts to better understand and address barriers to enrollment as well as improve education and outreach regarding clinical trials will need to be undertaken.
Acknowledgments
The authors thank Drs. Lori Muffly, Elysia Alvarez, Justine Kahn, and Lena Winestone for their contributions to this manuscript.
Correspondence
Theresa H.M. Keegan, Division of Hematology and Oncology, University of California Davis Comprehensive Cancer Center, 4501 X St, Suite 3016, Sacramento, CA 95817; e-mail: tkeegan@ucdavis.edu.
References
Competing Interests
Conflict of interest: The authors declare no competing financial interest.
Author notes
Off-label disclosure: None disclosed.