Abstract
This article reviews 1) the use of gene transfer methods to genetically manipulate hematopoietic stem cell targets, 2) recent advances in technology that are addressing problems that have prevented widespread successful translation of gene transfer approaches for the cure of disease, and 3) recent regulatory issues related to human gene therapy trials.
In Section I, Dr. Nienhuis describes the use of alternative viral envelopes and vector systems to improve efficiency of transduction of hematopoietic stem cells. Major limitations of stem cell transduction are related to low levels of viral receptors on the stem cells of large animal species and the low frequency of cycling stem cells in the bone marrow. Attempts to circumvent these limitations by exploiting non-oncoretroviral vectors and pseudotyping of Moloney vectors with alternative envelopes are discussed.
In Section II, Dr. Hawley addresses new strategies to improve the expression of transgenes in cells derived from long-term reconstituting hematopoietic stem cells. Transgene silencing in transduced hematopoietic stem cells remains an obstacle to gene therapy for some gene sequences. New generations of retroviral backbones designed to both improve expression and reduce silencing in primary cells are explored.
In Section III, Drs. Smith and Cornetta update regulatory issues related to human gene therapy trials. Increased scrutiny of human trials has led to changes in requirements and shifts in emphasis of existing regulations, which apply to human gene therapy trials. The current Food and Drug Administration's structure and regulations and the roles of the Recombinant DNA Advisory Committee of the NIH and other sponsors and partners in gene therapy trials are reviewed.
I. Gene Transfer into Hematopoietic Stem Cells
Arthur W. Nienhuis, M.D.**
St. Jude Children's Research Hospital, University of Tennessee, 332 North Lauderdale, Memphis TN 38105
The ability to achieve efficient gene transfer into human repopulating hematopoietic stem cells and to achieve lineage-specific expression would create many therapeutic opportunities.1 Many single-gene disorders, e.g. the hemoglobinopathies, the thalassemias, chronic granulomatous disease and various immunodeficiencies, affect derivatives of the hematopoietic stem cell. These diseases could, in principle, be cured by gene replacement. The results of studies in relevant murine models with oncoretroviral vectors strongly support the development of gene transfer into stem cells as an effective therapeutic approach for treatment of human diseases.2,3,4,5 Initial attempts to apply the methodology that has been used to successfully insert genes into murine stem cells in larger animal models or in human trials have been unsuccessful. This chapter will outline various strategies that have subsequently been pursued to overcome the barriers to human stem cell targeted gene transfer.
Oncoretroviral Vectors
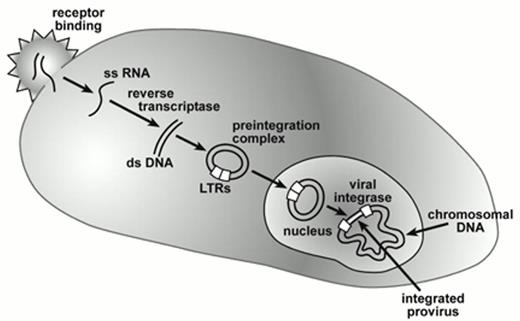
The methodology for developing oncoretroviral vector producer clones that generate 105-106 infectious vector particles/ml has been well established.6 Substantial experience in animal studies and clinical trials indicate that oncoretroviral vectors can be used safely to transduce human hematopoietic cells. However, there are several major barriers to successful transduction of primate hematopoietic stem cells with oncoretroviral vector particles (Figure 1). Particle uptake is initiated by the interaction of the viral envelope protein with cell surface molecules that act as a receptor.7 The receptor used by amphotropic vector particles, a sodium-dependent phosphate transporter, is expressed at a low level8 that is likely to be rate limiting, based on experimental studies relating receptor density to transduction efficiency.9 The generally quiescent nature of stem cells,10 most being in G0, also fails to provide an environment conducive for reverse transcription and vector integration.11 The oncoretroviral preintegration complex is quite unstable and cannot traverse the nuclear membrane12,13 so that cell division is required within several hours of vector uptake for successful integration. The cytokine mixtures used in early gene transfer studies may not have effectively initiated cell cycle progression among primitive hematopoietic cell populations.
Gene transfer by retroviral vectors.
Vector and cellular interactions are mediated by the viral envelope and cell surface receptors after which the diploid RNA genome is released into the cytoplasm as single-stranded (ss) RNA. Conversion into double-stranded complimentary DNA occurs through action of viral reverse transcriptase leading to formation of a circular double-stranded DNA intermediate as part of the pre-integration complex. This pre-integration complex reaches the nucleus either during mitosis in the case of oncoretroviral vectors or by direct nuclear transmigration in the case of lentiviral vectors. Subsequent integration of the proviral genome into chromosomal DNA is mediated by viral integrase. The long terminal repeats (LTRs) that contain transcriptional control and RNA processing signals, flank the integrated vector encoded genes and modulate their expression.
