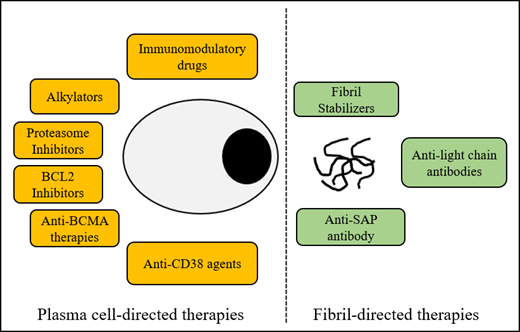
Abstract
Systemic light chain (AL) amyloidosis is a protein misfolding disorder characterized by the deposition of abnormal immunoglobulin light chains in fibrillary aggregates, resulting in end-organ damage. Several unique challenges face treating physicians, including delayed diagnosis, advanced vital organ involvement, and morbidity with treatment. Aggressive supportive care and risk-adapted application of plasma cell–directed therapies are the cornerstones of management. The therapeutic revolution in multiple myeloma will likely further expand the arsenal against plasma cells. Careful investigation of these agents will be critical to establish their role in this fragile population. The promise of fibril-directed therapies to restore organ function remains despite early disappointments. In this review, we discuss new therapies to tackle AL amyloidosis using a case-based approach.
Learning Objectives
Learn about the established and upcoming plasma cell–directed treatments in AL amyloidosis
Learn about anti-fibril-directed therapies in development to augment organ responses
CLINICAL CASE
Mr. X is a 49-year-old previously healthy man who noted dyspnea on exertion and exercise intolerance in July 2019. In April 2020, he was hospitalized with shortness of breath and was treated for an asthma exacerbation. He also had evidence of volume overload and was treated with diuresis with temporary improvement in his condition. In August 2020, he became progressively dyspneic, was unable to ambulate 20 yards, and sought the attention of a cardiologist. The evaluation included a nuclear stress test, which did not show ischemia but did reveal a left ventricular ejection fraction (LVEF) of 25%. Upon follow-up in October 2020, he was noted to have large right-sided pleural effusion, characterized as transudate on sampling. With no clear plan for further workup or management, he pursued a second opinion with a different cardiologist in November 2020. On echocardiogram, severe concentric left ventricular hypertrophy with a hypokinetic lateral wall and LVEF of 40% was appreciated. Electrocardiogram revealed normal sinus rhythm with 91 bpm, low voltage, and poor R-wave progression. Cardiac magnetic resonance imaging showed diffuse subendocardial late gadolinium enhancement. Based on these findings, infiltrative cardiomyopathy was suspected. He was started on diuretics and was referred to the hematology clinic in December 2020.
At the time of evaluation, Mr. X was relatively well appearing but had lower extremity edema. His Eastern Cooperative Oncology Group performance status was 1, with New York Heart Association class II. Laboratory values showed normal complete blood count with serum creatinine of 1.1 mg/dL, blood urea nitrogen of 21 mg/dL, albumin of 4.1 g/dL, alkaline phosphatase of 222 U/L, free κ light chain of 1.2 mg/dL, free λ light chain of 13 mg/dL, a κ/λ ratio of 0.10, and differential free light chain (dFLC) of 11.8 mg/dL. Serum protein electrophoresis did not reveal an M spike, and serum immunofixation did not show any monoclonal protein. N-terminal pro b-type natriuretic peptide (NT-proBNP) (5968 pg/mL, upper limit normal (ULN) ≤59 pg/mL), BNP (957 pg/mL, ULN ≤100 pg/mL), and troponin I (0.92 mg/mL, ULN ≤0.02 ng/mL) were elevated. A 24-hour urine protein electrophoresis did not reveal albuminuria. A 2-dimensional echo with strain revealed severely increased left ventricular wall thickness with an interventricular septal thickness at diastole of 1.9 cm, mild global hypokinesis, LVEF of 47%, grade 1 diastolic dysfunction, and global longitudinal strain of −6%. Bone marrow aspirate and biopsy specimen revealed 10% λ light chain–restricted plasma cells. There was no evidence of amyloid deposits by Congo red staining. Fluorescence in situ hybridization revealed t(11;14). The patient underwent a fat pad biopsy that was negative and subsequently had an endomyocardial biopsy, which revealed λ light chain amyloidosis. He was diagnosed with Mayo 2004 cardiac stage IIIA λ light chain amyloidosis in January 2021, approximately 18 months after his initial symptoms.
