Abstract
Targeted therapy is a powerful treatment option in chronic lymphocytic leukemia (CLL) that has outperformed conventional chemoimmunotherapy in most clinical settings. Except for selected young, fit patients with a mutated immunoglobulin heavy chain variable region gene, most patients benefit from targeted therapy with either a continuous BTK inhibitor or 1-year fixed-duration venetoclax-obinutuzumab as first-line treatment of CLL. Treatment selection is driven by patient-, treatment-, and disease-related factors, encompassing patient preference, concomitant medications, comorbidities, safety profile of the regimen, and TP53 aberration. Clinical trials are actively investigating the simultaneous inhibition of Bruton’s tyrosine kinase (BTK) and B-cell lymphoma 2 (BCL-2) proteins with or without a CD20 monoclonal antibody, which can achieve deep response in most patients (52%-89% undetectable minimal residual disease in bone marrow).
CLINICAL CASE
A 70-year-old woman with previously untreated chronic lymphocytic leukemia (CLL) presented with worsening fatigue, progressive lymphocytosis, and cytopenia (hemoglobin, 9 g/dL; platelets, 110 × 109/L). Prognostic markers were deletion 11q and unmutated immunoglobulin heavy chain variable region gene (U-IGHV). There was no evidence of deletion 17p by fluorescence in situ hybridization or TP53 mutation by targeted sequencing. Medical history was notable for myocardial infarction requiring a coronary artery bypass graft 10 years ago and paroxysmal atrial fibrillation. Current medications included aspirin and carvedilol (P-glycoprotein [P-gp] inhibitor).
Chemoimmunotherapy can be considered for IGHV-mutated CLL
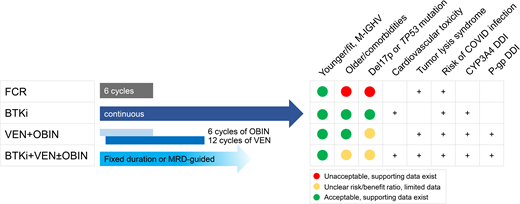
Fludarabine, cyclophosphamide, and rituximab (FCR) comprise one of the most commonly and globally used initial chemoimmunotherapy (CIT) regimens in CLL. A series of randomized studies in the 2000s and over a decade of follow-up experience demonstrated improved overall survival (OS) with the addition of rituximab to fludarabine plus cyclophosphamide1 and superior efficacy of FCR over bendamustine and rituximab (BR).2 Except for cellular therapy, FCR remains the only treatment option in CLL with a potential for cure. About half of patients with mutated IGHV (M-IGHV) substantially benefit from FCR by achieving durable treatment-free remissions, a finding supported by plateauing of progression-free survival (PFS) curves in 2 prospective trials1,3 and a retrospective analysis.4 In the M-IGHV subgroup, PFS rates were 67% at 5 years in the CLL8 study,1 54% at 12 years in the FCR300 cohort at MD Anderson,3 and 59% at 5 years in the Italian retrospective data set. Conversely, the risk of disease progression for the U-IGHV subgroup remains persistently elevated after FCR, leading to continuous relapse. Of independent prognostic markers relevant to FCR therapy, TP53 aberration has the strongest negative impact on survival.1 While deletion 11q and U-IGHV are associated with inferior PFS, extended treatment-free remission can be observed in a small subset of these subgroups.5 Efforts to improve the FCR regimen have focused on combinations with ibrutinib and/or substituting obinutuzumab for rituximab; in 3 separate studies, the PFS of these regimens is >95% at 3 to 5 years.
The observed benefit of FCR therapy is offset by the increased risk of treatment-related myeloid neoplasms (tMNs), neutropenia, and infection. tMNs are related to the DNA damage created by alkylating agents, which are key components of CIT regimens. Studies report that 1% to 5% of patients develop tMN, with a median time to onset of 40 months.1,6,7 Furthermore, FCR is poorly tolerated in older patients or those with reduced renal function due to high rates of neutropenia (85% with grade ≥3) and infections (38% with grade ≥3).2 Overall, fit, younger patients with M-IGHV are potential candidates for FCR therapy. Pretreatment bone marrow biopsy is desirable to rule out morphologic and/or genetic evidence of myeloid disorders before treatment with FCR. BR should not be substituted for FCR as BR therapy is not associated with the same durable benefit.