Abbreviations: LTRs, long terminal repeats
Gene transfer by retroviral vectors.
Vector and cellular interactions are mediated by the viral envelope and cell surface receptors after which the diploid RNA genome is released into the cytoplasm as single-stranded (ss) RNA. Conversion into double-stranded complimentary DNA occurs through action of viral reverse transcriptase leading to formation of a circular double-stranded DNA intermediate as part of the pre-integration complex. This pre-integration complex reaches the nucleus either during mitosis in the case of oncoretroviral vectors or by direct nuclear transmigration in the case of lentiviral vectors. Subsequent integration of the proviral genome into chromosomal DNA is mediated by viral integrase. The long terminal repeats (LTRs) that contain transcriptional control and RNA processing signals, flank the integrated vector encoded genes and modulate their expression.
Abbreviations: LTRs, long terminal repeats
A relevant question, given these barriers to primate stem cell transduction, is why gene transfer into murine stem cells can be so readily accomplished? The receptor used by ecotropic vector particles, a cationic amino acid transporter, is expressed at higher levels than the receptor utilized by amphotropic vectors.8 A polymorphic variant of this amino acid transporter, expressed in humans, does not bind ecotropic viruses. Murine bone marrow cells that serve as a target for retroviral-mediated gene transfer are typically harvested after administration of 5-fluoruracil (5-FU). The drug, when given 48 hours before marrow harvest from syngeneic donors, depletes the bone marrow of cycling cells and initiates activation of otherwise quiescent stem cells.14 This strategy increases murine stem-cell transduction 10-fold but has not been developed for effective use in other species. Co-culture of murine bone marrow with vector-producing cells increases transduction efficiency severalfold but allows recovery of only approximately 25% of the stem cells.14 These non-adherent stem cells may be the mitotically active subset that is most amenable to retroviral-mediated gene transfer. Substantial loss of stem cells is acceptable experimentally in the murine system since the use of additional syngeneic donor animals compensates for cell loss. In larger animals and humans, quantitative recovery of limiting numbers of autologous stem cells becomes a critical factor in experimental design. Murine repopulating cells apparently respond to the mixture of cytokines, including stem cell factor (SCF), interleukin-3 (IL-3), and interleukin-6 (IL-6) used in transduction protocols. Quantitative estimates obtained by using a competitive repopulation assay suggest that murine stem cells double in number during the two days of prestimulation and two days of co-culture with retroviral vector-producing cells.14 Greater expansion has been documented in serum-free medium containing SCF, Flk-3 ligand and thrombopoietin.15 A comparison of failed primate gene transduction protocols to successful murine transduction protocols has identified a number of important variables to manipulate in an effort to optimize oncoretroviral-mediated gene transfer into primate stem cells.
Optimization of Oncoretroviral Vector-Mediated Gene Transfer into Primate Stem Cells
Cytokine mobilization
In seeking an alternative to the use of 5-FU, Bodine and colleagues explored the use of cytokine administration prior to the marrow harvest. SCF and granulocyte-colony stimulating factor (G-CSF) were administered to donor mice for five days, resulting in mobilization of stem cells into the peripheral blood.16 These cytokine-mobilized peripheral blood stem cells were more efficiently transduced than post-5-FU bone marrow stem cells.17 The use of G-CSF- and SCF-mobilized CD34+ cells from peripheral blood of nonhuman primates has resulted in more successful transduction of stem cells with amphotropic vector particles.18 Recent studies documented that G-CSF-mobilized peripheral blood CD34+ cells from normal human volunteers express higher levels of the amphotropic receptor mRNA and have a greater proportion of cells in the G1 phase of the cell cycle compared to CD34+ cells from bone marrow,10 even though human mobilized primitive hematopoietic cells remain difficult to transduce.
