Abstract
As more children are appropriately being diagnosed, the burden of sickle cell disease is increasing greatly in Africa and in high-resource countries such as the United States and Europe. Early management is mandatory, but newborn screening is not implemented everywhere. Point-of-care testing devices are increasingly being used in low-resource countries, showing good sensitivity and specificity. Because the diagnosis is often traumatic for the families, the announcement should be made by an experienced person. The development of care networks is urgently required to facilitate daily life by defining the respective functions of nearby and highly specialized health care professionals, who should work in close collaboration. Comprehensive programs targeting the prevention of pneumococcal infections, malaria in infested zones, and stroke may substantially improve patient care. Hydroxyurea is increasingly being used, but whether it should be systematically prescribed in all children is debated, and its access is still limited in many African countries. Yearly checkups should be organized early in life in order to screen and then treat any organ impairment. Enhancing parents’ and patients’ knowledge and skills is mandatory.
Learning Objectives
Organize the management scheme for a child with sickle cell disease, coordinating nearby and highly specialized care
Create a long-term partnership with the family and patient
Create and provide specific therapeutic education programs
Adapt care programs to the facilities existing in the patient’s country
Introduction
By combining estimates of sickle cell disease (SCD) frequency with projected demographic data, researchers have predicted that the number of children affected by SCD will increase from about 300 000 in 2010 to about 400 000 in 2050.1 Quality of life and life expectancy vary widely, depending on where children are born and where they live. The overall survival rate in children is >94% in the United States and Europe.2-4 By contrast, the rate is highly variable in low-resource countries, especially in Africa, where the shortage of antibiotics, painkillers, and blood supplies, together with a poor knowledge of disease among many health workers and families, is responsible for delayed diagnosis and a high mortality rate in childhood. However, some African countries have implemented integrated approaches to manage SCD, which has led to significantly improved survival in children.5,6 This review presents programs of care in both high- and low-resource settings, emphasizing that some important care delivered routinely in high-resource countries is not deliverable in low-resource ones.
Clinical case: A 2-month-old boy receives a diagnosis of SCD by newborn screening (NBS). His parents are originally from Central Africa and moved to Europe 5 years ago. They tell you they have never heard about SCD, and they do not believe that their apparently well child is really affected by a severe disease.
Early diagnosis of the disease
NBS
Early introduction of daily penicillin, together with parental education, has reduced childhood mortality of SCD.2 Hence, universal NBS has long been implemented in the United States and in many European countries to provide appropriate care to infants with SCD.7,8 NBS was implemented more recently in several sub-Saharan countries (Nigeria, Ghana, Benin, Democratic Republic of the Congo, Tanzania, Gabon, Cameroon, Uganda, and Angola) as a pilot program but has not yet been implemented as part of systematic or sustainable national strategies.9
Point-of-care testing devices
The usual techniques for performing NBS (electrophoresis, isoelectric focusing, high-performance liquid chromatography, capillary electrophoresis, mass spectrometry, and molecular techniques) require highly trained staff, expensive equipment, reagents, and sustained electric power. Furthermore, results are often not available before the mother leaves the hospital maternity ward, and locating the family afterward is difficult. All these issues explain why NBS is extremely difficult to implement on a large-scale basis in low-resource countries. Over the past few years, electricity-free bedside tools have been developed. The most currently used are two immunoassays—Sickle SCAN (BioMedomics, Inc., Morrisville, NC) and HemoTypeSC (Silver Lake Research Corp., Azusa, CA)—requiring only 1 to 5 µL of blood from a fingerstick. Both have shown high sensitivity and specificity, especially in newborns.10-13
Clinical case: With the help of a psychologist and a therapeutic patient education nurse, we consult with the family again. Genetic transmission is re-explained. Finally, the parents agree to the treatment.
First contact with the parents: how to help them cope with the diagnosis
Announcing the diagnosis of SCD in a newborn always causes a deep psychological trauma for the parents and may have severe sequelae. Announcing the diagnosis during a face-to-face discussion is preferred. The presence of a psychologist may be very helpful when available. Some parents may have never heard of sickle cell disease (or drépanocytose in Francophone African countries) but may know the disease under local names or as “SS.” Many parents think that SCD always leads to early death and cannot make projections for their child. An important issue is helping them look ahead by talking about the expected achievements of their child in his/her future personal and professional life.