Comparative efficacy of targeted therapy regimens
Targeted therapy is preferred over CIT for most patients with CLL, including those with older age, comorbidities, and unfavorable prognostic markers. The current standard of care with targeted agents is largely divided into 2 approaches: continuous therapy with a Bruton’s tyrosine kinase (BTK) inhibitor (BTKi) ± anti-CD20 monoclonal antibody (mAb), or 1-year duration venetoclax plus obinutuzumab (VO). Superior efficacy and often improved tolerability of these approaches compared with CIT allow targeted agents to be applied to a broader patient population (Table 1). In terms of BTKis, ibrutinib-rituximab outperformed FCR by improving PFS in younger, fit patients in the UK FLAIR7 and the ECOG E1912 studies.8 Notably, OS advantage was demonstrated by the E1912 study but not the FLAIR study. The OS benefit in E1912 was small, with a 6% difference at 5 years, and appeared to be driven by early deaths in the FCR arm. The lack of OS difference in the FLAIR study is also explained by increased rates of arrhythmia and presumed cardiac deaths in the ibrutinib-containing arm. PFS of older adults or those with comorbidities consistently improved with BTKi-based therapy compared with CIT, but OS was not changed.9–11 It is important to note that the PFS differences in all of these trials were largely driven by differences among patients with U-IGHV while there were small to no differences in the M-IGHV subgroup. Favorable efficacy of VO was also demonstrated in randomized trials, leading to high rates of undetectable minimal residual disease (uMRD) and improved PFS in the CLL1312 and the CLL14 studies of the German CLL Study Group.13
The ongoing CLL17 study directly compares continuous BTKi and fixed-duration VO in treatment-naive (TN) CLL (NCT04608318). At present, in the absence of randomized data, these 2 approaches appear to have comparable efficacy. Estimated 4-year PFS was 76% with continuous ibrutinib14 and 74% with fixed-duration VO13 in older adults with TN-CLL. However, the data also suggest that there may be meaningful differences in efficacy among specific subgroups of CLL. For instance, 5-year PFS numerically differs by about 10% in the U-IGHV subgroup treated with ibrutinib (67%)15 compared with VO (56%).16 The PFS difference is larger for patients with TP53 aberration, favoring continuous treatment with ibrutinib (70% at 5 years)17 over VO (41% at 5 years).16 Although patient numbers remain small, and cross-trial comparisons are exploratory and lack statistical rigor, nonetheless, they raise an important question of whether the prolonged duration of therapy and/or BTK inhibition is necessary to fully overcome the poor prognostic impact of TP53 aberration in particular and, to a lesser extent, U-IGHV. In the setting of these very limited data, most investigators favor a BTKi for patients with TP53 aberration in the absence of a clinical trial. For U-IGHV, although fixed-duration therapy may result in a shorter initial PFS, patients may be eligible for VO retreatment that would extend the total benefit of the regimen. The opportunity to have long durations off therapy has intrinsic value and may reduce the development of resistance. More data are certainly needed to inform treatment selection in patients with higher-risk markers. These would include prospective trials specific to TP53 aberrant CLL and extended follow-up data that encompass 2 or more lines of therapy (and/or retreatment).
Selective BTKis appear to be as effective as ibrutinib, including in patients with TP53 aberration. In the acalabrutinib monotherapy arm of the ELEVATE-TN study, the estimated 4-year PFS was 76% for patients with TP53 aberration, similar to the 78% of the overall population.18 Zanubrutinib also demonstrated excellent efficacy in patients with deletion 17p, in the largest dedicated prospective cohort in this population, but the duration of follow-up is shorter than other BTKis (89% 18-month PFS).19
When anti-CD20 mAb is combined with other agents, obinutuzumab is preferred over rituximab in the first-line setting. The CLL13 study demonstrated higher rates of uMRD with VO (87%) than venetoclax-rituximab (57%), which was not more effective than CIT, indicating that obinutuzumab is a more potent antibody than rituximab.12 The role of combination CD20 mAb with a BTKi is more ambiguous, albeit well tolerated and supported by the National Comprehensive Cancer Network guidelines. The strongest data support the addition of obinutuzumab to acalabrutinib, which improved 5-year PFS by 12% in the ELEVATE-TN study.20 Intriguingly, the addition of rituximab to ibrutinib did not lead to any improvement in PFS in 2 randomized trials.9,21 These differences in outcome may be explained by greater cell killing and greater antibody-dependent cellular cytotoxicity (ADCC) of the type 2 mAb obinutuzumab. They may also be related to inhibition of ITK, which impairs ADCC, by ibrutinib but not acalabrutinib. The data combining ibrutinib with obinutuzumab are not randomized, hence leaving some doubt as to the exact benefit of the combination.
