Abstract
Erythrocyte alloimmunization is a major barrier to transfusion in sickle cell disease (SCD) because it can lead to transfusion deadlock and the development of life-threatening hemolytic transfusion reactions (HTRs). Several risk factors have been identified, such as blood group polymorphism in these patients of African ancestry frequently exposed to antigens they do not carry and an inflammatory clinical state of the disease. The most important preventive measure is prophylactic red blood cell antigen matching, and there is a consensus that matching for Rh (D, C, E, c, e) and K antigens should be performed for all SCD patients. However, some patients are high responders and more at risk of developing antibodies and HTRs. For these patients, the extension of matching to other blood groups, including variant antigens of the RH blood group, the use of genotyping rather than serology to characterize significant blood groups, and the prophylactic administration of immunosuppressive treatments remain a matter of debate due to low levels of certainty concerning their effects and the difficulty of determining which patients, other than those already immunized, are at high risk. These issues were recently addressed by a panel of experts established by the American Society of Hematology. Here, we review and stratify the various interventions for preventing alloimmunization, based on the literature and our experience and taking into account the obstacles to their implementation and any future developments required.
Learning Objectives
Describe the particularities of blood groups at risk for immunization in SCD patients
Understand the advantages of genotyping over phenotyping for blood group matching
Understand stratified prophylactic blood group matching and immunosuppressive therapy based on the potential risks of immunization and HTR
CLINICAL CASE
A 30-year-old man with sickle cell disease (SCD) was scheduled for a second hip replacement. His red blood cell (RBC) phenotype was D+ C+ E− c+ e+, K− Fya− b−, Jkb−, S−. He had had 2 previous transfusions. The second had taken place 3 years earlier, during his first hip replacement, with 2 RBC units matched for ABO, E, and K, and it had led to posttransfusion hyperhemolysis with anti-C and anti-Jkb antibodies. Molecular analyses showed that the patient carried an RHCE*CeRN allele, known to be associated with partial C antigen and a risk factor for anti-C alloimmunization. Preoperative transfusion was required on the day of surgery due to the risk of bleeding and a low baseline hemoglobin (Hb) concentration (7 g/dL). Pretransfusion screening detected only anti-C antibodies. A transfusion protocol and premedication to prevent anti-Jkb restimulation, new immunization, and hyperhemolysis were discussed. It was decided to provide serologically crossmatched extended-match RBCs (C-E-Fya-Jkb-S−). Rituximab (1000 mg) was administered 30 and 15 days before surgery. No adverse transfusion reactions occurred, and the patient was discharged 10 days after surgery.
Background and questions raised by this case
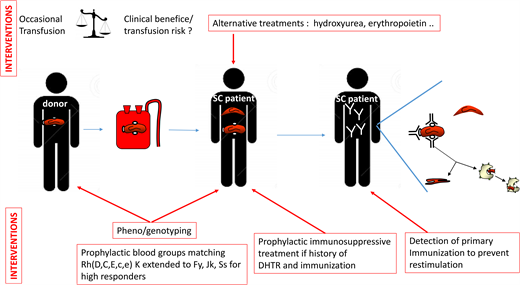
Transfusion therapy remains important for treating and preventing symptoms in SCD patients. It may, however, be complicated by RBC alloimmunization and hemolytic transfusion reaction (HTR),1 which can be life-threatening if hyperhemolysis occurs.2,3 Alloimmunization is also associated with delay in providing compatible blood due to the complexity of screening tests and with the risk of hemolytic disease of the newborn.4 Preventing alloimmunization is thus a major challenge in SCD. Alloimmunization is associated with blood group mismatches between blood donors and patients, most of whom are of African ancestry.5 In the case presented, the patient previously developed anti-C because he carries a partial C antigen. The anti-C was directed against the C epitopes absent from his own RBCs. He was likely exposed to conventional C from a Caucasian donor, as well as to Jkb, which is commonly expressed by donors of European descent. Inflammation associated with the surgical procedure was another risk factor for alloimmunization