The doctor’s speech may be perceived as contradictory because parents will be asked to trust the future and at the same time pay close attention to clinical signs prompting them to visit the emergency department. Psychological help, therapeutic education, and parent associations are important.
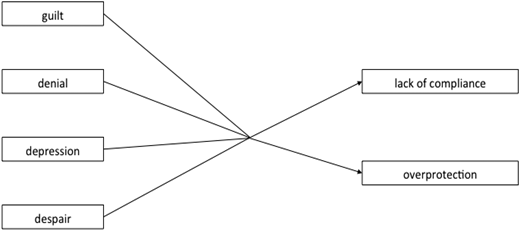
Parents may experience major feelings of guilt, even when genetic transmission is not fully understood. The notion of “healthy carrier” in particular is difficult to accept. Guilt is sometimes reinforced if one parent knew he/she was a carrier of abnormal hemoglobin and did not ask the partner to take the test. Some couples are aware that they are both carriers but prefer to trust in “providence” rather than undergo prenatal testing. The notion of “hereditary disease” is often confused with “malediction or witchcraft,” and some parents believe that their child’s sickness is related to sorcery or is the manifestation of ancestors’ anger. Thus, the disease may be perceived as a punishment or a curse from an enemy. The mother is often considered the only parent responsible for the child’s disease and may be ostracized.14
Denial of diagnosis, which is not a rare reaction, may lead the parents to refuse the recommended prescription for therapy (Figure 1). Noncompliance with penicillin therapy remains a cause of death in infants with SCD.3
Usual parental reactions after the announcement of a sickle cell disease diagnosis and possible consequences.
Usual parental reactions after the announcement of a sickle cell disease diagnosis and possible consequences.
Treatment
Prevention of invasive pneumococcal diseases
Children with SCD are at increased risk of invasive pneumococcal diseases, in part because of functional asplenia, dysfunctional antibody production, and poor opsonophagocytosis. Early studies documented that prophylactic penicillin significantly reduces the risk of pneumococcal infection and is particularly necessary in children under 5 years of age.15 Given the risk of poor adherence to daily prophylaxis and the development of penicillin-resistant Streptococcus pneumoniae strains, pneumococcal vaccination is recommended in addition to prophylactic penicillin therapy. The recommended immunization schedule for children with SCD consists of 4 doses of conjugated vaccine between 2 and 15 months of age, followed by a polysaccharide vaccine at 2 and 7 years of age.16 Pneumococcal conjugate vaccines have greatly reduced the incidence of invasive pneumococcal diseases in SCD, but nonvaccine serotypes are emerging.17-19 Therefore, parents must be repeatedly informed that fever in young children should prompt urgent medical examination, and health workers must be taught to start empiric antibiotics before the results of biological analyses are available. The emergence of nonvaccine serotypes raises the question of the total duration of penicillin prophylaxis, which may need to be revisited.16
Immunization
Other vaccines, in particular against Haemophilus influenzae and meningococcal infections, are also mandatory, and influenza vaccine is highly recommended. However, in some low-resource countries, the routine immunization schedule recommended by the World Health Organization is not yet fully implemented.
Malaria prophylaxis
Malaria can induce severe anemia in patients with SCD, and safe blood supplies are not always available in African countries. Chemoprophylaxis, insecticide bed nets, prompt diagnosis, and treatment are highly needed in integrated approaches to the disease in Africa.6,20
Stroke prevention
Stroke is one of the major complications of SCD, but most cases can be prevented by systematic screening and preventive therapies. Screening is based on yearly transcranial Doppler ultrasonography (TCD), which measures flow velocity in the large intracranial arteries of the circle of Willis. Arterial stenosis or flow turbulences induce a local acceleration detected by TCD. Hence, children can be stratified at low, intermediate, or high risk of stroke according to normal, conditional, or abnormal velocities, respectively.21 A monthly transfusion regimen efficiently prevents the risk of stroke in patients with pathological TCD findings.22 Alternatively, hydroxyurea (HU) has been used for primary and secondary stroke prophylaxis in low-resource settings.23
Clinical case: Because the family lives 60 km away from your reference hospital, you and the parents plan that regular consultations will occur; mild complications will be managed in a nearby hospital 2 km away from the parents’ home; and you will see the child once per year, combining your visit after 2 years of age with the TCD examination. All files, especially immunohematological data, will be shared between the nearby hospital and your unit.