Data for sequencing of targeted agents and retreatment strategies have been largely generated from patients who received targeted therapy for the treatment of relapsed or refractory disease. These populations differ from those who receive a BTKi or VO as their initial therapy. Furthermore, studies reporting sequencing of targeted agents had heterogeneous patient populations, such as patients treated with PI3K inhibitors, those who stopped targeted therapy due to acquired resistance, and those who discontinued therapy due to toxicity.22 Data for retreatment with time-limited venetoclax-based regimens are limited (4-39 evaluable patients in 3 studies, median treatment duration: 10-11 months).23,24 Despite limitations, all of these studies reported high rates of initial response, suggesting sequencing and retreatment after time-limited therapy is a viable treatment strategy in CLL. The overall response rate was 74% when venetoclax was given after ibrutinib or idelalisib in a retrospective pooled analysis22 and 100% when a BTKi was given after venetoclax-rituximab in the MURANO study.24 The overall response rate to venetoclax-based retreatment was 72% in the MURANO study24 and 80% to 100% in other reports.23,25
Comparative safety of frontline regimens
A common misconception is that elderly and frail patients should receive a BTKi rather than VO. However, data support either treatment approach for such patients. The CLL14 study testing VO against chlorambucil and obinutuzumab enrolled patients with comorbidities; one-third of the patients were 75 years or older.
Notable side effects of BTKis include cardiovascular toxicities, bleeding, and atypical infection. Of these, cardiac arrhythmia is the leading cause of treatment discontinuation and dose reduction for the first-generation BTKi ibrutinib.22 The mechanism of cardiovascular toxicity remains unclear. Preclinical data suggest arrhythmia can be induced by inhibition of unintended targets of ibrutinib, most notably C-terminal Src kinase (CSK) expressed in cardiac myocytes.26 In support of preclinical findings, head-to-head randomized clinical trials demonstrated lower rates of atrial fibrillation with more selective BTK inhibition through acalabrutinib (ELEVATE-RR study: 9% for acalabrutinib vs 16% for ibrutinib, median follow-up of 41 months)27 or zanubrutinib (ALPINE study: 3% for zanubrutinib vs 10% for ibrutinib, median follow-up of 15 months).28 Pirtobrutinib is another highly selective BTKi with a noncovalent binding mechanism and only a 1% rate of atrial fibrillation reported in a phase 1/2 study (albeit with median follow-up of 6 months).29 A recently launched phase 3 study will compare the efficacy and safety of pirtobrutinib and ibrutinib in CLL (NCT05254743). Existing data on the comparative safety of BTKis were generated in relapsed CLL but remain relevant to the first-line setting and support the use of selective BTKis over ibrutinib. Patients with known cardiovascular risk factors continue to be at risk of developing cardiac events even with the use of the more selective BTKi acalabrutinib.30 Treatment with VO can be considered over BTKis for these patients.
Increased risk of bleeding is a class effect toxicity of BTKis. BTK has a central role in platelet aggregation and adhesion, and modifiable risk factors for bleeding should be minimized during therapy.31 Patients should be advised to hold BTKis for 3 to 7 days before and after invasive procedures. Concurrent administration of warfarin or dual antiplatelet therapy is generally avoided; direct oral anticoagulants or aspirin can be safely used during BTKi therapy. While opportunistic infection is rare, some experts recommend Pneumocystis jirovecii and varicella-zoster virus prophylaxis during the first year of BTKi therapy, particularly in relapsed patients.