in this patient.6 Neither the primary immunization nor the partial C status had been known or considered in the previous transfusion. Fortunately, not all patients develop antibodies in mismatch situations. Some patients receive hundreds of RBC units without ever developing a single antibody. This patient was probably a high responder, accounting for the recommendation of extended phenotyping and preventive medication for the new transfusion episode. This case addresses many issues about the prevention of alloimmunization and its associated complications, such as the need for pretransfusion extended RBC antigen typing and the importance of blood group genotyping, transfusion protocols adapted to both the immunization and clinical status of the patient, and preventive immunosuppressive treatments. Alloimmunization management differs between countries, according to the ability of laboratories to perform an immunohematological workup, the prevailing policies concerning donor and recipient typing, and the availability of matched RBCs for implementing the recommended protocols.7 The American Society of Hematology (ASH) guidelines provide specific indications for managing alloimmunization, but most of the recommendations are conditional due to the paucity of evidence or low levels of certainty, given that most studies are observational and retrospective (Table 1).8
Blood groups involved in alloimmunization in SCD
Blood group mismatching between donors of European descent and patients of African descent constitutes a major risk for alloimmunization. Common antigens are heterogeneously distributed between these 2 groups, with some (eg, C and E, Jkb, Fya, Fyb, and S) less frequent in SCD patients than donors. Patients also have high frequencies of blood group variants, especially for the RH system (Figure 1). As in our case, up to 22% of patients carry partial RH antigens, associated with a nonnegligible risk of immunization against epitopes they do not express.9-11 These variants account for Rh alloimmunization despite antigen matching for Rh(D, C, E, c, e).12 Some antigens are expressed by all but a very small proportion of the population. The absence of these high-frequency antigens characterizes rare RBC phenotypes, several of which occur in individuals of African descent: eg, U−, Hr−, HrB−, Sec−, and CEVF−. Transfusion exposes carriers of rare phenotypes to high-frequency antigens, with a variable risk of developing the corresponding antibody, causing transfusion deadlock. Moreover, some antigens expressed only in individuals of African ancestry, and therefore considered “low-frequency” antigens in the European population, are also involved in blood group polymorphism. They can cause alloimmunization in SCD patients receiving RBCs from donors of African ancestry. The associated antibodies are frequently undetectable in the standard pretransfusion screening test. Panels of RBCs differ between hospital transfusion services and may or may not be able to detect these alloantibodies.13,14
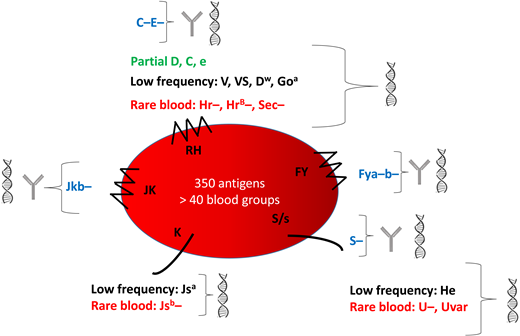
Characteristics of the main blood groups in SCD patients and their identification. This figure shows the 5 blood group systems most frequently involved in alloimmunization. The following are shown for each blood group: blue, the common antigens rarely expressed; green, the partial antigens; black, the low-frequency antigens most frequently expressed; red, the high-frequency antigens most frequently absent (rare blood groups). They can be identified by serology  and genotyping
and genotyping  or genotyping alone.
or genotyping alone.
Characteristics of the main blood groups in SCD patients and their identification. This figure shows the 5 blood group systems most frequently involved in alloimmunization. The following are shown for each blood group: blue, the common antigens rarely expressed; green, the partial antigens; black, the low-frequency antigens most frequently expressed; red, the high-frequency antigens most frequently absent (rare blood groups). They can be identified by serology  and genotyping
and genotyping  or genotyping alone.
or genotyping alone.
