Abstract
Venous thromboembolism (VTE) is a common complication in ambulatory cancer patients receiving chemotherapy. Current clinical guidelines recommend against the use of routine primary thromboprophylaxis in unselected ambulatory cancer patients. The Khorana score is a risk assessment tool derived and prospectively validated for the identification of cancer patients at high risk of thrombotic complications. Recently, 2 randomized, controlled trials have assessed the use of low-dose direct oral Xa inhibitors, apixaban and rivaroxaban, for the prevention of cancer-associated thrombosis in ambulatory patients at intermediate to high risk of VTE (Khorana score ≥2). Taken together, these trials have shown that low-dose direct oral Xa inhibitors reduce the risk of VTE in this patient population without a significant increase in major bleeding. These results should encourage clinicians to consider the use of primary thromboprophylaxis in ambulatory cancer patients at intermediate to high risk of VTE who do not have any apparent risk factors for bleeding. The direct oral Xa inhibitors have also been assessed in the acute management of cancer-associated thrombosis. Current evidence suggests that these drugs are a convenient, effective, and safe option for the management of acute VTE in many cancer patients. Low-molecular weight heparin, however, may continue to be the treatment of choice depending on the presence of bleeding risk factors, the type of cancer, drug-drug interactions, and patient preferences.
Learning Objectives
Understand the incidence and risk factors associated with venous thromboembolism in cancer patients
Learn the management options for the prevention of venous thromboembolism in ambulatory cancer patients receiving chemotherapy
Review the management of cancer-associated thrombosis
Clinical case 1
A 58-year-old man has been diagnosed with advanced pancreatic cancer. He does not have any other comorbidities requiring active medical care apart from hypertension. Bilirubin is <1.5 times the upper limit of normal, albumin is 3.5 g/L, international normalized ratio is 1.1, and creatinine is within the normal limit. White blood cell count is 9.8 × 109/L. Hemoglobin is 13.4 g/dL, and platelets are 420 × 109/L. Body mass index is 28 kg/m2. The patient has never suffered a venous thromboembolic or major bleeding event. Eastern Cooperative Oncology Group Performance Status is 1. He is to start a platinum-based chemotherapy regimen next week. His Khorana score is 3.
Epidemiology of cancer-associated thrombosis
Cancer is a hypercoagulable state associated with an estimated increase in the risk of venous thromboembolism (VTE) of 4 to 7 times the baseline risk of the general population.1 A nested case-control study from the Olmsted County cohort reported an odds ratio (OR) of 4.1 for the diagnosis of VTE in patients with cancer. In patients with cancer requiring chemotherapy, the OR increased to 6.5.2 In a recent large database study, which followed patients for a total of 112 738 active cancer-associated person-years, cancer patients were found to have a VTE rate of 5.8 (95% confidence interval [95% CI], 5.7-6.0) per 100 person-years.3 The most common types of cancers, prostate in men and breast in women, contributed most heavily to the overall burden of cancer-associated thrombosis (CAT). The highest VTE incidence rate, however, was found in patients with pancreatic cancer (14.6 per 100 person-years; 95% CI, 12.9-16.5) followed by brain cancer (12.1 per 100 person-years; 95% CI, 10.3-14.0) and ovarian cancer (11.9 per 100 person-years; 95% CI, 10.6-13.2). Furthermore, this same study found that the rate of recurrent VTE after the diagnosis of CAT was substantial at 9.6 (95% CI, 8.8-10.4) per 100 person-years and that CAT was associated with an overall mortality rate of 67.7 (95% CI, 65.9-69.7) per 100 person-years. Tellingly, 64.5% of mortality occurred in the first year after VTE diagnosis. These data are consistent with previous studies, which found that thromboembolism is the second leading cause of death in cancer patients receiving chemotherapy4 and that the annual death rate for VTE in cancer patients is 47 times that of the general population.5
Primary prevention of CAT in ambulatory patients