Key toxicities of VO therapy are tumor lysis syndrome (TLS), infusion-related reactions to obinutuzumab, cytopenias, and infection. TLS is a potentially fatal side effect of venetoclax and, less commonly, obinutuzumab. Reduction of tumor burden decreases the risk of TLS, making this side effect almost exclusive to the treatment initiation period. Because obinutuzumab precedes venetoclax in the VO regimen and can effectively debulk CLL, only 3 patients in the CLL14 study developed TLS (1.4%), and all 3 events occurred during the obinutuzumab lead-in period. Venetoclax should be initiated with 5 weekly ramp-ups coupled with close monitoring and supportive care with uric acid–lowering agents and oral ± intravenous fluids. Infusion-related reactions to obinutuzumab are also relevant to treatment initiation and can be effectively managed by premedication with corticosteroids, acetaminophen, and an antihistamine. Cytopenias are commonly observed during therapy and are generally responsive to supportive care and treatment interruption. The incidence of serious infection remains low.
Drug-drug interaction is an important consideration for all targeted therapy approved for the treatment of CLL. Both BTKis and venetoclax require dose reduction during concurrent treatment with a moderate CYP3A inhibitor (ie, ciprofloxacin, diltiazem). Strong CYP3A inhibitors (ie, most azoles, grapefruit juice) or inducers (ie, rifampin) should be avoided. Additionally, venetoclax requires 50% dose reduction and dosing 6 hours after a P-gp inhibitor. Carvedilol, amiodarone, and verapamil are P-gp inhibitors that are commonly prescribed for patients with cardiac arrhythmia. The use of venetoclax in patients with cardiovascular comorbidities requires a comprehensive review of concurrent medications and adjustment of the dose and timing of venetoclax.
CD20 mAbs and BTKis abrogate humoral and cellular response to vaccination and increase the risk of serious coronavirus disease 2019 (COVID-19) infection.32,33 Patients should be advised to receive at least 1 dose of messenger RNA COVID-19 vaccine before treatment initiation. The US Centers for Disease Control and Prevention recommends that immunocompromised adults receive up to 5 doses of the COVID-19 vaccine. In addition, tixagevimab-cilgavimab (Evusheld; AstraZeneca) is available in the United States for preexposure prophylaxis of immunocompromised patients under Emergency Use Authorization. Patients with CLL who have mild to moderate COVID-19 infection can receive antiviral treatment with an oral (nirmatrelvir/ritonavir or Paxlovid [Pfizer]) or intravenous (remdesivir or Veklury [Gilead]) agent. Of these, Paxlovid is a CYP3A inhibitor, and the patient should have stable enough disease to be able to hold BTKis or venetoclax during antiviral therapy. When it is unsafe to hold treatment, bebtelovimab is an alternative treatment for mild to moderate COVID-19.
Novel combinations
Attainment of uMRD at the end of fixed-duration therapy has a prognostic impact in CLL and is associated with prolonged PFS after VO.16 An area of active investigation is whether novel combinations, often coupled with MRD-guided treatment cessation, can induce higher rates of uMRD and ultimately improve outcomes (Table 2). Initial results from studies testing the combination of a BTKi and venetoclax ± CD20 mAb reported high rates of uMRD in blood and bone marrow compartments. The all-oral, doublet combination of ibrutinib plus venetoclax (IV) has been tested in TN CLL. A single-center phase 2 study conducted at MD Anderson enrolled patients with unfavorable prognostic markers or older age (≥65 years).34 The GLOW study is a phase 3 study in patients with older age (≥65 years) or comorbidities.35 The CAPTIVATE study is a phase 2 study in younger patients (median age 60 years) with treatment arms for fixed-duration therapy (15 cycles) and MRD-driven therapy (15 cycles + continuous therapy based on randomization related to MRD status).36,37 The rate of best bone marrow uMRD (BM-uMRD) was 52% to 66% in these trials, comparable to the 57% rate reported from the VO arm of the CLL14 study.16 Older age and comorbidities appear to be associated with increased rates of cardiovascular toxicities during treatment with IV. In the GLOW study, 14% had atrial fibrillation of any grade and 7% (6 patients) had cardiac or sudden deaths,35 which were higher than the rates reported from the CAPTIVATE study with younger patients (4% atrial fibrillation, <1% or 1 patient with sudden death).36