Cytokine stimulation
Primitive human hematopoietic cells as defined by surface antigens undergo self-renewal divisions while retaining repopulating potential when stem cell factor and Flt-3 ligand are used in high concentration along with one or more additional cytokines including IL-3, IL-6, G-CSF or thrombopoietin.19,20 A period of pre-stimulation enhances retroviral-mediated transduction of primitive hematopoietic cells since many such cells do not cycle until after 48 hours in culture.21 Loss of homing potential has been reported for cytokine-stimulated primitive human hematopoietic cells assayed in immunodeficient mice.22,23,24 A 48-hour period of incubation in SCF may facilitate restoration of the homing potential of successfully transduced cells.25
Stromal support
Co-culture with bone marrow stromal cells has been reported to enhance retroviral-mediated transduction of primitive human hematopoietic cells.26 The discovery that extracellular matrix molecules, specifically fibronectin, enhance gene transfer provided a much more feasible strategy than the use of whole stromal cells.27,28 Fibronectin fragments appear to bind both the vector particles and target cells; co-localization apparently enhances transduction efficiency.29 Furthermore, the interaction of primitive hematopoietic cells with fibronectin may provide a growth-promoting signal.30,31 The appropriate fragment, CH-296, is available commercially as RetroNectin™ and strategies have been devised to incorporate the fibronectin fragment, retronectin, into high-volume systems for clinical gene transfer protocols.32
Summary
The use of cytokine-mobilized peripheral blood cells as targets for gene transfer, culture in complex cytokine mixtures including high concentrations of SCF and Flt-3 ligand, the use of a pre-stimulation period before exposure to vector particles, and multiple exposures to conditioned medium containing vector particles in Retronectin™-coated culture plates have generally improved transduction of stem cells with amphotropic vector particles. When these manipulations are used, approximately 25% of the human hematopoietic cells in non-obese diabetic/severe combined immunodeficient (NOD/SCID) murine recipients of transduced, mobilized peripheral blood cells were genetically modified.33 More efficient gene transfer into repopulating cells has been achieved in nonhuman primates34,35,36,37 as reflected by percentages of genetically modified peripheral blood cells ranging from 12-47%, at least transiently. Recent studies documented that many multipotential gene-marked stem cell clones contribute to hematopoiesis in nonhuman primates transplanted under these conditions.38 Approximately 5-10% of bone marrow progenitors contained the retroviral vector genome in such animals.37 Finally, a recent study has reported successful transduction of human repopulating cells with long-term marking of progenitor cells at levels of 5-10% for up to a year in several patients.39 The first successful use of stem cell-targeted gene transfer to treat a human disease, severe combined immune deficiency, has been reported using similar conditions of transduction.40
Pseudotyping of Oncoretroviral Vector Particles with Alternative Envelope Proteins
The use of alternative envelope proteins (“pseudotyping”) for vector particles has been a frequent approach to improve transduction efficiency.
Gibbon ape leukemia virus envelope protein
The receptor for the gibbon ape leukemia virus (GALV), Glvr1, is a sodium-dependent phosphate transporter that is closely related to the receptor for amphotropic viruses.41 However, the two proteins have different patterns of expression, and many hematopoietic cells express higher levels of Glvr1 than Ram1, the receptor for amphotropic vector particles.42 GALV-pseudotyped particles are more efficient than amphotropic particles at transducing human cells capable of establishing hematopoiesis in immunodeficient mice43,44 and stem cells from baboons.45 Subsequent studies in the baboon model using GALV-pseudotyped vector particles in optimized culture with Retronectin™-coated plates resulted in higher efficiencies of gene transfer into stem cells as reflected by up to 20% of genetically modified peripheral blood and bone marrow cells for more than 20 weeks post engraftment.46 These results led to the prediction that substitution of GALV-pseudotyped vector particles for amphotropic particles in human stem cell-targeted transduction protocols should result in improved efficiency of gene transfer.
Vesicular stomatitis virus-G envelope protein
The use of this vector has been studied extensively.47,48,49 Although vesidular stomatitis virus-G (VSV-G) pseudotyped particles are quite stable and can be concentrated to titers of 109 infectious particles/ml, transduction of progenitor cells with VSV- or amphotropic envelope pseudotyped vectors were comparable.50 Attempts to utilize VSV-G-pseudotyped particles to transduce human primitive hematopoietic cells resulted in marking of 25% of the progenitors in transplanted immunodeficient murine animals, but the conditions of transduction were toxic to repopulating cells.51 Other investigators have found that CD34+CD38- cells are poorly transduced with VSV-G-pseudotyped particles.52 Overall, despite vector stability and high titers, VSV-G-pseudotyped oncoretroviral vector particles seem to afford no advantage in transducing primitive primate hematopoietic cells. Indeed, the near universal use of the VSV-G protein to pseudotype lentiviral vector particles for transduction of human hematopoietic cells may have resulted in an underestimate of their potential for this purpose (see below).
RD114 envelope protein
The feline endogenous retrovirus, RD114, utilizes a neutral amino acid transporter as its receptor.53 This receptor is expressed at higher levels on primitive human hematopoietic cells than the phosphate transporters that serve as receptors for amphotropic or GALV-pseudotyped vector particles (D. Bodine, personal communication). Oncoretroviral vector particles pseudotyped with the RD114 envelope protein transduce T-lymphocytes more efficiently than either amphotropic or GALV- pseudotyped vector particles.54 The cells in the cord blood CD34+ population that are capable of establishing human hematopoiesis in immunodeficient murine mice are efficiently transduced by RD114-pseudotyped vector particles as reflected by the presence of the proviral genome in as many as 90% of the myeloid and lymphoid cells in transplant recipients.55 The human sarcoma cells (HT1080) used to derive a packaging cell line for RD114-pseudotyped particles produce a substance(s) that depletes immunodeficient murine repopulating cells. This undesirable effect could largely be avoided by preloading RD114 particles onto Retronectin™-coated plates. Even with a single exposure to preloaded vector particles, transduction of immunodeficient murine repopulating cells was far higher than that achieved with multiple exposures to amphotropic vector particles.55 Initial results in the rhesus autologous transplant model suggest high-efficiency transduction of repopulating cells,56 although derivation of a new producer clone that is free of the differentiation-inducing factor present in HT1080 cell-conditioned media will undoubtedly be necessary to fully evaluate RD114-pseudotyped oncoretroviral vector particles in larger animal models and humans.