Current guidelines do not recommend the general use of thromboprophylaxis in ambulatory cancer patients, although this intervention may be considered in certain patients at high risk for CAT.6,7 Furthermore, the American Society of Clinical Oncology guidelines recommend that cancer patients should be periodically reassessed for CAT risk and that these patients should be educated regarding the presenting signs and symptoms of VTE.6 Several studies have assessed the role of prophylactic dose low-molecular weight heparin (LMWH) or ultra-LMWH in the prevention of CAT in ambulatory cancer patients receiving chemotherapy.8-17 Based on high-quality evidence, a Cochrane systematic review on this subject found a risk ratio (RR) for the development of symptomatic VTE in unselected cancer patients treated with LMWH prophylaxis compared with no thromboprophylaxis of 0.54 (95% CI, 0.38-0.75; I2 = 0%).18 Based on low-quality evidence, this finding was associated with a statistically nonsignificant increase in major bleeding (RR = 1.44; 95% CI, 0.98-2.11; I2 = 0%) but a significant increase in clinically relevant nonmajor bleeding (CRNMB; RR = 3.40; 95% CI, 1.20-9.63; I2 = 78%). The 1-year overall mortality was unchanged between the 2 groups (RR = 0.93; 95% CI, 0.80-1.09; I2 = 62%). Therefore, although this meta-analysis suggested that primary thromboprophylaxis in cancer patients was effective and safe, the limited number of both thrombotic and bleeding events leading to small absolute differences between the treatment and observation or placebo groups led the authors to conclude that additional studies were needed before the general use of LMWH prophylaxis in cancer patients could be recommended.
Assessment for the risk of VTE in ambulatory cancer patients
Identifying cancer patients at high risk of VTE complications for which thromboprophylaxis might lead to a larger absolute difference in efficacy is warranted. Multiple factors have been associated with the risk of CAT, including patient-related risk factors, cancer-associated risk factors, therapy-related risk factors, and the presence of certain biomarkers19 (Table 1). Unfortunately, as highlighted by the American Society of Clinical Oncology guidelines,20 individual risk factors cannot reliably predict the risk of thrombosis in cancer patients. Therefore, validated risk assessment tools must be used in clinical practice to identify those patients who are most at risk for CAT.
The Khorana risk score integrates several patient, tumor, and hematological biomarkers into 1 risk assessment tool to identify patients at high risk of VTE21 (Table 2). This risk score was prospectively validated in a large cohort study, the Vienna CATS cohort, in 2010.22 In this Vienna CATS cohort study, the risk of VTE at 6 months in patients with a Khorana score of 2 or more, representing a high-intermediate score, was found to be elevated at 9.4%. Of note, this same study also proposed an expanded Khorana score (Table 3). In clinical practice, a dichotomized Khorana score using a score of 2 or more to identify patients at intermediate-high risk of VTE was also found to be both practical and readily capable of risk-stratifying cancer patients into 2 clinically relevant groups.23 Since then, the Khorana score has been validated retrospectively and prospectively in >18 000 patients internationally.24
Can the use of risk stratification and thromboprophylaxis in selected cancer patients reduce the risk of CAT?
Prophylaxis of High-risk Ambulatory Cancer Patients Study (PHACS) was a multicenter, randomized, controlled trial (RCT) assessing LMWH (dalteparin 5000 U daily) vs observation in ambulatory cancer patients receiving chemotherapy, with a Khorana score of 3 or more.13 Before randomization, patients underwent screening compression ultrasonography of the lower extremities and chest computed tomography to rule out preexisting VTE. Repeat compressive ultrasonography screening of the lower extremities was performed at weeks 4, 8, and 12. Of the 117 patients enrolled in the study, 10 patients were diagnosed with a VTE at baseline, and 9 were not randomized. Therefore, 50 patients were randomized to the LMWH arm, and 48 were randomized to the observation arm. The trial was unfortunately terminated early because of poor accrual. In the included patients, 37% had pancreatic cancer, 27% had lung cancer, and 25% had gastroesophageal cancer. In the LMWH arm, 6 VTE events were diagnosed compared with 10 in the observation arm for a hazard ratio (HR) of 0.69 (95% CI, 0.23-1.89). Of note, there were only 4 symptomatic VTE events diagnosed. The primary safety outcome of clinically relevant bleeding occurred in 7 patients in the dalteparin arm compared with 1 in the observation arm for an HR of 7.02 (95% CI, 1.24-131.6). Major bleeding, however, only occurred once in each arm.