Monoclonal immunoglobulin/light chain (AL)–associated systemic amyloidosis is caused by clonal plasma cells, which produce abnormal immunoglobulins/fragments that misfold into amyloid fibrils and deposit in vital organs, causing disruption of organ function and ultimately death. Given its insidious onset and relatively nonspecific symptomatology, delay in diagnosis is unfortunately typical. Most patients see multiple specialists, and a third are diagnosed more than a year after the onset of symptoms similar to our patient.1 The prognosis of patients is primarily driven by vital organ, specifically cardiac, involvement.2,3
Historically, therapy targeting the underlying clonal plasma cells was borrowed from related plasma cell dyscrasia, multiple myeloma. Melphalan with steroids were the first agents used to treat amyloidosis.4 Given the efficacy of proteasome inhibition in targeting malignant plasma cells, bortezomib was evaluated in amyloidosis. The off-label standard of care in the United States consisted of a combination of a proteasome inhibitor (PI) (bortezomib), an alkylator (cyclophosphamide), and steroids (dexamethasone) (VCD).5,6 In the largest retrospective series of 230 patients treated with VCD, the overall hematologic response rate (ORR) was 60% (complete response, [CR] 23%). Organ responses were suboptimal and often delayed.7 In 2015, the anti-CD38 monoclonal antibody daratumumab was approved by the US Food and Drug Administration (FDA) to treat relapsed/refractory multiple myeloma. Daratumumab is a human IgG1κ monoclonal antibody that binds to CD38-expressing cells and induces tumor cell death through various immune-mediated actions. CD38 is overexpressed on malignant plasma cells in patients with AL amyloidosis, making it a rational target for treatment. In a small series of 25 patients, ORR was 76%, with one-third of patients achieving CR.8 Since then, prospective studies of daratumumab in relapsed disease have showed rapid (median time to response 1-4 weeks) and deep hematologic responses.9 Given impressive efficacy in the relapse setting, daratumumab was evaluated in a large randomized phase 3 study that included VCD with or without daratumumab (ANDROMEDA, NCT03201965). The addition of the monoclonal antibody resulted in significant improvement in ORR (92% vs 77%; CR, 53% vs 18%), and major organ deterioration progression-free survival favored the quadruplet arm (hazard ratio for major organ deterioration, hematologic progression, or death, 0.58; 95% CI, 0.36-0.93; P = .02). The quadruplet arm was also associated with doubling responses at 6 months (cardiac response, 42% vs 22%; renal response, 53% vs 24%).10 Patients achieved responses faster and stayed on therapy longer in the quadruplet arm. On the basis of these data, the FDA endorsed daratumumab in combination with bortezomib, cyclophosphamide, and dexamethasone (dara-VCD) in a historic approval on January 15, 2021.
CLINICAL CASE (continued)
Our patient was treated with dara-VCD and did well other than mild insomnia, which was managed with attenuated steroid dosing. After 1 cycle of quadruplet therapy, Mr. X achieved a partial response (dFLC 12 mg/dL → 5.35 mg/dL) and a very good partial response (VGPR) (dFLC 3.74 mg/dL) after 3 cycles. The patient also achieved a cardiac response (reduction in NT-ProBNP by 30% and ≥300 ng/L), with NT-ProBNP reduced from 5968 to 1280 ng/L.