The data from different studies on the specificity of the antibodies found in patients are consistent with these blood group characteristics of patients. However, the incidence of alloimmunization ranges from 4.4% to 76%.15 This disparity reflects differences in phenotype-matching transfusion protocols, the use of leukocyte-reduced blood, adult and pediatric populations, transfusion indications, the clinical status of the patient, and transfusion methods. Many studies have confirmed a higher risk for episodic transfusions, the first transfusion after the age of 5, and transfusion during inflammatory events or in the presence of autoantibodies.6,15-18
Is pretransfusion extended red blood cell antigen profiling beneficial in SCD patients?
The ASH guidelines (Table 1) suggest obtaining an extended RBC antigen profile in addition to ABO/RhD typing, as soon as possible, for all patients with SCD. This profile should include at least C/c, E/e, K, Fya/Fyb, Jka/Jkb, M/N, and S/s. We perform extended phenotyping, and now systematic RH genotyping as well, at the initial assessment of each patient.10 This initial analysis detects all the negative and partial Rh antigens and some of the rare blood phenotypes mentioned above.19 In the event of alloimmunization in a recently transfused patient, this pheno/genotype is immediately made available, making it possible to resolve complex polyimmunization situations more rapidly. In accordance with the ASH guidelines, it is essential that this pheno/genotype be shared between the various centers managing the patient.
What are the advantages of genotyping over serology?
Genotyping provides information about a larger number of blood groups, including specific features inaccessible by serology for some (Figure 1). It is also more accurate than commercial phenotyping reagents, particularly for Fyb and partial C antigens, for example.20 The case presented, with a partial C antigen, provides a perfect illustration. The most common partial C antigen in SCD patients, due to the RHD*DIIIa-CE (4-7)-D or RHCE*CeRN allele, has only slightly weaker expression, which may be missed on standard phenotyping. In the absence of genotyping, partial C antigens may therefore be missed, potentially leading to anti-C antibody formation. Over the last decade, genotyping has been performed on C+ SCD patients in France and has resulted in a sharp decrease in anti-C antibody formation in patients with partial C antigens, demonstrating the benefits of following ASH guidelines.10,11 Genotyping strategies for SCD patients are usually designed to detect only the most common variants, based on well-known polymorphisms,21 to ensure cost-effectiveness, and such strategies are accurate in most patients. More comprehensive and dependable methods may become available in the future,22,23 but unless they can be performed cheaply, combined serology and genotyping will remain the most reliable approach.
What matching should be used for transfused patients?
The ASH guidelines strongly recommend prophylactic Rh(C, E or C/c, E/e) and K antigen matching, in preference to ABO/RhD matching alone, for patients with SCD (Table 1). There is a consensus on this first level of prevention, limited to the most immunogenic antigens. The high incidence of Rh and K antibodies in most studies strongly supports this recommendation.15,24 The next step is extending matching to other blood groups. The ASH guidelines state that extended RBC antigen matching (Jka/Jkb, Fya/Fyb, S/s) may provide further protection. However, such matching is not possible for every patient because it requires sufficient pheno/genotyped units from donors of African ancestry and would be of limited benefit for the many patients who are never immunized. The indications must, therefore, be hierarchical. Patients already producing antibodies can be considered high responders, at high risk of producing additional antibodies.25 Such patients would benefit from extended matching. Our patient initially developed anti-C and anti-Jkb antibodies after transfusion. This drove the decision to extend matching to Jkb-negative RBCs, to prevent restimulation, and to Fya and S-negative cells, for the prevention of new immunization. The clinical status of the patient should also be considered in decisions about prophylactic matching. Chronically transfused patients in a steady state who are already immunized have a lower risk of developing new antibodies than patients undergoing occasional transfusions for acute conditions.6 For the former, phenocompatibility may be limited to the antibodies produced, as for non-SCD patients undergoing transfusion. The ASH guidelines do not differentiate acute and chronic transfusion regimens. Because of the limited resource of pheno/genotyped RBCs, the practice we have implemented is to transfuse immunized patients in an acute condition with RBCs matched for all FY, JK, and Ss antigens but to transfuse patients in a chronic transfusion program (likely in a steady state) matching only for the blood group for which the patient has already produced an antibody.