Primary prevention of CAT in ambulatory patients using direct oral anticoagulants
The Efficacy and Safety of Rivaroxaban Prophylaxis Compared with Placebo in Ambulatory Cancer Patients Initiating Systemic Cancer Therapy and at High Risk for Venous Thromboembolism (CASSINI) trial was an international, double-blind, placebo-controlled, randomized, superiority trial of intermediate-high risk (Khorana score ≥2) ambulatory cancer patients.25 Patients underwent prerandomization lower-extremity compressive sonography screening for preexisting VTE. If no deep vein thrombosis (DVT) was found, participants were randomized to rivaroxaban 10 mg daily or placebo for up to 6 months, with stratification for the presence of pancreatic cancer. Planned repeat compressive ultrasonography was to be done every 8 weeks. The primary efficacy outcome was a composite of incidental or symptomatic proximal DVT or pulmonary embolism (PE), symptomatic upper-extremity DVT or symptomatic distal DVT, or VTE-related death during the 6-month follow-up period. The primary safety outcome was major bleeding as defined by the International Society on Thrombosis and Haemostasis.26 In total, 1080 patients were enrolled, but 49 (4.5%) patients were diagnosed with DVT at first screening, and 190 were excluded from the trial for other causes. In the intention-to-treat analysis, a total of 841 patients were included: 420 in the rivaroxaban group and 421 in the placebo group. Overall, 32.6% of patients had pancreatic cancer, 20.9% had gastric or gastroesophageal junction cancer, and 15.9% had lung cancer. In the intention-to-treat analysis, the primary efficacy outcome occurred in 6.0% of the patients in the rivaroxaban group compared with 8.8% in the placebo group for an HR of 0.66 (95% CI, 0.40-1.09; P value = .10). In a pe-specified on-treatment analysis, the primary efficacy outcome took place in 2.6% of patients in the rivaroxaban group and 6.4% in the placebo group for an HR of 0.40 (95% CI, 0.20-0.80). The primary safety outcome, major bleeding, occurred in 2.0% of the patients receiving rivaroxaban and 1.0% of the patients receiving placebo (HR = 1.96; 95% CI, 0.59-6.49). CRNMB was ascertained in 2.7% of patients receiving rivaroxaban compared with 2.0% of those receiving placebo for an also nonsignificant HR of 1.34 (95% CI, 0.54-3.32).
The Apixaban for the Prevention of Venous Thromboembolism in High-Risk Ambulatory Cancer Patients (AVERT) trial was a double-blind, placebo-controlled RCT assessing the use of apixaban 2.5 mg twice daily in the thromboprophylaxis of intermediate-high risk (Khorana score ≥2) ambulatory cancer patients receiving chemotherapy.27 Eligible patients were randomized in a 1:1 ratio to receive apixaban or placebo. No screening compressive ultrasonography of the lower extremities was done at baseline and throughout the follow-up period of 6 months. The primary efficacy outcome was a composite of symptomatic or incidental proximal upper-extremity or lower-extremity DVT, symptomatic or incidental PE, or PE-related death. The main safety outcome was major bleeding.26 Overall, 574 patients were randomized, and 563 were included in the modified intention-to-treat analysis. The most common cancer types were gynecologic (25.8%), lymphoma (25.3%), and pancreatic (13.6%). The primary efficacy outcome occurred in 4.2% of the patients in the apixaban group and 10.2% of patients in the placebo group for an HR of 0.41 (95% CI, 0.26-0.65; P value ≤ .001). Major bleeding was diagnosed in 3.5% of the patients in the treatment arm and 1.8% of patients in the comparator arm for an HR of 2.00 (95% CI, 1.01-3.95; P value = .046). CRNMB was noted in 7.3% of the patients receiving apixaban and 5.5% of those receiving placebo (HR = 1.28; 95% CI, 0.89-1.84). In the secondary on-treatment analysis, the primary outcome occurred in 1.0% of the patients in the apixaban group compared with 7.3% in the placebo group for an HR of 0.14 (95% CI, 0.05-0.42), and major bleeding occurred in 2.1% in the apixaban group and 1.1% in the placebo group for an HR of 1.89 (95% CI, 0.39-9.24).