The importance of early and deep responses to therapy in patients with AL amyloidosis cannot be overstated. In addition to causing tissue disruption by deposition, the amyloidogenic free light chain is independently toxic, and rapid clearance from circulation is of paramount importance. Palladini et al11 showed the importance of early hematologic response at 3 months as a predictor of survival for all patients with AL amyloidosis (Table 1). This was true even in the group of patients with very advanced cardiac involvement (stage IIIB disease, NT-proBNP >8500 ng/L). Patients achieving CR/VGPR at the end of first month of therapy experienced a survival benefit even in this group otherwise destined for poor outcomes.13 Therefore, we and others advocate for a change in therapy for those patients who do not achieve a VGPR after 3 cycles of treatment. However, the optimal end point of therapy remains debatable, and novel definitions of deep hematologic responses have been proposed.10 Among patients treated in the ANDROMEDA trial, those who received dara-VCD more frequently achieved a CR and had involved free light chain (FLC) <20 mg/L or dFLC <10 mg/L. Patients who achieved deep responses by any of these criteria had superior outcomes compared with patients with less FLC suppression. These and other definitions of optimal serologic response (suppression of the involved FLC to <10 mg/L) have not been compared, so which one will be associated with the best long-term outcomes remains unknown.14 The clinical relevance of bone marrow minimal residual disease (MRD) assessment and its association with organ responses is also of great interest but has not been uniformly studied in AL amyloidosis.15 Bone marrow–based MRD estimation has the potential to enable deep response assessment even in patients with low paraprotein production. Novel technologies such as mass spectrometry can detect monoclonal components in serum and urine with profound sensitivity and may prove more clinically useful than bone marrow–based MRD assays but await validation in AL amyloidosis.16
Response assessment in AL amyloidosis
| Hematologic response . | CR . | VGPR . | PR . | PD . |
|---|---|---|---|---|
| Serum protein electrophoresis/immunofixation | Serum negative for monoclonal proteins by immunofixation | ≥50% reduction in current serum monoclonal protein levels >0.5 g/dL | If progressing from CR, any detectable monoclonal protein If progressive from PR or SD, ≥50% increase in the serum M protein to >0.5 g/dL | |
| Urine protein electrophoresis/immunofixation | Urine negative for monoclonal proteins by immunofixation | ≥50% reduction in current urine M protein levels >100 mg/d with a visible peak | If progressive from PR or SD, ≥50% increase in urine M protein to >200 mg/d with visible peak present | |
| Serum free light chains | Normal serum free light chain ratio or the uninvolved serum free light chain concentration is greater than the involved serum free light chain concentration with or without an abnormal free light chain ratio | Reduction in the difference between the involved and uninvolved serum free light chain to <40 mg/L | ≥50% reduction in current free light chain levels >10 mg/dL | Serum free light chain increase of ≥50% to >10 mg/dL (100 mg/L) |
| Organ response | Response | Stable disease | PD | |
| Cardiac | Requires any of the following: ≥2-mm decrease in mean intraventricular septal wall thickness by echocardiogram ≥20% increase in LVEF ≥2 grade decrease in New York Heart Association functional class without an increase in diuretic use and no increase in wall thickness Reduction (≥30% and ≥300 ng/L) of NT-proBNP in patients whom the eGFR is ≥45 mL/min/1.73 m2 | Does not meet the criteria for cardiac response or progressive disease | Requires any of the following: ≥2-mm increase from baseline in the intraventricular wall thickness by echocardiogram ≥10% decrease in the LVEF ≥1 grade increase in New York Heart Association functional class | |
| Renal | ≥50% decrease of at least 0.5 g/d (500 mg/24 h) in 24-h urine protein of >0.5 g/d (500 mg/24 h) pretreatment and Creatinine clearance or serum creatinine must not have worsened by ≥25% over baseline | Does not meet the criteria for renal response or progressive disease | Requires any of the following: ≥50% increase of at least 1 g/d (1000 mg/24 h) for urine protein to >1 g/d (1000 mg/24 h) 25% worsening of serum creatinine or creatinine clearance | |
| Hepatic | Requires all of the following: ≥2-cm decrease in liver span if hepatomegaly present (liver >15 cm) ≥50% decrease and/or normalization of serum ALP level | Does not meet the criteria for hepatic response or progressive disease | Requires the following: ≥50% increase in the serum ALP level | |
| Autonomic nervous system | Resolution of symptomatic orthostatic hypotension | Does not meet the criteria for autonomic neuropathy response or progressive disease | Worsening of symptomatic orthostatic hypotension | |
