Abstract
Chronic graft-versus-host disease (GVHD) is the leading cause of late morbidity and mortality after allogeneic hematopoietic cell transplantation. Symptoms and manifestations of chronic GVHD are heterogeneous and pleomorphic, and there are no standard treatments beyond corticosteroids. Therapy is typically prolonged, and chronic GVHD and its treatment are associated with adverse effects that have a significant impact on long-term quality of life and functional status. Several advances have been made over the last 2 decades to define the diagnosis of chronic GVHD as well as its severity and response criteria for clinical trials. Further understanding into the biologic mechanisms of the development of chronic GVHD has led to the investigation of several novel immunomodulatory and targeted therapies. Multi-institutional collaboration and pharmaceutical support in the development of therapies based on sound biologic mechanisms and clinical trials with defined end points and responses have led to several promising agents on the horizon of approval for treatment of chronic GVHD. This article reviews advances in our knowledge of chronic GVHD and its biologic framework to improve approaches to prevention and treatment.
Learning Objectives
Describe biologic phases and mechanisms of chronic GVHD development
Describe novel approaches to chronic GVHD prevention and treatment
Understand the continued need for high-quality multicenter clinical trials based on biologic rationale and using chronic GVHD end points
CLINICAL CASE
A 64-year-old male with a history of relapsed acute myelogenous leukemia in second complete remission underwent a reduced-intensity matched unrelated-donor peripheral blood stem cell transplant. Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus and mycophenolate mofetil (MMF). In the setting of tapering immunosuppression, 4 months after transplant he developed worsening fatigue, anorexia, skin rash, dry eyes, and dry mouth. Skin biopsy and ocular evaluation confirmed chronic GVHD. Tacrolimus was increased to therapeutic levels, and he was started on 1 mg/kg prednisone. Topical therapies including dexamethasone oral rinses and ocular agents were used, including scleral lenses. The GVHD symptoms resolved on steroids; however, he had several adverse effects to steroids, such as hyperglycemia, irritability, edema, and cytomegalovirus reactivation. Systemic corticosteroids were subsequently successfully tapered off. The patient was eager to get off all immunosuppression, but again, after a taper of tacrolimus, he had a flare of GVHD with oral, eye, and skin involvement. There was some improvement with maximizing topical therapy and increasing tacrolimus. The patient was very reluctant to consider systemic corticosteroids or other medications. He developed worsening fatigue, anorexia, shortness of breath (pleural effusions), lower-extremity swelling, dysphagia, and anemia/thrombocytopenia. Additional workup to rule out other etiologies was consistent with a GVHD flare. He was restarted on 0.5 mg/kg prednisone with plans for a quick taper and started on ibrutinib. He had an excellent response; his counts improved and symptoms resolved. He was able to taper off prednisone without a flare in GVHD. He remains on low-dose tacrolimus and ibrutinib.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is the only potentially curative therapy for many high-risk hematologic malignancies, metabolic and immunodeficiencies, and bone marrow failure syndromes, and the number of transplant procedures continues to increase. Despite significant advances in donor selection, conditioning regimens, and supportive care, chronic GVHD remains the leading cause of late morbidity and mortality.1,2 The incidence of chronic GVHD ranges from 30% to 50%, depending on GVHD prophylaxis regimens and the donor graft source.1 Chronic GVHD, once established, is a heterogeneous and pleomorphic syndrome in which most patients have at least 3 involved organs, and treatment typically requires the prolonged (median 2-3.5 years) use of immunosuppressive agents.3 Chronic GVHD and its treatment are thus often associated with late mortality as well as several adverse effects with significant impact on long-term quality of life and functional status among HCT survivors.2,4
The National Institutes of Health (NIH) chronic GVHD projects of 2005 and 2014 have provided a critical framework for substantial advances in the transplant field over the last decade,5,6 outlining standard guidelines and definitions for chronic GVHD diagnosis, grading, and response criteria for clinical trials.7,8 Further refinement of response definitions has enhanced the reliability and practical utility of these measures in clinical trials, ultimately leading to the first US Food and Drug Administration (FDA) approval of treatment for chronic GVHD.9 In 2020, the NIH Chronic GVHD Consensus Conference further set a forward-thinking agenda to address gaps and needs in future research over the next 3 to 7 years. This article summarizes recent advances in our understanding of chronic GVHD and its (1) etiology and prevention, (2) development and diagnosis, and (3) novel treatments.