The rate of BM-uMRD ranges between 66% and 89% when CD20 mAb is added to the time-limited combinations of venetoclax and a BTKi, which has been tested with ibrutinib, acalabrutinib, and zanubrutinib.38–41 A similarly high rate of BM-uMRD was observed in patients with TP53 aberration, as demonstrated by the CLL2-GIVe study, which exclusively enrolled 41 patients with deletion 17p (66% BM-uMRD with IV plus obinutuzumab [IVO])39 and by subgroup analyses of individual studies (ie, 92% BM-uMRD in 10 patients with TP53 aberration treated with acalabrutinib, venetoclax, and obinutuzumab [AVO]).40
The addition of ibrutinib to VO may not substantially improve the depth of response while it adds to toxicity. In the CLL13 study, VO-containing arms of the study achieved the highest rates of BM-uMRD with a minimal difference between VO (73%) and IVO (78%).12 Three-year PFS was similar in the 2 VO-containing arms (88% for VO, 91% for IVO). The IVO arm of the study did have higher rates of adverse events compared with other arms, including cytopenia, infection, and cardiovascular toxicity. The rate of cardiovascular toxicity is lowered by using a selective BTKi; only 3% of the patients treated with AVO40 or zanubrutinib plus VO41 developed atrial fibrillation in single-arm studies.
While none of the novel combinations have been approved by the US Food and Drug Administration, existing data support that targeted combinations can induce deep response in over half of patients with CLL, including those with TP53 aberration. Critical to the application of these regimens in practice is a better understanding of the comparative risk of novel combinations and their long-term benefit. Specifically, can we achieve durable treatment-free remission and cure at least a subset of patients with treatment intensification? Two randomized phase 3 studies with identical treatment arms (ibrutinib and obinutuzumab, IV, IVO) will compare the efficacy of ibrutinib-based combinations in older (NCT03737981: Alliance A041720) and younger (NCT03701282: ECOG E9161) patients. A phase 3 study incorporating acalabrutinib in place of ibrutinib has completed accrual (NCT03836261: acalabrutinib plus venetoclax vs AVO vs FCR). To identify the best partner for venetoclax, 2 studies will compare the doublets of venetoclax plus a BTKi or obinutuzumab (NCT04608318, the CLL17 study for ibrutinib vs VO vs IV; NCT05057494, the MAJIC study for acalabrutinib plus venetoclax vs VO). To determine the impact of a BTKi in generating durable response in high-risk CLL, the CLL16 study will compare VO vs AVO specifically in patients with TP53 aberration or complex karyotype (NCT05197192). Ongoing clinical trials and longer follow-up will be required to understand the characteristics of patients who would benefit from novel combinations.
CLINICAL CASE (Continued)
The selection of initial treatment for CLL is an individualized decision with consideration of patient-, treatment-, and disease- related factors. For the clinical case herein, we would propose treatment with either a BTKi or VO while avoiding CIT for the patient with deletion 11q and U-IGHV. VO is not associated with cardiovascular toxicity and can be discontinued after 1 year, which may be appealing for patients who prefer to avoid indefinite therapy. Continuous BTKi therapy is also a reasonable choice, as long as a selective BTKi rather than ibrutinib is used given the patient's age and cardiovascular comorbidities. Aspirin and direct oral anticoagulant are permitted during treatment with either a BTKi or VO. Carvedilol is a P-gp inhibitor, which would require a 50% dose reduction of venetoclax and to be taken 6 hours before venetoclax due to drug-drug interactions.
Conflict-of-interest disclosure
Inhye E. Ahn has no relevant conflict of interest.
Jennifer R. Brown has served as a consultant for Abbvie, Acerta/Astra-Zeneca, BeiGene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Eli Lilly, Genentech/Roche, Hutchmed, iOnctura, Janssen, MEI Pharma, Morphosys AG, Novartis, and Pharmacyclics and received research funding from BeiGene, Gilead, Loxo/Lilly, MEI Pharma, SecuraBio, Sun, and TG Therapeutics.
Off-label drug use
The current article discusses off-label uses of zanubrutinib for the treatment of CLL and targeted combination therapy with a BTK inhibitor and venetoclax with or without an anti-CD20 monoclonal antibody.