Organization of care
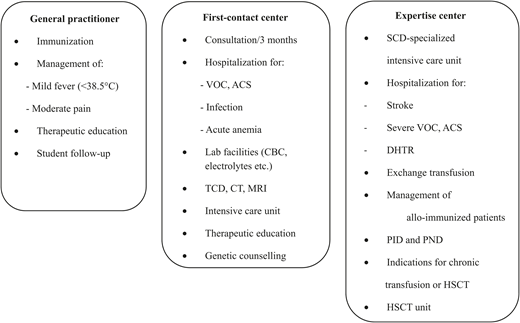
The management scheme must reconcile two distinct imperatives: (1) facilitating family life, enabling patients to come regularly to scheduled visits, and (2) the ability to reach highly specialized care within a short time. A hierarchy in health care centers is always needed and must be determined at the first consultation. Among crucial issues, the general practitioner should know when to refer a child to the emergency department, and the first-contact treatment center should determine when and where to perform an exchange transfusion (Figure 2). All health care professionals involved in the care of a child with SCD must share information and protocols and ideally should use the same tools of therapeutic education. A structured medical note and personalized pain management plan that the patient or family carries is very helpful.
Example of management scheme for SCD in children in high-resource countries. ACS, acute chest syndrome; CBC, complete blood count; CT, computed tomography; DHTR, delayed hemolytic transfusion reaction; HSCT, hematopoietic stem cell transplantation; MRI, magnetic resonance imaging; PID, preimplantation diagnosis; PND, prenatal diagnosis; SCD; sickle cell disease; TCD, transcranial Doppler ultrasonography; VOC, vaso-occlusive crisis.
Example of management scheme for SCD in children in high-resource countries. ACS, acute chest syndrome; CBC, complete blood count; CT, computed tomography; DHTR, delayed hemolytic transfusion reaction; HSCT, hematopoietic stem cell transplantation; MRI, magnetic resonance imaging; PID, preimplantation diagnosis; PND, prenatal diagnosis; SCD; sickle cell disease; TCD, transcranial Doppler ultrasonography; VOC, vaso-occlusive crisis.
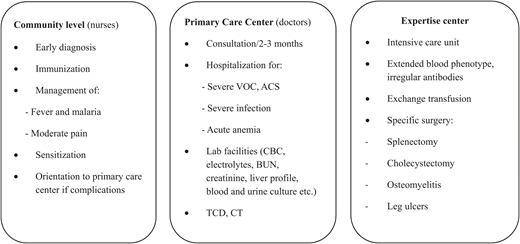
In Africa, centers with specialized care are few and exist only in the largest cities; increasing efforts are made to integrate the SCD management into the minimal package of care at the first-contact level of the health care system (Figure 3). In most cases, treatments are to be paid for by parents, which may represent an unaffordable cost for most of them. Depending on the center, penicillin and immunization can be provided free of charge. Very few centers offer TCD screening. Prevention of blood-transmitted infections is progressing, but most blood banks do not perform extended blood group phenotyping and do not look for irregular antibodies. Likewise, red blood cell transfusions usually respect only ABO and D matching, which contributes to a high rate of alloimmunization.
Example of management scheme for SCD in children in low-resource countries. ACS, acute chest syndrome; BUN, blood urea nitrogen; CBC, complete blood count; TCD, transcranial Doppler ultrasonography; VOC, vaso-occlusive crisis.
Example of management scheme for SCD in children in low-resource countries. ACS, acute chest syndrome; BUN, blood urea nitrogen; CBC, complete blood count; TCD, transcranial Doppler ultrasonography; VOC, vaso-occlusive crisis.
Clinical case: At 2 years of age, the child has an acute chest syndrome (ACS), and you start HU therapy.