Etiology and prevention
Advances in our understanding of chronic GVHD have led to the recognition of several donor, recipient, and transplant factors that contribute to its initiation and development. Established clinical risk factors include growth factor-mobilized peripheral blood stem cells, mismatched or unrelated donor grafts, female-to-male transplantation, older recipient age, and acute GVHD history.10 Preclinically, although no murine model adequately mimics the clinical spectrum of chronic GHVD,11 biologic insights from multiple models with distinct pathophysiologies have laid a foundation leading to further clinical studies and new treatment paradigms. Several detailed and comprehensive reviews of chronic GVHD biology and preclinical models already exist and are outside the scope of this article.12-14 Key elements in the pathophysiology, however, will be briefly summarized here to provide a framework for the updates on novel therapeutic targets for prevention and treatment.
Etiology and pathogenesis
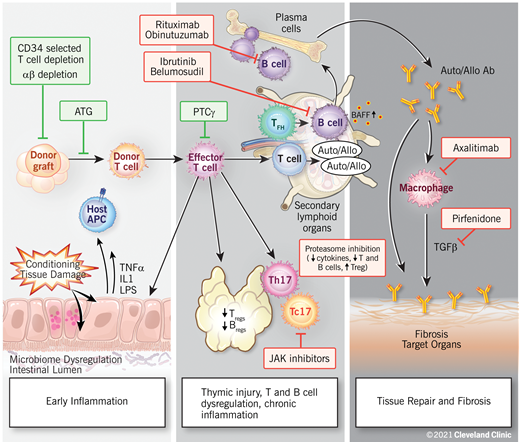
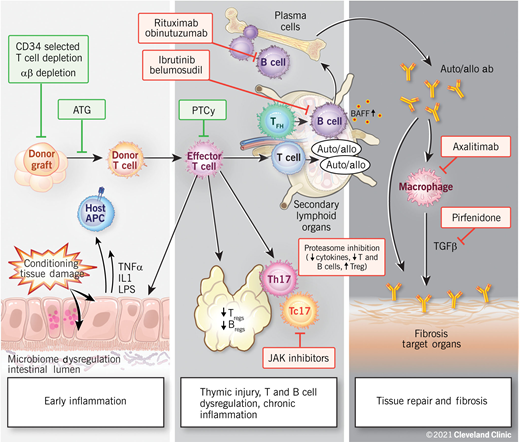
Chronic GVHD is a result of complex and dynamic mechanisms and can be thought to occur within 3 biologic phases: (1) early inflammation due to tissue injury, (2) thymic injury and T- and B-cell dysregulation, and (3) tissue repair and fibrosis (Figure 1).14 Although these processes are often overlapping and dependent, they do not necessarily need to occur in sequence, or even occur at all. As with acute GVHD, experimental models suggest that the initial phase of chronic GVHD also begins with damage of host tissues by pretransplant conditioning and the subsequent release of inflammatory cytokines. Damage to gut tissues and the release of microbial contents result in the activation of antigen-presenting cells, and inflammatory cytokines stimulate the activation of donor alloreactive T cells, driving helper T (Th1)/T-cytotoxic 1 (Tc1), and Th17/Tc17 differentiation and expansion of effector T cells, causing further cytotoxicity to host target cells.12
Proposed biologic phases of chronic GVHD. A few novel therapeutic approaches targeting biologic pathways are highlighted. Reproduced with permission from the Cleveland Clinic Center for Medical Art and Photography.
Proposed biologic phases of chronic GVHD. A few novel therapeutic approaches targeting biologic pathways are highlighted. Reproduced with permission from the Cleveland Clinic Center for Medical Art and Photography.
Tissue damage to the thymus and the secondary lymphoid organs predisposes patients to subsequent chronic GVHD. The second phase is thus characterized by thymic injury, which allows for the emergence of and the selection of auto- and alloreactive T-cell populations.13 A loss of central and peripheral tolerance leads to impaired or deficient regulatory T (Treg) and B cells.15 Donor T follicular helper (Tfh) cells have been shown to expand in the secondary lymphoid organs and promote the survival, expansion, and differentiation of donor B cells into aberrant anti-host-immunoglobulin-producing plasma cells via cytokines such as IL-21 and B-cell activating factor.16 Chronic inflammation is thought to be maintained by Th17 cells that have escaped immune regulation.12
The third phase is marked by aberrant tissue repair. Activated macrophages dependent on IL-17 and colony-stimulating factor (CSF-1) have been shown to play an important role in this by producing transforming growth factor β and platelet-derived growth factor alpha.17 This leads to fibroblast activation, resulting in chronic GVHD manifestations such as scleroderma or bronchiolitis obliterans. Thus, it is increasingly clear that subclinical pathogenic processes critical to the development of chronic GVHD begin long before the specific clinical manifestations become evident.