| Peripheral nervous system | Requires any of the following: Resolution of abnormal physical findings Resolution or improvement of abnormal EMG and/or NCV findings | Does not meet the criteria for peripheral neuropathy response or progressive disease | Requires any of the following: Worsening of physical findings Worsening of EMG and/or NCV findings |
| Hematologic response . | CR . | VGPR . | PR . | PD . |
|---|---|---|---|---|
| Serum protein electrophoresis/immunofixation | Serum negative for monoclonal proteins by immunofixation | ≥50% reduction in current serum monoclonal protein levels >0.5 g/dL | If progressing from CR, any detectable monoclonal protein If progressive from PR or SD, ≥50% increase in the serum M protein to >0.5 g/dL | |
| Urine protein electrophoresis/immunofixation | Urine negative for monoclonal proteins by immunofixation | ≥50% reduction in current urine M protein levels >100 mg/d with a visible peak | If progressive from PR or SD, ≥50% increase in urine M protein to >200 mg/d with visible peak present | |
| Serum free light chains | Normal serum free light chain ratio or the uninvolved serum free light chain concentration is greater than the involved serum free light chain concentration with or without an abnormal free light chain ratio | Reduction in the difference between the involved and uninvolved serum free light chain to <40 mg/L | ≥50% reduction in current free light chain levels >10 mg/dL | Serum free light chain increase of ≥50% to >10 mg/dL (100 mg/L) |
| Organ response | Response | Stable disease | PD | |
| Cardiac | Requires any of the following: ≥2-mm decrease in mean intraventricular septal wall thickness by echocardiogram ≥20% increase in LVEF ≥2 grade decrease in New York Heart Association functional class without an increase in diuretic use and no increase in wall thickness Reduction (≥30% and ≥300 ng/L) of NT-proBNP in patients whom the eGFR is ≥45 mL/min/1.73 m2 | Does not meet the criteria for cardiac response or progressive disease | Requires any of the following: ≥2-mm increase from baseline in the intraventricular wall thickness by echocardiogram ≥10% decrease in the LVEF ≥1 grade increase in New York Heart Association functional class | |
| Renal | ≥50% decrease of at least 0.5 g/d (500 mg/24 h) in 24-h urine protein of >0.5 g/d (500 mg/24 h) pretreatment and Creatinine clearance or serum creatinine must not have worsened by ≥25% over baseline | Does not meet the criteria for renal response or progressive disease | Requires any of the following: ≥50% increase of at least 1 g/d (1000 mg/24 h) for urine protein to >1 g/d (1000 mg/24 h) 25% worsening of serum creatinine or creatinine clearance | |
| Hepatic | Requires all of the following: ≥2-cm decrease in liver span if hepatomegaly present (liver >15 cm) ≥50% decrease and/or normalization of serum ALP level | Does not meet the criteria for hepatic response or progressive disease | Requires the following: ≥50% increase in the serum ALP level | |
| Autonomic nervous system | Resolution of symptomatic orthostatic hypotension | Does not meet the criteria for autonomic neuropathy response or progressive disease | Worsening of symptomatic orthostatic hypotension | |
| Peripheral nervous system | Requires any of the following: Resolution of abnormal physical findings Resolution or improvement of abnormal EMG and/or NCV findings | Does not meet the criteria for peripheral neuropathy response or progressive disease | Requires any of the following: Worsening of physical findings Worsening of EMG and/or NCV findings |
ALP, alkaline phosphatase; eGFR, estimated glomerular filtration rate; EMG, electromyography; NCV, nerve conduction velocity; PD, progressive disease; PR, partial response; SD, stable disease.
Treatment intensification with high-dose melphalan is an option in a subset of patients. High-dose therapy followed by autologous stem cell transplantation (ASCT) is the cornerstone of therapy in multiple myeloma, and when applied to carefully selected patients, it has been a treatment strategy with durable responses in AL amyloidosis. With this onetime treatment, several groups have shown survival exceeding 15 years in a third of patients.17 The obvious challenge is a high burden of vital organ involvement and treatment-related mortality. More recently, with a deeper understanding of disease biology, improvements in supportive care, and refined patient selection criteria, the treatment-related mortality is now less than 3% (Table 2).18 Functional assessment with global longitudinal strain by 2-dimensional speckle tracking echocardiography has emerged as a strong predictor of survival and further risk-stratifies patients with AL amyloidosis undergoing high-dose therapy.20 When ASCT is used together as a consolidative strategy after induction with a bortezomib-containing regimen, this approach is associated with deeper responses and improved progression-free and overall survival.21,22 As dara-VCD is incorporated into initial therapy and more patients achieve hematologic CR, the role of high-dose therapy and ASCT will necessarily evolve.