HU
For >20 years, HU has been an essential treatment of SCD.9 Many studies support HU in children with SCD, including pivotal controlled studies and registry studies in the United States, France, and Belgium.24-30 Also, more recent observational studies in the United Kingdom, Italy, France, and Belgium have reported the effectiveness and safety of HU.3,31,32 However, the indications and management scheme differ between the United States and Europe. The US recommendations are to propose the drug to all children after the age of 9 months, whatever the disease severity, and to increase the dosage up to the maximum tolerated dose.33 This strategy has not been adopted by all centers in Europe, owing to uncertainties about a possible impact on fertility of boys. Indeed, young boys treated with HU have shown reduced sperm count.34 However, male patients with SCD who received HU have had children (Laure Joseph, unpublished data), and the reversibility of HU toxicity on sperm function in boys receiving early treatment and for a long duration is under study. Therefore, in many European centers, the drug is given for selected indications (see Table 1) at the minimum therapeutic dose, increased only in case of recurrence of vaso-occlusive crises or ACS. Accordingly, cryopreservation of sperm may be proposed to boys who have reached the age of puberty before implementing the treatment.
Medical and ethical debates in Africa must take into account the fact that SCD may be life-threatening and that optimal care with antibiotics, painkillers, and safe blood supplies is rarely accessible. Most centers cannot perform monthly exchange transfusion in children with cerebral vasculopathy, especially because of the cost of this procedure. Several studies have assessed the safety and efficacy of HU in Africa.35,36 They raise the critical question of whether treatment with HU should be offered to all children with SCD in Africa, even those who are not yet symptomatic.
Hematopoietic stem cell transplantation
Allogeneic transplantation from an HLA-matched sibling can cure SCD, with a mortality rate of 5% and event-free survival >90%.37 In high-resource countries, transplantation is systematically proposed to severely affected children with an HLA-identical sibling donor. Whether this procedure should be proposed in mild SCD forms and whether higher-risk procedures such as haploidentical transplantation should be considered more frequently in children with severe disease are debated.
Yearly checkup
SCD-related organ damage may occur early in life. In many cases, screening and prevention of organ dysfunction can alleviate the outcome of the disease. Table 2 lists examinations to be performed regularly.38 Brain magnetic resonance imaging can reveal silent cerebral infarcts, which are associated with decreased cognitive ability, and some centers recommend performing magnetic resonance imaging in students at least once.39 We agree with this proposal, given that early diagnosis of silent cerebral infarct usually leads deeper evaluation of academic achievements and implementation of educational and psychological support if required, but it may also be considered an indication for intensification of SCD treatment with HU.
Clinical case: When the patient reaches 13 years of age, he has a new ACS. He tells you it is too difficult for him to take a pill every day. He agrees to share his experience in a therapeutic education group.
Empowerment of parents and patients
SCD is a lifelong disease, and parents are always at the front line. A high proportion of parents experience posttraumatic stress disorder, which may disturb the child’s development and enhance his/her feeling of pain. A study found that the presence of posttraumatic stress disorder in parents was not correlated with the frequency of hospitalizations or hospitalization in an intensive care unit, but was correlated to their feeling of powerlessness over their child’s illness.40 Parental exhaustion explains in part why many value curative treatments versus continuing with current treatments or experimenting with new drugs.
Therapeutic education aims to transfer expertise from physicians to patients so that they may become partners in their child’s own care. Enhancing parents’ and patients’ skills is all the more necessary, given the growing number of new pharmaceutical treatments and curative therapies (eg, hematopoietic stem cell transplantation, gene therapy).
In conclusion, management of SCD in children requires offering appropriate quality care but also giving parents and, progressively, the child the tools to become true actors in the program of care. This is a key element to improve prognosis and to allow transition to adult life.
Correspondence
Mariane de Montalembert, Hopital Necker, 149 rue de Sevres, 75015 Paris, France; e-mail: mariane.demontal@aphp.fr
References
Competing Interests
Conflict-of-interest disclosure: M.d.M. has received consultancy fees and honoraria from Novartis and Addmedica. L.T. and S.A. declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.