Chronic GVHD prevention
Calcineurin inhibitor (CNI)-based prophylaxis, typically in combination with methotrexate (MTX) or MMF, has been standard practice over the past 3 decades for GVHD prevention.18 Although these regimens may have relative benefit in limiting acute GVHD, they are not effective against chronic GVHD and, paradoxically, may support its development by blocking thymic central tolerance and Treg function.19-21 The need for improvements in GVHD prevention strategies has been recognized as a priority by the field,22 and several novel strategies and end points have been investigated.
Although several prophylaxis strategies have been shown to decrease the incidence of chronic GVHD, their effects on immune reconstitution and disease relapse remain problematic. Because donor-derived effector T cells are critical initiators of chronic GVHD, approaches to deplete T cells from the hematopoietic cell graft have been investigated, demonstrating significant reductions in the incidence and severity of chronic GVHD. While the ex vivo removal of T cells by the positive selection of CD34+ cells has become the predominant approach in clinical use,23 other novel approaches, such as αβ-T-cell graft depletion, have demonstrated significant reductions in moderate and severe chronic GVHD.24 Although T-cell depletion has not been shown to increase relapse risk, it is associated with delayed immune reconstitution and higher rates of infection-related mortality,23 which was recently confirmed in a phase 3 multicenter Blood and Marrow Transplant Clinical Trials Network (BMT CTN) trial.25 In vivo T-cell depletion with antithymocyte globulin (ATG) has also been effective in reducing the incidence of chronic GVHD. Several phase 3 studies have confirmed reductions in chronic GVHD with ATG, with subsequent superior quality of life and GVHD-free survival.26-28 However, controversy remains on its effect on overall survival due to increases in relapse and nonrelapse mortality, as well as optimal dosing, formulation, and dependency on lymphocyte count at the time of administration.28
The role of donor B cells in chronic GVHD biology has also provided a strong rationale for the evaluation of B-cell depletion strategies for chronic GVHD prevention. Prior studies have incorporated monoclonal anti-B-cell therapy as part of the conditioning regimen or the peritransplant period with the intent of reducing relapse of B-cell malignancies.29 Although significant depletion of B cells and reduced rates of chronic GVHD have been reported,30 concerns over the delayed reconstitution of B-cell immunity and excess infections remain a concern.
Posttransplant cyclophosphamide (PTCy) is a promising approach used successfully to prevent GVHD in the HLA-mismatched/haploidentical setting.31 Recent work has proposed that the efficacy of PTCy is due to the reduction of alloreactive CD4+ effector T-cell proliferation, impaired functionality of surviving CD4+ and CD8+ effector T cells, and preferential recovery of CD4+ Treg cells.32 PTCy has been investigated both with and without CNI in the fully HLA-matched setting and has shown itself to be a potentially effective strategy for both acute and chronic GVHD prevention.