Other strategies
Simultaneous transduction with vectors targeting both the amphotropic and GALV receptors resulted in higher efficiencies of gene transfer than with either vector alone in human CD34+ cells.57 Only the cost and effort required to derive preparations of vector particles that can be certified for clinical use limit this approach to improving transduction of human repopulating stem cells. Another strategy utilizes the incorporation of cytokine peptide sequences into envelope protein molecules. For example, chimeric envelopes harboring interleukin-2 (IL-2) peptide sequences were more efficient at transducing IL-2-dependent cells than vector particles with only the amphotropic envelope protein. The chimeric envelope protein may both enhance binding to specific target populations and activate the target cells via cytokine-mediated receptor signaling.58
Summary
GALV-pseudotyped vector particles are more efficient at transducing primate repopulating hematopoietic stem cells than are conventional amphotropic vector particles. Accordingly, GALV-pseudotyped vector particles are likely to be preferred for future human gene therapy trials involving gene transfer into bone marrow cells. The high efficiency of gene transfer into cord blood cells achieved with RD114-pseudotyped vector particles seems encouraging. More effort will be required to fully evaluate and potentially exploit this novel envelope protein for human gene therapy. Available data do not support the use of VSV-G-pseudotyped oncoretroviral vector particles for transduction of primitive human hematopoietic cells and call into question the use of this envelope protein to pseudotype lentiviral vector particles for that purpose. Additional envelope proteins, e.g. those for the Mus dunni59 and 10A1 viruses,60 have yielded interesting preliminary data with respect to transduction of various hematopoietic target populations and should be studied further.
Alternative Vector Systems
Lentiviral vectors
Lentiviruses such as the human immunodeficiency virus (HIV), are retroviruses with a similar life cycle to those of murine oncoretroviruses. These viruses have two potential inherent biological advantages over oncoretroviruses for the infection of quiescent cells. The pre-integration DNA protein complex is more stable than that of oncoretroviruses, allowing its persistence in quiescent cells and subsequent integration upon resumption of cell cycling.13 Furthermore, the preintegration complex possesses nuclear transport signals in several proteins, which facilitates its translocation through the nuclear membrane. Stable transduction of non-dividing cells by lentiviral vectors has been documented.13 HIV vectors have been modified to eliminate accessory proteins involved in HIV pathogenesis and to improve safety.61,62 Packaging lines have been developed that, in principle, could be used for derivation of producer clones.63
Direct comparisons between oncoretroviral and lentiviral vectors pseudotyped with the VSV-G protein at comparable multiplicities of infection (MOI) demonstrated significant superiority of the HIV-1-based vector.64,65 Similarly, lentiviral vectors pseudotyped with VSV-G were far superior at transducing cord blood, immunodeficient murine repopulating cells than comparable oncoretroviral vector particles during short-term culture in the absence of cytokines.66 However, the frequency of genetically modified human hematopoietic cells in transplant recipients was significantly lower than that achieved with GALV- or RD114-pseudotyped vector particles under conditions optimized for these vectors.43,44, 55 The initial results obtained in the nonhuman primate model with VSV-G-pseudotyped, lentiviral vector particles were also unimpressive; although marker expression was detected in multiple lineages, only 1-2% of mononuclear cells expressed the lentiviral vector-encoded gene.67
As noted above, the VSV-G envelope protein may not be the optimal pseudotype for lentiviral vector-mediated gene transfer into primitive hematopoietic cells. Furthermore, recent results clearly demonstrate that cell cycle progression is required for lentiviral integration.68 Indeed, available evidence indicates that transduction efficiency of primitive human hematopoietic cells is severalfold greater once the cell exits G0 and enters G1.69 Optimization of transduction conditions and the use of alternative pseudotyped vector particles will undoubtedly be necessary to fully evaluate the potential of lentiviral vectors for transduction of human stem cells.
Foamy virus
Human foamy virus appears to be non-pathogenic and has a broad host range.70 The LTR depends on a virally encoded trans-activator, Bell, which can be provided in trans during virus production so that the long terminal repeat (LTR) is silent in target cells that have been successfully transduced with vector particles. Foamy virus may be less dependent on cell division than oncoretroviruses, although this point has not yet been definitively established.71 Foamy virus vectors are able to transduce human hematopoietic cells,72 and helper-free virus stocks have recently been used to transduce murine stem cells, with resulting multilineage engraftment in transplant recipients.73 This vector system continues to hold promise for gene transfer into hematopoietic stem cells.