Should primary prevention of VTE in ambulatory cancer patients receiving chemotherapy be adopted in clinical practice?
Taken together, the results of the CASSINI trial and the AVERT trial offer novel and compelling evidence for the use of prophylactic dose direct oral Xa inhibitors in the primary prevention of VTE in ambulatory cancer patients receiving chemotherapy at intermediate-high risk of VTE. As previously reported by Agnelli,28 when combining the efficacy results of both trials in an intention-to-treat analysis, the RR was 0.56 (95% CI, 0.38-0.83) for a number needed to treat to prevent 1 VTE event of 24. The evidence for major bleeding, however, was quite reassuring, with an RR of 1.96 (95% CI, 0.88-4.33) for a number needed to harm of 77. Furthermore, the risk of death from any cause seemed to be unchanged with the use of thromboprophylaxis, with an RR of 0.92 (95% CI, 0.73-1.16).
The results of the CASSINI trial and the AVERT trial, therefore, offer a better understanding of the VTE burden and efficacy of thromboprophylaxis in the general population of cancer patients at elevated risk of VTE. However, they do not provide specific knowledge on the risk and benefit of thromboprophylaxis in specific cancer types given the limited number of patients with each particular cancer diagnosis included in these studies. This limitation may only be addressed with more studies assessing the use of primary thromboprophylaxis in specific cancer types. Furthermore, when combining the results of both studies, it is important to bear in mind that the proportion of each cancer type differed significantly between the AVERT trial and the CASSINI trial, with the AVERT trial having included a large proportion of hematological and gynecological cancer patients and the CASSINI trial having included a large proportion of pancreatic and gastric or gastroesophageal cancer patients. These differences may be explained in part by the different inclusion and exclusion criteria of the 2 studies as well as the use of the expanded Khorana score from Ay et al22 in the AVERT trial compared with the original Khorana score used in the CASSINI trial. As such, patients with primary brain tumors, brain metastases, or hematological cancers other than lymphoma were excluded from the CASSINI trial, whereas the AVERT trial included a relatively important number of hematological cancer patients (allowing for the both lymphoma and plasma cell myeloma cancer patients to be randomized) and a small proportion of patients with brain cancer. Patients with acute leukemia and myeloproliferative neoplasms, however, were excluded from both studies.
Certain questions remain, which when elucidated, would help with the full integration of preventive dose direct oral Xa inhibitors in the care of cancer patients.
First, although both the AVERT trial and the CASSINI trial managed to identify a patient population at high risk for VTE by using the Khorana score or an expanded Khorana score with a threshold of ≥2 (with a combined incidence of VTE in the placebo groups of 9.3%), other adjustments to the Khorana score may lead to an improved risk stratification strategy. Indeed, in a recent meta-analysis assessing the performance of the Khorana score during a 6-month follow-up period in both prospective and retrospective studies including >34 000 patients, it was shown that the score’s predictive capacity differed significantly across the various cancer types and that using a cutoff of ≥3 to identify patients at high risk of VTE resulted in only 23% of the VTE events in the follow-up period occurring in this high-risk group.29 By comparison, using a cutoff of ≥2 in a sensitivity analysis found an incidence of VTE in the 6-month follow-up period similar to what was reported in the AVERT trial and the CASSINI trial at around 9%, accounting for 55% of the VTE events in the follow-up period. Therefore, it remains to be seen whether the Khorana score may further be adapted to better inform the decision on primary thromboprophylaxis, especially as it pertains to the risk of VTE across different cancer types.