The place of Rh variants in the matching strategy is also a key issue. The ASH guidelines limit matching recommendations to patients with partial C antigens. But what about other variants? Partial C antigens are the most common in SCD patients, present in 1 in 3 C+ SCD patients.11 However, 1 in 6 SCD patients also have a partial D antigen, and about 1 in 15 have a partial e antigen.9,10 These partial antigens are associated with a high risk of antibody formation and HTRs.9,10,19,26,27 Our strategy also aims to prevent antibody formation in carriers of partial D and e antigens by genotyping all SCD patients for common partial RH antigens and providing antigen-negative RBCs for transfusion before the occurrence of alloimmunization.10 This prophylactic genotyping strategy for RH is shared by others,28 but it remains challenging due to the requirement of additional D-negative or e-negative donors. The risk/benefit ratio of such strategies may, nevertheless, be favorable.
When and how to use immunosuppressive therapy
The ASH guidelines suggest the use of rituximab in patients with an acute need for transfusion at high risk of acute HTR or with a history of multiple or life-threatening delayed HTRs (DHTR). We recently reported our experience of rituximab use in 58 adult SCD patients with a history of DHTR.29 This treatment is recommended based on a shared decision-making process for the transfusion of patients at high risk of developing antibodies and DHTR.30 For scheduled surgery, two 1000-mg infusions are administered 15 days apart, and corticosteroids, usually administered with rituximab to prevent rituximab-induced adverse reactions, are limited to 10 mg to prevent triggering vaso-occlusive complications. In combination with extended matching, this treatment prevented severe intravascular hemolysis in all patients following transfusion, except in cases of acute DHTR, despite a decrease in transfusion yield with time in some patients. Efficacy was much lower for rituximab administered for new transfusions in patients with active DHTR. No new antibody production was detected in this study. French and ASH guidelines are concordant, but additional prospective studies are required to confirm the efficacy of this treatment. Bortezomib, a proteasome inhibitor, has also been used to prevent alloimmunization.31 A number of other molecules, targeting different stages of alloimmunization and already used in other antibody-dependent diseases or B- and plasma cell-associated diseases (Table 2), could be considered in the future.
Should we restrict the access of high-risk patients to transfusion based on benefit/risk ratios?
Two clinical situations are considered in the ASH guidelines (Table 1). For the first—pregnant women with SCD—ASH guidelines suggest prophylactic transfusion or standard care (transfusion when clinically indicated for a complication). For the second—patients with SCD undergoing surgery lasting more than 1 hour under general anesthesia—ASH guidelines suggest that preoperative transfusion should be preferred over no transfusion.
The risks of alloimmunization and DHTR are significantly higher in patients receiving occasional transfusions—for example, in the clinical situations mentioned above. A prospective study identified having received few transfusions in the past, alloimmunization, and previous DHTR as risk factors.37 However, infrequent transfusion is less of a risk in itself than the resulting lack of knowledge regarding the risk relative to patients transfused frequently with no adverse reaction. Indeed, responder status is unknown in patients who have received few transfusions. For this reason, the benefit/risk ratio should always be considered whenever transfusion is indicated, particularly for occasional transfusions. In 1 series of 99 cases of DHTR, 33% of the patients were pregnant, and 33% had received intraoperative transfusions.38 All had received very few previous transfusions (<13 units). Other measures, such as hydroxyurea and erythropoietin treatments, may therefore be important to overcome the need for transfusion.
We designed a score for stratifying DHTR risk in adults and the associated preventive interventions.37 In this study the score attributed to a history of DHTR probably underestimated the magnitude of the risk because patients with a history of DHTR had rarely undergone retransfusion.37 At our sickle cell center, indications for preventive transfusion in high-risk patients are considered on a case-by-case basis. For example, preventive transfusions are never performed in patients undergoing cholecystectomy but may be performed for hip replacement surgery in patients with low baseline Hb concentrations, as in our case, and extended RBC matching and rituximab are recommended in this setting.