Refined transplantation eligibility criteria across transplant centers
| Boston University, USA16 . | Mayo Clinic, USA18 . | National Amyloidosis Center, UK19 . |
|---|---|---|
| Cardiac ejection fraction ≥40% | Age ≤70 years | No symptomatic gastrointestinal involvement |
| Absence of symptomatic pleural effusions | Creatinine clearance ≥30 mL/min | Estimated glomerular filtration rate ≥45 mL/min |
| Absence of uncompensated heart failure or arrhythmias resistant to medical management | New York Health Association functional class I or II | No symptomatic cardiac involvement |
| Oxygen saturation ≥95% on room air | ≤2 Organs significantly involved | ≤2 Organs significantly involved |
| Lung diffusion capacity ≥50% predicted | Troponin T ≤ 0.06 ng/mL | High sensitivity troponin T ≤ 0.06 ng/mL |
| Supine systolic blood pressure ≥90 mm Hg | Systolic blood pressure ≥90 mm Hg | No autonomic neuropathy |
| Southwest Oncology Group performance status score ≤2 unless limited by peripheral neuropathy | Eastern Cooperative Oncology Group performance score ≤2 | Eastern Cooperative Oncology Group performance score ≤1 |
| Boston University, USA16 . | Mayo Clinic, USA18 . | National Amyloidosis Center, UK19 . |
|---|---|---|
| Cardiac ejection fraction ≥40% | Age ≤70 years | No symptomatic gastrointestinal involvement |
| Absence of symptomatic pleural effusions | Creatinine clearance ≥30 mL/min | Estimated glomerular filtration rate ≥45 mL/min |
| Absence of uncompensated heart failure or arrhythmias resistant to medical management | New York Health Association functional class I or II | No symptomatic cardiac involvement |
| Oxygen saturation ≥95% on room air | ≤2 Organs significantly involved | ≤2 Organs significantly involved |
| Lung diffusion capacity ≥50% predicted | Troponin T ≤ 0.06 ng/mL | High sensitivity troponin T ≤ 0.06 ng/mL |
| Supine systolic blood pressure ≥90 mm Hg | Systolic blood pressure ≥90 mm Hg | No autonomic neuropathy |
| Southwest Oncology Group performance status score ≤2 unless limited by peripheral neuropathy | Eastern Cooperative Oncology Group performance score ≤2 | Eastern Cooperative Oncology Group performance score ≤1 |
Among patients who are ineligible for ASCT, other plasma cell–directed therapies have shown benefit. Second-generation PIs, carfilzomib and ixazomib, have shown efficacy in previously treated patients. Carfilzomib, an irreversible PI, was studied in a dose-finding phase 1/2 study among patients with Mayo stage I/II disease and LVEF >40%, where over a third of patients were PI refractory. The maximum tolerated dose (MTD) was 36 mg/m2 administered on standard schedule days 1, 2, 8, 9, 15, and 16. The ORR was impressive at 63% (CR, 12%) but not without a high incidence of grade 3 adverse events, including a reduction in LVEF, an increase in NT-proBNP, and acute kidney injury.23 An ongoing study from the United Kingdom is evaluating carfilzomib in combination with thalidomide and dexamethasone. At the time of publication, 7 of the 10 recruited patients had completed 3 cycles of therapy with an ORR of 70% (VGPR, 40%). No MTD was reached, and the recommended dose of carfilzomib was 45 mg/m2.24 Ixazomib, an oral PI with a manageable safety profile, generated much interest when, in a phase 1/2 study, an ORR of 52% and an organ response of 56% were seen at the MTD of 4 mg with patients treated for up to 12 cycles of therapy.25 These data led to a phase 3 randomized study comparing ixazomib and dexamethasone with physician's choice in the relapsed setting. Although the study failed to significantly improve the overall hematologic response frequency, which was the primary end point, the CR rate, duration of hematologic response, vital organ progression-free survival, and time to next treatment were superior in the ixazomib arm.26 Currently, several ongoing studies are evaluating ixazomib in the relapsed (with daratumumab and dexamethasone, NCT03283917), newly diagnosed (with cyclophosphamide and dexamethasone, NCT03236792 and NCT01864018), and maintenance (NCT03618537) settings.
Although immunomodulatory agents are the backbone of myeloma therapy, they have not been readily incorporated into treatment regimens for AL amyloidosis due to their adverse safety profile in this population. The dosing of both lenalidomide and pomalidomide must be significantly attenuated to allow tolerability (no higher than 15 mg for lenalidomide and 2 mg for pomalidomide in most patients). At these doses, ORRs of 50% to 70% have been achieved in different series.27-29 Unfortunately, renal dysfunction, cardiac arrhythmias, and increased cardiac biomarkers have tempered the adoption of immunomodulatory agents in this fragile population.