Second, the goal of primary thromboprophylaxis is to decrease the risk of VTE while minimizing the risk of bleeding. As shown above, the Khorana score may be used to characterize a patient’s individual risk of VTE and therefore, assess the potential benefit of primary thromboprophylaxis. The evaluation of the bleeding risk while receiving thromboprophylaxis, however, remains less clear. Both the AVERT trial and the CASSINI trial excluded patients at high risk of bleeding. In these studies, the risk of bleeding was not assessed systematically, but they relied on expert judgement and the presence of generally accepted criteria for increased risk of bleeding, such as severe renal dysfunction, severe thrombocytopenia, bleeding diathesis, or the presence of a hemorrhagic lesion. In clinical practice, however, the capacity to identify cancer patients at risk of bleeding while receiving primary thromboprophylaxis through a more systematic evaluation would help clinicians better balance the risk-benefit of primary thromboprophylaxis in their patients. Additional evidence on the risk of bleeding in cancer patients receiving primary thromboprophylaxis as well as greater knowledge regarding the characteristics associated with increased bleeding risk on primary thromboprophylaxis will be very helpful in addressing this issue. For now, thankfully, the evidence available to us regarding the risk of bleeding on prophylactic dose direct oral Xa inhibitor for this indication in a selected population of cancer patients is quite reassuring.
Third, the duration of primary thromboprophylaxis in ambulatory cancer patients receiving chemotherapy has not yet been well defined. In both the AVERT trial and the CASSINI trial, a follow-up period of 6 months was chosen, which corresponds to the highest-risk period for VTE postcancer diagnosis30 and generally coincides with the length of many chemotherapy regimen. Therefore, after a decision is made to initiate primary thromboprophylaxis, continuation for a period of up to 6 months would be consistent with the current available evidence while affording VTE risk reduction during the period most at risk for VTE. The risk-benefit of primary thromboprophylaxis, however, must be regularly reassessed during the patient’s cancer journey to confirm the ongoing appropriateness of this intervention. After 6 months of thromboprophylaxis, a clinician may choose to continue using primary thromboprophylaxis based on an individual patient’s risk assessment of VTE and bleeding, but this decision would be based on the extrapolation of the data presented here. It is important to note that the Khorana score was not validated for the ongoing risk stratification of patients at this 6-month postchemotherapy initiation period. Additional studies are necessary to address this question.
Fourth, the usefulness of serial VTE screening imaging techniques in the care of cancer patients is not well established. Evidence suggests that the prognosis of incidental VTE is similar to that of symptomatic VTE in cancer patients,31-35 which supports anticoagulation therapy in proximal DVT or PE with high-burden thrombus (multiple subsegmental PE or more).36 Still, the role of screening is less well defined, and the decision regarding management of VTE identified by screening must be extrapolated from evidence regarding the treatment of incidental PE. Additionally, it remains unclear whether the strategy of serial screening is cost effective, despite the burden of repeat imaging, and whether it alters the disease’s natural history in a beneficial way. In a recent pilot study assessing the use of an automated electronic medical record alert to identify patients at high risk of VTE (Khorana score ≥3), a significant proportion of patients (12.5%) was diagnosed with DVT from screening compressive ultrasonography alone. In patients who were identified as being at high risk for VTE but who did not undergo screening compressive ultrasonography, 8.3% were diagnosed with a symptomatic DVT, and 4.5% were diagnosed with a PE within 90 days of the alert.37 However, additional prospective studies assessing the benefit of serial compressive ultrasonography in cancer patients are necessary before this practice can be recommended in clinical practice.
Fifth, the complexity of cancer care and the multiple expectations put on cancer patients must be taken into consideration before recommending the implementation of another treatment strategy, such as thromboprophylaxis. The difficulty of adding additional interventions in the care of cancer patients was illustrated by the high rate of premature discontinuation noted in the CASSINI trial and the AVERT trial. One may argue that the administration of a direct oral Xa inhibitor is more convenient than LMWH, and therefore, the threshold to implement this intervention should be lower than before. It is important to note, however, that cancer patients receiving treatment of CAT seem to be more concerned by the interactions between the anticoagulants and their antineoplastic regimen than the inconvenience of self-injection.38 Unlike LMWH, direct oral Xa inhibitors have known interactions with combined P-glycoprotein and strong cytochrome P450 3A4 inducers and inhibitors,39 medications that are commonly used in the care of cancer patients. Therefore, despite the convenience of direct oral anticoagulants, these medications bring forward a different set of challenges for both patients and clinicians.