The optimal GVHD prophylaxis regimen for the prevention of chronic GVHD remains elusive. A benchmark analysis of novel GVHD prevention was conducted through the Center for International Blood and Marrow Transplant Research to select promising agents and new, clinically meaningful outcome measurements for further investigation through the BMT CTN.33 Several composite GVHD end points were explored, including chronic GVHD relapse-free survival (CRFS), which includes survival without development of chronic GVHD, disease relapse, or death, and GVHD relapse-free survival (GRFS), which includes survival without acute grade 3 to 4 GVHD, chronic GVHD, disease relapse, or death. The results of these analyses led to the development of 2 multicenter BMT CTN clinical trials. PROGRESS 1 (BMT CTN 1203) was a randomized phase 2, 3-arm trial investigating bortezomib/Tac/MTX, maraviroc/Tac/MTX, and PTCy/Tac/MMF in reduced-intensity HCT, evaluating GRFS as a primary end point. PTCy was associated with superior GRFS and had the lowest incidence of chronic GVHD requiring immunosuppression compared to other arms.34 This led to the ongoing BMT CTN trial 1703 (NCT03959241) phase 3 trial comparing PTCy/Tac/MMF with Tac/MTX, with the primary end point of GRFS, potentially establishing a new standard of care for GVHD prevention. PROGRESS 2 (BMT CTN 1301) was a randomized phase 3, open-label, 3-arm trial comparing CNI-free approaches of CD34 selection, PTCy, and Tac/MTX, with a primary end point of CRFS. While there were no statistically significant differences in CRFS between the 3 approaches, CD34+ selection was found to be associated with worse overall survival, driven by increased infection-related mortality.25
Other novel strategies
As we work to further understand the development of GVHD, we now recognize several additional potential targets. For example, instead of blanket B- or T-cell depletion, prevention strategies to block the development of pathogenic B cells, or to inhibit Tfh cells, may better prevent chronic GVHD without affecting other outcomes. Further, strategies to augment Tregs, as have been reported with novel agents such as IL-2,35 ruxolitinib,36 hypomethylating agents,37 extracorporeal photopheresis,38 proteasome inhibitors,39 and adoptive transfer of Tregs,40 may need additional exploration. Table 1 provides a summary of several recently published and ongoing studies that include chronic GVHD as a primary or as part of a primary composite end point.
Development and diagnosis
While the 2005 and 2014 NIH Chronic GVHD Consensus Conference papers established and refined standard definitions for chronic GVHD diagnosis,5,6 the 2020 Chronic GVHD Consensus Conference further highlighted several important considerations in the development and diagnosis of chronic GVHD, focusing especially on the necessity of early recognition.41 These include the need for earlier clinical identification by both providers and patient/caregivers, potentially with the use of technology such as telehealth and apps, as well as the need for better identification of preceding symptoms or biomarkers associated with subsequent more severe/morbid forms of chronic GVHD—including biomarkers from blood and tissue or findings from imaging or functional testing. Trials specifically investigating the development of chronic GVHD with increased monitoring using blood, fluid, tissue, imaging, and patient-reported symptoms at time points throughout transplant to detect early signs of disease are currently ongoing (NCT04372524, NCT04188912).
The early identification of symptoms or subclinical indicators of chronic GVHD is necessary for the proposed approach of preemptive treatment of chronic GVHD,42 potentially allowing for the prevention of the more severe and morbid phenotypes of chronic GVHD. In a preventative/preemptive approach with rituximab posttransplant, a modest decrease in chronic GVHD and steroid-requiring chronic GVHD was found.30 Ideally, future preemptive approaches will have the advantage of directing treatment at patients who have been identified as at greatest risk, allowing for early, more targeted and subsequently less long-term, damaging therapy.
Treatment of established chronic GVHD
Given the heterogeneous manifestations of established chronic GVHD, clear guidelines for treatment are lacking. High-dose corticosteroids, typically 0.5 to 1 mg/kg/d, remain the first-line standard treatment for chronic GVHD.3 While there is no current evidence to support any benefit from additional agents in the up-front setting, they are often considered in severe cases. The response rate to steroids alone is about 50%, with more than half of patients requiring second-line therapy within 2 years. A number of second-line agents of varying efficacy and toxicities have been investigated over the past several decades, highlighting the heterogeneity in practice, variability in response rates, and challenges in determining the best treatment options.43 Corticosteroids thus remain the standard despite continuing to be associated with several short- and long-term toxicities, including infection, myopathy, edema, hyperglycemia, bone loss, avascular necrosis, cataracts, and sleep/mood disturbances. In clinical practice, topical therapies and supportive measures to minimize these adverse effects are of critical importance.44
The ideal treatment for chronic GVHD continues to be elusive. Goals of therapy are several in number but should include symptom burden reduction and improvement of quality of life, prevention of progression and inflammatory activity, prevention of fibrosis and disability, preservation of response to allow for withdrawal of immunosuppression, repair and modulation of the immune system, and, ultimately, improvement of chronic GVHD mortality.
With these aims in mind, the focus of novel chronic GVHD treatment has thus shifted from the use of broad, long-term immunosuppression toward the investigation of immunomodulatory agents that target pathways relevant to the pathophysiology of the disease (Figure 1). Several multicenter phase 2 and 3 clinical trials have now investigated multiple agents in the treatment of up-front or steroid-refractory chronic GVHD, and a few recently reported trials are briefly reviewed here.