Recurrent cytogenetic abnormalities are present within the clonal plasma cells in AL amyloidosis, with t(11;14) being the most common abnormality. Compared with an incidence of 15% to 20% in multiple myeloma, it is present in about half of all cases with amyloidosis.30 The presence of t(11;14) in AL amyloidosis is associated with inferior event-free survival and, possibly, overall survival when treated with bortezomib, yet favorable response rates and event-free survival have been demonstrated when patients with t(11;14) are treated with high-dose melphalan and stem cell transplantation.31 Venetoclax is an oral selective small-molecule BCL-2 inhibitor that has shown remarkable efficacy in patients with multiple myeloma who harbor t(11;14).32 Based on the overrepresentation of this cytogenetic subset in amyloidosis, several groups have explored the role of venetoclax in this disease. Sidiqi et al33 reported on 12 patients with the relapsed disease (1-4 prior lines of therapy) who received venetoclax off-label (11 with t(11;14)) and showed an impressive ORR of 88%. Premkumar et al34 reported the largest series to date with 43 patients (31 with t(11;14)) from 14 centers across the United States and Europe. The ORR was 68% (63% ≥ VGPR) for the entire cohort and 81% (80% ≥ VGPR) for the t(11;14) cohort. Among evaluable patients, the cardiac and renal response rates were 30% and 40%, respectively, and 10 of the 12 responders (88%) harbored t(11;14). Prospective studies evaluating venetoclax combinations in AL amyloidosis are under way (NCT04847453).
Other plasma cell–directed antibodies targeting CD38 are in development. Isatuximab has been explored in the relapsed, refractory setting in the Southwest Oncology Group S1702 study, which is fully accrued. Preliminary results presented at the American Society of Hematology meeting in December 2020 demonstrated an ORR of 77% and a 1-year estimated PFS and OS of 85% and 97%.35 Isatuximab is also being evaluated in the newly diagnosed setting among patients with advanced cardiac disease (NCT04754945). Anti-CD38 antibody drug conjugate STI 6129, comprising a fully human anti-CD38 antibody conjugated to the microtubule inhibitor duostatin, is also being studied in a phase 1b study in the relapsed setting (NCT04316442).
To date, the treatment for AL amyloidosis has been focused on the eradication of the abnormal plasma cell clone. Despite achieving deep and durable hematologic responses, the prognosis of AL amyloidosis is driven by the degree of organ dysfunction. It is sobering to admit that our understanding of the organ-specific biology, the potential for recovery, and the available response criteria are limited. Patients continue to experience morbidity secondary to organ impairment, and slow, suboptimal organ responses impede quality and quantity of life. Kaufman et al36 showed a median time to organ response of 10.4 months, which did not differ between transplant and nontransplant strategies. In the safety cohort of the ANDROMEDA study, time to cardiac, renal, and hepatic response was approximately 4 months, 2 months, and 1 year, respectively.10 Safe and efficacious therapies to target and remove the pathologic resident amyloid deposits are lacking but are critical to reducing morbidity and mortality in AL patients. The first of this class of drugs was birtamimab (NEOD001), a humanized form of murine monoclonal antibody 2A4, which binds to an epitope unique to the misfolded light chain protein, not available in light chain's native conformation or in fully formed immunoglobulin.37 Despite promising results in a phase 1/2 clinical trial, in 2018, the development of this agent was discontinued after the phase 2b PRONTO study in previously treated patients failed to meet its primary (cardiac response) or secondary end points (change in physical component summary, 6-minute walk test, and NT-proBNP rate of change). At that time, the ongoing phase 3 VITAL study in the newly diagnosed population was also discontinued for futility.
Serum amyloid P (SAP) is a nonfibrillary protein that is present in all types of amyloid deposits, is agnostic to underlying offending protein type, and serves as a chaperone, stabilizing and protecting the fibrillary protein. The small molecule miridesap cross-links SAP molecules in plasma, which triggers their clearance by the liver. When combined with an anti-SAP antibody, dezamizumab, which targets residual SAP in tissues, amyloid was resorbed from visceral organs. After this strategy was found to be promising in preclinical models, the combination was found to be safe and resulted in organ responses as measured by liver stiffness, extracellular liver volume, and SAP scans in a phase 1 dose-escalation study.38 However, a phase 2 trial in patients with cardiac disease was terminated early based on toxicity, although the exact nature of the adverse events has not been reported.