Sixth, it remains to be seen whether, in a setting of limited health care resources (such as publicly funded health care systems) where opportunity costs must be carefully evaluated before attributing restricted funds to a certain intervention, the use of direct oral Xa inhibitors in primary prevention of VTE in ambulatory cancer patients is a cost-effective initiative.
The CASSINI and AVERT trials provide reliable evidence for the use of a dichotomized Khorana score (<2 or ≥2), which can dependably identify ambulatory cancer patients receiving chemotherapy who may most benefit from primary thromboprophylaxis. The use of prophylactic doses of apixaban and rivaroxaban is effective at preventing VTE in this patient population. Overall, the risk of major bleeding with this intervention is low in a selected patient population. These findings should encourage clinicians to assess an individual patient’s risk of thrombosis and bleeding when initiating chemotherapy and consider the use of primary thromboprophylaxis in patients with a Khorana score ≥2. Ongoing studies regarding the use of primary thromboprophylaxis in cancer patients are presented in Table 4. Finally, the results discussed here should inform future guideline recommendations regarding the use of primary thromboprophylaxis in higher-risk cancer patients.
Back to clinical case 1
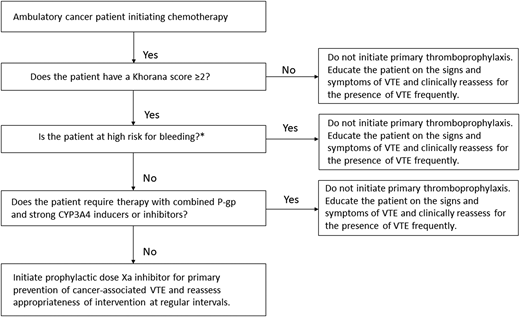
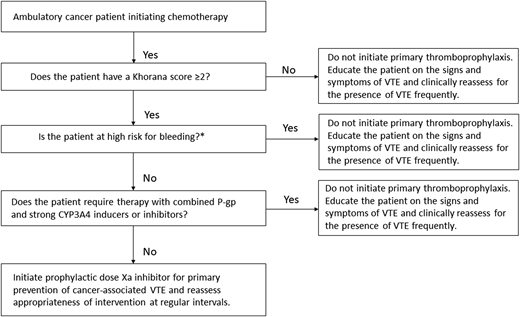
After ensuring that the patient is not actively bleeding or at unacceptably elevated risk for bleeding, discussing his preferences, and confirming that there are no important interactions between direct oral Xa inhibitors and the planned chemotherapy regimen (Figure 1), the patient is started on a prophylactic direct oral Xa inhibitor for the primary prevention of VTE. The use of primary thromboprophylaxis is reassessed regularly during the treatment period. He successfully completes chemotherapy without any thrombotic or major bleeding complications.
Initiating primary thromboprophylaxis in an ambulatory cancer patient receiving chemotherapy. CYP3A4, cytochrome P450 3A4; P-gp, P-glycoprotein. *Characteristics associated with high bleeding risk to consider: previous major bleeding, known hemorrhagic lesion, active bleeding, severe renal dysfunction, severe thrombocytopenia, and bleeding diathesis.
Initiating primary thromboprophylaxis in an ambulatory cancer patient receiving chemotherapy. CYP3A4, cytochrome P450 3A4; P-gp, P-glycoprotein. *Characteristics associated with high bleeding risk to consider: previous major bleeding, known hemorrhagic lesion, active bleeding, severe renal dysfunction, severe thrombocytopenia, and bleeding diathesis.