Adeno-associated virus vectors
Adeno-associated virus (AAV) is a replication-defective parvovirus that depends on a helper virus, either adenovirus or herpes virus, for virus production during lytic infection.74 AAV has a single-stranded DNA genome of 4680 nucleotides, which includes 145 base pair inverted terminal repeats (ITRs), which are the only viral elements required for vector transmission. Recent progress in the production of recombinant (rAAV) vectors has yielded preparations free of both helper adenovirus and wild-type AAV.75,76 Transduction of non-dividing cells in liver, brain and muscle with rAAV has been well documented.77,78,79 In livers of animals receiving rAAV via the portal vein, uniform transduction of hepatocytes is observed, although only 5-6% of cells ultimately come to contain integrated rAAV sequences.80 The process of rAAV integration occurs over 2-3 weeks and involves annealing of complimentary single-stranded rAAV genomes leading to episomal gene-expressive, rate-limiting formation of a dimer and then concatamerization, resulting in integration of multiple genomes linked head to tail.81,82
Our growing understanding of the biology of rAAV is consistent with the fact that this vector system no longer appears to hold promise for stem cell-targeted gene transfer in humans. A very high ratio of viral particles to target cells is required,83 presumably because of low levels of expression of the AAV receptor, heparan sulfate proteoglycan.84 Transient rAAV-mediated gene expression in the absence of genome integration has been observed, as is predictable for episomes in rapidly proliferating cell populations.83 Sequential transduction of primary primitive hematopoietic cells with a rAAV vector encoding the ecotropic receptor followed by transduction with an ecotropic retrovirus results in integration of the retroviral genome in the context of transient expression of the rAAV-encoded sequences.85 Reports of long-term persistence and expression of rAAV-encoded genes in murine and human hematopoietic cells86,87 have yet to be confirmed and are difficult to explain in the context of the emerging biology of rAAV.
Non-integrating DNA viruses
Adenoviral vectors have also been used to transduce primitive human hematopoietic cells. Sequential transduction can be used to transiently express functional proteins,88 but adenovirus is toxic to human hematopoietic cells.89 SV40 vectors have been reported to transduce human hematopoietic cells very efficiently,90 but the lack of a mechanism for genome integration would seem to limit the usefulness of this vector system for gene therapy applications.
Summary
Consistent efforts to adapt amphotropic oncoretroviral vectors for transduction of human hematopoietic stem cells has resulted in gradually improving efficiencies in animal models and the first successful use of such vectors for human gene therapy. Pseudotyping of oncoretroviral vectors with alternative envelope proteins, particularly those from the GALV or RD114, appear to result in higher efficiencies of gene transfer into primitive primate hematopoietic cells than conventional amphotropic vector particles. Lentiviral vectors have inherent biological advantages over oncoretroviral vectors for transducing non-dividing cells, namely the stability of the pre-integration complex and its ability to transverse the nuclear membrane. Translation of these theoretical advantages into improved stem cell transduction frequencies has yet to be realized and may require pseudotyping with envelope proteins other than VSV-G and use of cytokine mixtures that activate stem cells into the G1 phase of the cell cycle. Overall progress in the past 5 years has enhanced the probability that stem cell-targeted gene transfer will ultimately become a useful and widely applied therapeutic modality.
II. Vector Designs for Stem Cell Expression
Robert G. Hawley, Ph.D.*
Hematopoiesis Department, Jerome H. Holland Laboratory for the Biomedical Sciences, American Red Cross, 151601 Crabbs Branch Way, Rockville MD 20855-2743
Advanced Oncoretroviral Vector Systems
Although there were some encouraging exceptions, until recently the results of the majority of the human hematopoietic stem cell (HSC) gene therapy trials conducted with oncoretroviral vectors had been disappointing, paralleling the limited success achieved in preclinical gene transfer studies in nonhuman primates and with human HSCs in xenogeneic transplant assays.1 However, new advances in retroviral gene transduction methodology that have led to significant improvements in gene transfer efficiencies in animal transplant models have culminated of late in the successful treatment by gene therapy of four patients with X-linked severe combined immunodeficiency disease (SCID-X1).2 SCID-X1 disease is a fatal disorder characterized by genetic defects in the common cytokine receptor gamma chain (γc) for interleukin-2, -4, -7 -9, and -15 that lead to a block in T cell and natural killer cell development. Reinfusion into the SCID-X1 patients of CD34+ bone marrow cells that had been transduced with an MFG oncoretroviral vector expressing the wild-type γc gene corrected the disease phenotype, presumably by restoring cytokine growth and survival signals to early lymphoid progenitors. This