Cael-101 (11-1F4) is an amyloid fibril-reactive monoclonal antibody designed to target amyloid deposits by directly binding to a conformational epitope present on human light chain amyloid fibrils. When administered to mice bearing subcutaneous human AL amyloidomas, the antibody bound to the pathologic material and initiated an inflammatory response that led to the elimination of the induced tumors.37 Results from a phase 1a/1b study in relapsed AL amyloidosis demonstrated no dose-limiting toxicity up to a maximum dose of 500 mg/m2, and 67% had organ responses. Responses were fast (median time to response was 2 weeks) and sometimes independent of hematologic response.39 More recently, Cael-101 at 1000 mg/m2 was recommended in combination with VCD based on pharmacokinetics and a favorable toxicity profile in a dose-escalation study, which showed promising organ responses. There are now 2 ongoing randomized phase 3 studies of bortezomib-based initial therapy with or without Cael-101 in patients with advanced cardiac involvement (NCT04512235, NCT04504825), including stage IIIB, a population universally excluded from clinical trials. Additional studies of Cael-101 in different combinations and in distinct patient populations are also planned.
Doxycycline has antifibril properties, including inhibition of amyloid deposition and reduction in the number of intact fibrils in preclinical models. In a single-arm phase 2 study that administered doxycycline along with induction chemotherapy, safety and high transplant utilization (60% of patients) were shown by D'Souza et al.40 An ongoing study is evaluating its role in a randomized study compared with standard supportive care in bortezomib-treated patients (NCT03474458). Table 3 summarizes the ongoing studies of novel agents in AL amyloidosis.
Ongoing studies of novel therapies in AL amyloidosis
| Drug . | Mechanism of action . | Trial identifier . | Phase . | Population . | Enrollment target . |
|---|---|---|---|---|---|
| Cael-101 | Fibril-directed therapy Anti–light chain antibody | NCT04304144 | 2 (part A with VCD; part B with DVCD) | NDAL | 25 |
| Cael-101 | Fibril-directed therapy Anti–light chain antibody | NCT04512235 | 3 (randomized, double blind) | NDAL (IIIA) | 267 |
| Cael-101 | Fibril-directed therapy Anti–light chain antibody | NCT04504825 | 3 (randomized, double blind) | NDAL (IIIB) | 111 |
| Doxycycline | Fibril-directed therapy Fibril stabilizer | NCT03474458 | 2/3 | NDAL | 120 |
| Isatuximab | Plasma cell–directed therapy Anti-CD38 monoclonal antibody | NCT04754945 | 1 | NDAL (high risk = Mayo 2012 stage IV, Mayo 2004 IIIB BUMC 2019 3B) | 25 |
| Isatuximab | Plasma cell–directed therapy | NCT03499808 | 2 | R/R | 43 |
| Venetoclax | Plasma cell–directed therapy BCL2 inhibitor | NCT02994784 | 1 | R/R | 24 |
| Belantamab | Plasma cell–directed therapy Anti-BCMA antibody drug conjugate | NCT04617925 | 2 | R/R | 35 |
| Melflufen | Plasma cell–directed therapy Alkylating agent | NCT04115956 | 1/2 | R/R (after 1L) | 46 |
| STI-6129 | Plasma cell–directed therapy Anti-CD38 antibody drug conjugate | NCT04316442 | 1 | R/R (≥2) | 60 |
| Drug . | Mechanism of action . | Trial identifier . | Phase . | Population . | Enrollment target . |
|---|---|---|---|---|---|
| Cael-101 | Fibril-directed therapy Anti–light chain antibody | NCT04304144 | 2 (part A with VCD; part B with DVCD) | NDAL | 25 |
| Cael-101 | Fibril-directed therapy Anti–light chain antibody | NCT04512235 | 3 (randomized, double blind) | NDAL (IIIA) | 267 |
| Cael-101 | Fibril-directed therapy Anti–light chain antibody | NCT04504825 | 3 (randomized, double blind) | NDAL (IIIB) | 111 |
| Doxycycline | Fibril-directed therapy Fibril stabilizer | NCT03474458 | 2/3 | NDAL | 120 |
| Isatuximab | Plasma cell–directed therapy Anti-CD38 monoclonal antibody | NCT04754945 | 1 | NDAL (high risk = Mayo 2012 stage IV, Mayo 2004 IIIB BUMC 2019 3B) | 25 |
| Isatuximab | Plasma cell–directed therapy | NCT03499808 | 2 | R/R | 43 |
| Venetoclax | Plasma cell–directed therapy BCL2 inhibitor | NCT02994784 | 1 | R/R | 24 |
| Belantamab | Plasma cell–directed therapy Anti-BCMA antibody drug conjugate | NCT04617925 | 2 | R/R | 35 |
| Melflufen | Plasma cell–directed therapy Alkylating agent | NCT04115956 | 1/2 | R/R (after 1L) | 46 |
| STI-6129 | Plasma cell–directed therapy Anti-CD38 antibody drug conjugate | NCT04316442 | 1 | R/R (≥2) | 60 |
DVCD, daratumumab, bortezomib, cyclophosphamide, dexamethasone; NDAL, newly diagnosed AL amyloidosis; R/R, relapsed/refractory; 1L, first line.