Clinical case 2
A 66-year-old woman with locally advanced cervical cancer presents to the emergency department with pleuritic chest pain and dyspnea on exertion. She is not hypoxic, hypotensive, or tachycardic. She is found to have bilateral pulmonary segmental and subsegmental emboli on computed tomography angiography. Laboratory investigations reveal normal blood counts, coagulation parameters, renal function, and hepatic function. She is not suffering from any bleeding. She is currently being treated with cisplatin and concurrent radiotherapy. She asks whether her CAT may be treated with an oral medication.
New evidence in the treatment of cancer-associated VTE
Clinical guidelines currently recommend the use of LMWH for the treatment VTE in cancer patients.6,40,41 In this patient population, LMWH compared with vitamin K antagonists has been shown to produce a greater reduction in the risk of recurrent VTE, with a recent meta-analysis reporting an RR of 0.58 (95% CI, 0.43-0.77).42 More recently, studies have compared the use of direct oral Xa inhibitors with LMWH in the treatment of CAT.
The HOKUSAI-VTE CANCER trial was a large, international, open-label, noninferiority RCT comparing edoxaban with LMWH for the treatment of VTE in cancer patients.43 The primary outcome was a composite of recurrent VTE or major bleeding during the 12-month follow-up period. In the edoxaban group, 12.8% of patients developed a recurrent VTE or major bleeding compared with 13.5% in the LMWH group for an HR of 0.97 (95% CI, 0.70-1.36; P value for noninferiority of .006). There were fewer episodes of recurrent VTE in the edoxaban group (HR = 0.71; 95% CI, 0.48-1.06; P value = .09) but more episodes of major bleeding (HR = 1.77; 95% CI, 1.03-3.04; P value = .04). The imbalance in major bleeding between the 2 groups was owing to an excess of upper gastrointestinal bleeding complications, occurring mostly in gastrointestinal cancer patients.
The SELECT-D study was an open-label, multicenter, randomized pilot study that compared rivaroxaban with LMWH for the treatment of CAT.44 The study’s primary outcome was recurrence of VTE during a 6-month follow-up period. The cumulative incidence of VTE was 4% (95% CI, 2%-9%) in the rivaroxaban group compared with 11% (95% CI, 7%-16%) in the LMWH group (HR = 0.43; 95% CI, 0.19-0.99). The 6-month cumulative incidence of major bleeding was 6% (95% CI, 3%-11%) in patients receiving rivaroxaban compared with 4% (95% CI, 2%-8%) in those receiving LMWH (HR, 1.83; 95% CI, 0.68-4.96). There was also a disproportionate number of bleeding events in this trial, which were gastrointestinal or urologic in nature. As a precautionary measure, after an interim safety analysis, patients with esophageal and gastroesophageal cancer were excluded from the study.
The results of the ADAM-VTE trial were presented at the American Society of Hematology’s 60th Annual Meeting and Exposition.45 In this open-label RCT, cancer patients diagnosed with VTE were randomized to apixaban or LMWH. No patient suffered a major bleeding event in the apixaban arm compared with 2 patients (1.4%) in the LMWH arm (P value = .14). In the apixaban group, 1 patient (0.7%) was diagnosed with recurrent VTE, whereas 9 patients (6.3%) in the LMWH group experienced this complication (P value = .03).