progress can be attributed to key modifications of earlier transduction protocols: the inclusion of flt3 ligand to cytokine cocktails, which helps preserve the long-term hematopoietic repopulating ability of HSCs during the ex vivo transduction procedure;3 and the use of certain fibronectin fragments, which co-localize vector particles and cells while stimulating HSC survival and/or proliferation.4 Another development that is anticipated to contribute to improved clinical gene transfer efficacy is the generation of vector particles pseudotyped with envelope proteins from the GALV or the feline endogenous virus RD114, both of which appear to be more efficient than amphotropic vector particles for transduction of primate and human HSCs (see above).5,6,7 Efficient transduction of candidate human HSCs with oncoretroviral vectors pseudotyped with the VSV-G envelope protein has also been reported.8
Despite the aforementioned marked improvements in HSC gene transfer efficiency, a second potential obstacle to effective gene therapy using HSCs, which has repeatedly been observed in preclinical studies with standard oncoretroviral vectors based on Moloney murine leukemia virus (MoMLV), is positional variegation and extinction of transgene expression.9 Indeed, position effects and transgene silencing have been found to occur even after ex vivo preselection of transgene-expressing HSCs.10,11 For this reason, it will be of great interest to learn whether the MoMLV-based MFG vector expressing a γc cytokine receptor transgene will be subject to transcriptional down-regulation during long-term follow up of the SCID-X1 patients who received transduced CD34+ bone marrow cells.2 The MFG vector was designed to achieve high level transgene expression by controlled splicing of vector transcripts, but transcription is driven by an unmodified MoMLV LTR.12 Using a serial bone marrow transplantation assay in lethally irradiated mice, Kohn and colleagues documented a high rate of MoMLV vector expression failure in secondary recipients concomitant with methylation of the LTR.13 Essentially identical frequencies of silencing were observed irrespective of whether the MFG vector backbone or another MoMLV-based vector backbone (N2) was employed.14
Non-function of the enhancer as well as negative regulatory factors that bind to the LTR and the 5′-untranslated region have been implicated as the control mechanisms responsible for restriction of expression of MoMLV-based vectors in murine embryonal carcinoma (EC) cells and embryonic stem (ES) cells.15,16,17,18,19,20,21,22 The silencers identified include the CGCCATTTT and TCAAGGTCA elements at the 5′ end of the LTR and the retroviral tRNA primer binding site, TGGGGGCTCGTCCGGGAT, in the 5′-untranslated region. The CGCCATTTT element, the core motif of an upstream conserved region present in over 90% of mammalian type C oncoretrovirus isolates (originally referred to as the negative control region), is bound by the YY-1 (Yin-Yang 1) repressor/activator, a member of the GLI-Krüppel zinc finger transcription factor family.15,20 Transcriptional repression by YY-1 may be mediated by interaction with a chromatin-modifying histone deacetylase complex targeted to methylated CpG (cytosine-guanine) dinucleotides within the promoter region.23 The TCAAGGTCA element is bound by a factor originally termed embryonal LTR-binding protein (ELP), which was subsequently demonstrated to be an isoform of the murine SF-1 protein, an orphan member of the nuclear steroid receptor superfamily.21,24 Whereas ELP functions as a repressor in embryonal cells, alternatively spliced murine SF-1 transcripts encode positive regulators of gene expression in EC and steroidogenic cells.25 Wild-type MoMLV uses a cellular tRNAPro to prime synthesis of the first segment of the minus DNA strand during reverse transcription. The tRNAPro primer-binding site region contains a silencer element that suppresses MoMLV expression in EC and ES cells and directs the methylation of viral and adjacent cellular DNA.17,26 A factor (factor A) that binds to a consensus GGRGGCTCGTYYGGGAT sequence, which overlaps with 17 of the 18 nucleotides of the tRNAPro primer-binding site, has been identified but not molecularly characterized.22 Besides abrogation of these negative regulatory mechanisms, transcriptional inactivity of the MoMLV LTR in murine embryonal cells can be circumvented by insertion of an active enhancer region.27
The murine embryonic stem cell virus (MESV) created by Ostertag and colleagues contains modifications in several of the above cis-acting elements that allow effective transgene expression in murine EC and ES cells.28 Specifically, MESV harbors a point mutation in the LTR at position -345 with respect to the transcriptional start site that destroyed the ELP-binding site29 and another point mutation in the enhancer region at position -166 that created a functional binding site for the transcription factor Sp1 (GGGCGG), which was shown to activate the MoMLV LTR in EC and ES cells.18,19 The MESV vector also possesses a 5′ untranslated region from the dl587 rev virus containing a primer binding site for tRNAGln instead of tRNAPro, which removed the factor A repressor binding site.30 Hypothesizing that mechanisms similar to those