CLINICAL CASE (continued)
Our patient, Mr. X, had a hematologic VGPR and cardiac biomarker response after 3 cycles of dara-VCD. He is functionally doing very well with New York Heart Association class I symptoms and will complete 6 cycles of dara-VCD. Despite achieving an excellent response, the best management of this patient after completing initial therapy remains elusive. Unanswered questions include the role of maintenance therapy in this setting, whether the patient would benefit from attempting to achieve a deeper response than VGPR with alternate therapy (such as venetoclax), and, if so, with when and with what method should MRD be measured in this disease.
Future directions
Plasma cell disorder therapeutics is a rapidly expanding field. Understanding how to optimize the use of currently available treatments to maximize benefit and improve outcomes is essential. Identifying new targets and novel ways of approaching known targets through immunotherapeutic strategies such as chimeric antigen receptor T-cell therapies, T-cell engagers, and antibody drug conjugates has revolutionized the outcomes of refractory myeloma. Among the latest therapies, B-cell maturation antigen (BCMA) targeting antibody drug conjugates and chimeric antigen receptor T cells are furthest along, and 2 such drugs, belantamab mafodotin and idecabtagene vicleucel, have recently received FDA approval. BCMA has also been shown to be expressed on the surface of clonal plasma cells in amyloidosis. Belantamab mafodotin is being studied by the European Myeloma Network in a phase 2 study in relapsed amyloidosis (NCT04617925). Innovative therapies targeting GPRC5D, FCRH5, and XPO inhibitors and repackaged alkylating agents have shown promise in refractory multiple myeloma. Our group is evaluating the expression of these and other targets on clonal plasma cells in amyloidosis (unpublished work). Several characteristics of amyloidosis, such as small clonal disease burden in bone marrow, a lower proliferative index, and a reduced incidence of high-risk mutations, lend themselves favorably to immunotherapeutic strategies. Therefore, it is possible that some if not all of these therapies could be effective in AL amyloidosis. The evaluation of their safety and efficacy in well-designed clinical trials will be critical.
With the availability of novel therapies and improving survival, the role of high-dose therapy and ASCT will need to be refined. We envision that for patients achieving rapid and deep responses, ASCT could be deferred given the short- and long-term toxicities associated with high-dose melphalan.41 However, it will continue to remain a very important complementary strategy to deepen responses for fit patients with suboptimal response, including those with persistent MRD.
Last, patients in whom organ function is compromised without expectation for meaningful recovery at the time of diagnosis or after adequate control of the hematologic disease could be candidates for solid organ transplantation, prolonging survival and offering a much-needed improvement in quality life.42 Data supporting the benefit of cardiac and renal transplant are widely available and should be offered to patients in the right clinical scenario.
Acknowledgment
This work was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Conflict-of-interest disclosure
Susan Bal has received funding from the Amyloidosis Foundation.
Heather Landau has received research support for clinical trials from Takeda. She served on the advisory boards of Takeda, Celgene, Janssen, Sanofi, and Caelum Biosciences and has been a consultant for Karyopharm and Pfizer.
Off-label drug use
Susan Bal: nothing to disclose.
Heather Landau: nothing to disclose.