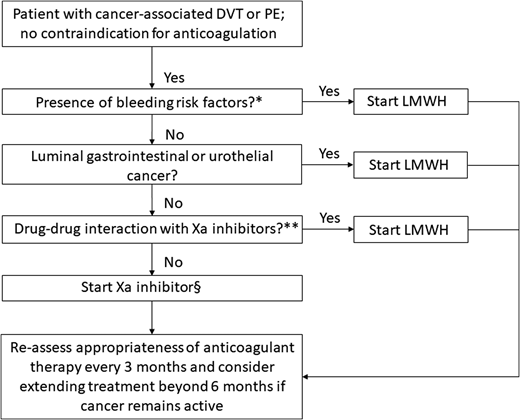
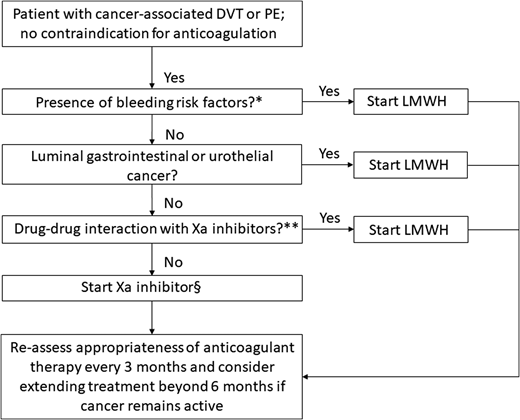
This recent body of evidence for the use of direct oral Xa inhibitors in the management of VTE in cancer patients offers clinicians the opportunity to tailor anticoagulation therapy based on their patient’s values and needs. Although direct oral Xa inhibitors are a convenient, effective, and generally safe alternative to LMWH for the treatment of CAT, several factors (such as the presence of bleeding risks, the type of cancer, and potential drug-drug interactions) must be taken into consideration when choosing a particular therapy (Figure 2). Ongoing studies on the acute treatment of CAT and secondary thromboprophylaxis are described in Tables 5 and 6, respectively.
Algorithm for the treatment of cancer-associated VTE. *High risk factors for bleeding include thrombocytopenia (platelet count is <50 × 109/L), renal dysfunction (creatinine clearance of 30-50 mL/min by the Cockcroft–Gault formula), liver dysfunction, presence of hemorrhagic lesion, recent major hemorrhage, or use of antiplatelet agent. **Combined P-glycoprotein and strong cytochrome P450 3A4 inducers and inhibitors interact significantly with rivaroxaban and apixaban. These combinations should be avoided. P-glycoprotein inducers should be avoided with edoxaban, whereas a reduced dose (edoxaban 30 mg once daily) should be used with concomitant P-glycoprotein inhibitors. §Based on the current published data, edoxaban and rivaroxaban have the highest quality of evidence for use in CAT, although additional studies with apixaban are underway. Adapted from Carrier et al46 with permission.
Algorithm for the treatment of cancer-associated VTE. *High risk factors for bleeding include thrombocytopenia (platelet count is <50 × 109/L), renal dysfunction (creatinine clearance of 30-50 mL/min by the Cockcroft–Gault formula), liver dysfunction, presence of hemorrhagic lesion, recent major hemorrhage, or use of antiplatelet agent. **Combined P-glycoprotein and strong cytochrome P450 3A4 inducers and inhibitors interact significantly with rivaroxaban and apixaban. These combinations should be avoided. P-glycoprotein inducers should be avoided with edoxaban, whereas a reduced dose (edoxaban 30 mg once daily) should be used with concomitant P-glycoprotein inhibitors. §Based on the current published data, edoxaban and rivaroxaban have the highest quality of evidence for use in CAT, although additional studies with apixaban are underway. Adapted from Carrier et al46 with permission.
Back to clinical case 2
After assessing the patient’s risk of bleeding and ensuring that there are no drug-drug interactions between direct oral Xa inhibitors and the patient’s chemotherapy regimen or supportive care, she begins a 5-day treatment with LMWH followed by edoxaban. This anticoagulation regimen is well tolerated, and the patient’s pleuritic chest pain and dyspnea resolve.
Acknowledgment
M.K. received grant funding from the CanVECTOR network for her fellowship in adult thrombosis medicine.
Correspondence
Marc Carrier, University of Ottawa at The Ottawa Hospital, General Campus, Department of Medicine, Division of Hematology, Thrombosis Program, 501 Smyth Rd, Box 201A, Ottawa, ON K1H 8L6, Canada; e-mail: mcarrier@toh.ca.
References
Competing Interests
Conflict-of-interest disclosure: M.C. received research funding from BMS, Leo Pharma, and Pfizer and has received honoraria from Bayer, BMS, Leo Pharma, Servier, and Pfizer. M.K. declares no competing financial interests.
Author notes
Off-label drug use: Use of apixaban and rivaroxaban for primary prevention of VTE in ambulatory cancer patients initiating chemotherapy.