responsible for the restriction of expression of MoMLV-based vectors in undifferentiated murine EC and ES cells might be operative in HSCs, Hawley and colleagues derived the murine stem cell virus (MSCV) retroviral vector from MESV and their HMB vector, which had previously been shown to direct stable gene expression in these cell types from an internal murine phosphoglycerate kinase (pgk) promoter.31,32 A number of investigators have confirmed that the MSCV retroviral vector system is highly efficient at expressing transgenes in murine HSCs.33,34,35,36,37 This group subsequently created safety-modified versions of the basic MSCV backbone for potential use in human HSC gene therapy protocols.38,39 Preclinical studies have documented sustained MSCV-directed transgene expression following transplantation of transduced human CD34+ hematopoietic precursors into sublethally irradiated NOD/SCID mice and SCID-human (SCID-hu) chimeric mice.6,40,41,42,43 Stable in vivo expression from an MSCV-based vector in T and B lymphocytes for as long as 35 weeks was also observed following engraftment of rhesus macaques with transduced peripheral blood CD34+ cells.44 On the basis of these results, the MSCV vector platform is being tested in two phase I gene therapy trials at the Indiana University School of Medicine. The intent of one trial is to insert the O6-methylguanine DNA methyltransferase (MGMT) gene into peripheral blood CD34+ cells transplanted into patients with brain tumors as a prelude to future therapeutic studies designed to increase the dose of the chemotherapy regimen.45 The MGMT protein repairs DNA damage caused by the nitrosourea-type alkylating agents that are commonly used to treat this type of cancer. A major side effect limiting the use of these drugs is severe myelosuppression due to bone marrow sensitivity. Preclinical studies in mice suggest that overexpression of MGMT in hematopoietic progenitor and precursor cells may provide multilineage protection against the chemotherapy-induced toxicity.45 The second trial involves patients with X-linked chronic granulomatous disease (X-CGD).46 X-CGD patients have an inherited disease of host defense in which the generation of superoxide by the respiratory burst oxidase of phagocytic leukocytes is absent or markedly deficient. As a result, they are susceptible to life-threatening bacterial and fungal infections. The study addresses the safety of retroviral-mediated gene transfer of a functional version of the defective gene, gp91phox, into peripheral blood CD34+ cells, with the eventual goal being to restore respiratory burst oxidase activity.
Other groups have also made MoMLV oncoretroviral vectors with mutant LTRs and variant 5′-untranslated regions.47,48,49 Kohn and colleagues created the vector MND (myeloproliferative sarcoma virus [MPSV] enhancer, negative control region deleted, dl587rev primer-binding site substituted) and observed a higher frequency of expression in the reconstituted hematopoietic systems of mice compared with a standard MoMLV-based vector.50 Like the MESV/MSCV LTR, the MND LTR contains an activating Spl-binding site in the enhancer region due to a point mutation at position -166. It differs slightly from the former in that the ELP-binding site has been retained while the YY-1-binding site has been deleted. The MPSV/MND LTR enhancer is generally stronger than the MESV/MSCV LTR enhancer, which is from PCC4 EC cell-passaged MPSV and contains only one direct repeat element,29 although it may not function more efficiently in HSCs.37,51 Ostertag and colleagues have gone on to combine the U3 regions of the LTRs from other retroviruses with the ES cell-permissive dl587rev primer-binding site to create hybrid retroviral vectors, which they postulate may express better in HSCs than the MSCV or MND vectors.49,52 Their FMEV vector backbone, incorporating the U3 region from the polycythemic strain of the spleen focus-forming virus, is especially noteworthy in that it contains an altered enhancer core and Sp1-activating mutations that disrupt the ELP-binding site.53 Xenogeneic transplant experiments in NOD/SCID mice of human CD34+ cells transduced with GALV-pseudotyped FMEV-derived vectors have demonstrated persistence of transgene-expressing cells for up to 120 days post engraftment.11,54
Multiply Attenuated Lentiviral Vector Systems
Because of their ability to transduce nondivided cells,55 lentiviral vectors are promising tools for ex vivo genetic modification of HSCs, which reside almost exclusively in the G0/G1 phase of the cell cycle.56,57 VSV-G-pseudotyped HIV-1-based vectors have been shown to readily introduce exogenous genes into human hematopoietic progenitors detectable in clonal culture assays58,59,60,61,62 as well as into more primitive precursors capable of regenerating lymphomyelopoiesis in NOD/SCID mice63 and thymopoiesis in the SCID-hu thymus-liver mouse model.64 Current generation lentiviral vectors contain a 400-bp deletion of enhancer and promoter sequences in the U3 region of the 3′ HIV-1 LTR, which, after transfer to the 5′ LTR by reverse transcription, yields a `self-inactivated' proviral form incapable of synthesizing vector transcripts.65 In addition to improving the biosafety of lentiviral-mediated gene delivery, removal of these