Ibrutinib is a small-molecular tyrosine kinase inhibitor targeting Bruton's tyrosine kinase (BTK), which plays an important role in B-cell receptor signaling and activation of B cells and T cells. A phase 2 trial of ibrutinib in 42 patients with steroid-refractory chronic GVHD led to the first FDA approval of a second-line therapy for chronic GVHD, demonstrating an overall response rate of 67%. Approximately 71% of responders continued to show a response for at least 20 weeks, with responses generally observed across all organ systems involved.9 Although the number of patients treated on this trial was relatively small, the FDA approval of this drug highlights the success of multi-institutional collaboration and pharmaceutical support in developing a trial based on preclinical data and clear biologic mechanisms, with defined clinical end points.
Ruxolitinib is a JAK 1/2 inhibitor that suppresses T-cell activation by inhibiting cytokine receptor-mediated signaling for a variety of proinflammatory cytokines and is currently FDA approved for the treatment of steroid-refractory acute GVHD.36 A phase 3 study of ruxolitinib vs best available therapy (BAT) in steroid-refractory chronic GVHD was recently reported,45 demonstrating an overall response rate of 50% compared to 26% in the BAT arm. Failure-free survival was significantly longer for ruxolitinib-treated patients, and symptom improvement was greater with ruxolitinib compared to BAT (24% vs 11%). Based on the results of this study, ruxolitinib has been granted priority review by the FDA for the treatment of chronic GVHD, and an FDA decision on drug approval is expected before the end of the year.
Belumosudil is an oral selective rho-associated coiled-coil kinase 2 (ROCK) inhibitor targeting Tfh-cell generation and thus B-cell differentiation and survival. Results of the phase 2 trial of belumosudil in steroid-refractory chronic GVHD were also recently reported,46 demonstrating an overall response rate of 74% in heavily pretreated patients. Responses were seen across all affected organs, and of responders, 49% maintained response for at least 20 weeks. Failure-free survival was 77% at 6 months. Based on the results of this study, belumosudil has also been granted priority review by the FDA.
The need to investigate other novel therapies either targeted toward fibrosis or already being developed for other fibrotic diseases has been recognized. For example, pirefenidone, which inhibits transforming growth factor β signaling, is currently FDA approved for idiopathic pulmonary fibrosis and has been shown in mouse models to restore pulmonary function and reverse lung fibrosis. Its study in chronic GVHD-related bronchiolitis obliterans is ongoing (NCT03315741). Axatilimab is a monoclonal antibody against CSF-1, which regulates monocyte differentiation into macrophages promoting fibrosis, and has been shown to have potential efficacy in heavily pretreated chronic GVHD. A phase 2 study further evaluating this is ongoing (NCT03604692). Table 2 summarizes several other up-front and steroid-refractory chronic GVHD trials.
Despite our improved understanding of chronic GVHD, it remains a complex disease marked by significant immune dysfunction, disability, and toxicities, and chronic GVHD-related non-relapse mortality keeps rising over time.47 Practically, the choice of first- and second-line therapy continues to depend on physician preferences and clinical experience, patient disease history and comorbidities, cost/availability of treatment, and access to clinical trials. As we progress away from broad immunosuppression, there remains a need to investigate agents that target the biologic pathways relevant to the pathophysiology of disease and chronic GVHD symptoms; to ultimately understand which drug will work when. The identification and use of blood and tissue biomarkers, imaging, and other novel approaches can better identify specific clinical and biologic phenotypes to differentiate chronic GVHD and work toward a more tailored, personalized therapy for patients.
Reflecting back to our clinical case, this scenario demonstrates several aspects of chronic GVHD development, evolution, and treatment challenges and highlights the importance of understanding the goals of therapy from both a patient and provider perspective. While broad immunosuppression may reduce symptom burden and decrease inflammatory activity in the short term, there remains a need to further investigate targeted agents to preserve function, provide a prolonged response to allow for the withdrawal of medications, prevent disability, and ultimately improve quality of life and overall mortality. The continued understanding of biologic mechanisms through multi-institutional collaborative preclinical studies, clinical trials with careful correlative studies of blood, fluid, tissue samples, imaging, and patient-reported symptoms—are necessary to achieve a more personalized approach to the prevention and treatment of chronic GVHD.
Conflict-of-interest disclosure
Betty K. Hamilton: consultancy: Syndax, Equilium.
Off-label drug use
Betty K. Hamilton: none.