Final remarks
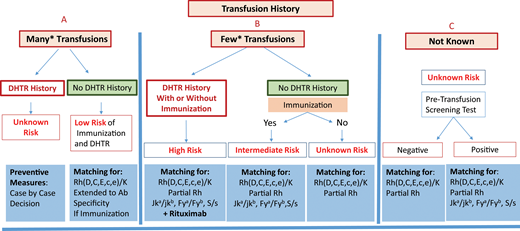
Preventing alloimmunization in SCD remains a real challenge. Prophylactic Rh(C, E or C/c, E/e) and K matching for transfusion is consensual, but other preventive measures remain a matter of debate due to a lack of strong evidence and certainty in many studies. Additional preventive measures may be specific, such as extended antigen matching and genotype matching for RH variants, or nonspecific, such as the introduction of immunosuppressive treatment.39 However, the main problem is our inability to identify patients likely to become immune and to develop a reaction at first presentation. Transfusion history is essential for risk assessment. It is essential to distinguish between 2 situations: chronically transfused patients and patients receiving occasional transfusions, for whom a higher risk has been demonstrated. Figure 2 shows an attempt at preventive measure stratification for patients undergoing occasional transfusions, once transfusion avoidance has been considered, based on recommendations and experience. The preventive measures proposed range from RH/K matching only to extended RH/K pheno/genotyping plus rituximab. However, the application of these measures requires pheno/genotyped blood from donors of African ancestry. Table 3 also summarizes possible measures for preventing alloimmunization, with many areas of investigation remaining open. Altogether, prevention of alloimmunization is an important step toward preventing posttransfusion hemolysis, the most frightening and underrecognized reaction to transfusion in SCD. Follow-up of transfusion to diagnose these reactions with a proactive approach, including HbA assessment, and the management of hemolysis are 2 other very important issues. See the accompanying article by Hendrickson.41
Stratification of preventive measures for patients undergoing occasional transfusions. Preventive measures are recommended according to the risk of developing a new alloimmunization and DHTR, a risk that is itself evaluated from the patient's history of immunization and DHTR. Three situations are considered: (A) known history, with many transfusions in the past; (B) known history but with few transfusions in the past, and finally; (C) history unknown. In (C), the protocol may be based exclusively on the screening test result, with a risk that previous antibodies will be missed. *For (A) and (B), the cutoff between many and few transfusions is based on published data concerning the mean number of RBCs transfused at which the first antibody appears and on the risk being higher below 13 units. Few studies have addressed this issue, which remains a matter of debate. A count of 13 RBCs transfused is routinely used as the cutoff at our center.
Stratification of preventive measures for patients undergoing occasional transfusions. Preventive measures are recommended according to the risk of developing a new alloimmunization and DHTR, a risk that is itself evaluated from the patient's history of immunization and DHTR. Three situations are considered: (A) known history, with many transfusions in the past; (B) known history but with few transfusions in the past, and finally; (C) history unknown. In (C), the protocol may be based exclusively on the screening test result, with a risk that previous antibodies will be missed. *For (A) and (B), the cutoff between many and few transfusions is based on published data concerning the mean number of RBCs transfused at which the first antibody appears and on the risk being higher below 13 units. Few studies have addressed this issue, which remains a matter of debate. A count of 13 RBCs transfused is routinely used as the cutoff at our center.
Acknowledgment
We thank Dr. C. Tournamille for the review of the manuscript.
Conflict-of-interest disclosure
France Pirenne: no competing financial interests to declare.
Aline Floch: no competing financial interests to declare.
Anoosha Habibi: no competing financial interests to declare.
Off-label drug use
France Pirenne: no competing financial interests to declare.
Aline Floch: no competing financial interests to declare.
Anoosha Habibi: no competing financial interests to declare.