LTR sequences eliminates the possibility that they might negatively influence the transcriptional activity of the internal promoter driving transgene expression.66,67 While the utility of HIV-1-based lentiviral vectors for gene transfer into human HSC subsets has been established, the majority of the lentiviral vectors presently in use are not optimized for transgene expression in HSCs. This is because the internal promoter typically utilized, the human cytomegalovirus immediate early enhancer-promoter, does not perform well in hematopoietic cells.63,64,68 For this reason, HIV-1-based lentiviral vectors carrying other internal promoters, such as the MESV/MSCV LTR, have recently been constructed by a number of groups and are being evaluated in HSC gene transfer studies in the context of a central DNA flap that acts as a cis-determinant of HIV-1 DNA nuclear import.55 ,69,70,71,72
III. Regulatory Oversight of Gene Therapy Clinical Trials: A Matter of Balance
Franklin O. Smith, M.D.* and Kenneth Cornetta, M.D.*
Blood and Bone Marrow Transplantation Program, Herman B Wells Center for Pediatric Research, Indiana University School of Medicine, Cancer Res. Bldg., Room 402, 1044 West Walnut Street, Indianapolis IN 46202
The past year has seen the great potential of gene transfer technology, as French investigators have reported the first use of gene therapy to cure several children with a rare immune deficiency.1 At the same time, the lay press has aggressively publicized alleged errors in the conduct of several gene therapy clinical trials conducted at prestigious research institutions. These concerns arose after a death that is allegedly attributable to the process of gene transfer. As a result of possible lapses in Good Clinical Practice (GCP) guidelines2 and alleged failures to adhere to the Federal Code of Regulations3 and the National Institutes of Health's (NIH) Guidelines for Research Involving Recombinant DNA Molecules4 (hereafter referred to as NIH Guidelines) there is again increased scrutiny of gene therapy clinical trials by institutional and federal government regulatory agencies, physicians, scientists, ethicists, public health policy makers, the lay press and patients. These concerns also bring to light the complex ethical issues, potential conflict of interest by physician-scientists, and other concerns that, if not carefully addressed, may further erode the public's trust in the process of clinical investigation. Furthermore, these events have raised questions regarding the ability of the regulatory community to adequately protect research subjects.
This review will focus on institutional and federal government regulatory structures and identify the current, although rapidly changing, regulatory requirements imposed upon clinical gene therapy trials. Issues that may serve as barriers to more effective regulation will also be presented.
Regulatory Agencies
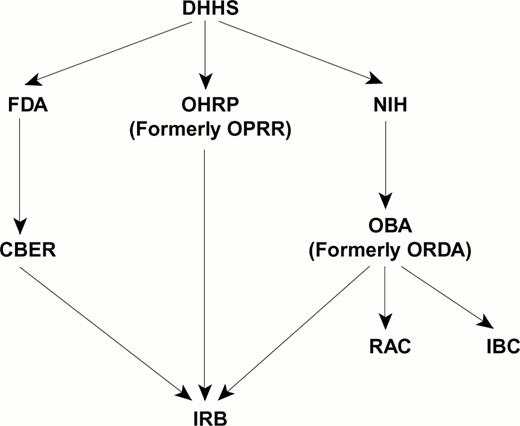
Oversight of clinical gene therapy trials occurs at the local institution and nationally (Figure 2). The oversight structure is complex, interactive, and constantly changing.
National regulatory agencies and local institutional committees involved in the oversight of gene therapy trials.
Abbreviations: DHHS, Department of Health and Human Services; OHRP, Office for Human Research Protection; OPRR, Office for Protection of Research Risks; FDA, Federal Drug Administration; CBER, Office for Biologics Evaluation and Research; NIH, National Institute of Health; OBA, Office of Biotechnology Activities; ORDA, Office of Recombinant DNA Activities; RAC, Recombinant DNA Advisory Committee; IBC, Institutional Biosafety Committee.
National regulatory agencies and local institutional committees involved in the oversight of gene therapy trials.
Abbreviations: DHHS, Department of Health and Human Services; OHRP, Office for Human Research Protection; OPRR, Office for Protection of Research Risks; FDA, Federal Drug Administration; CBER, Office for Biologics Evaluation and Research; NIH, National Institute of Health; OBA, Office of Biotechnology Activities; ORDA, Office of Recombinant DNA Activities; RAC, Recombinant DNA Advisory Committee; IBC, Institutional Biosafety Committee.
National Institutes of Health, Office of Biotechnology Activities
As a result of the development of recombinant DNA technology in the 1970s, a number of complex issues relating to the safety of genetic research were raised by the public and within the scientific community, leading eventually to a self-imposed moratorium on research involving recombinant DNA. In response to these safety concerns, the NIH created the Recombinant DNA Advisory Committee (RAC). With advice from the RAC, the NIH developed policies and procedures (e.g., NIH Guidelines) intended to ensure maximal safety for basic research using recombinant DNA. Prior to the first human experiment that transplanted genetically modified cells, a subcommittee of the RAC was formed to provide guidance in the design of pre-clinical research and clinical gene therapy trials. This subcommittee also delineated the responsibilities for those involved in gene therapy trials, including national oversight by the NIH Office of Biotechnology Activities (OBA) (formerly the Office of Recombinant DNA Activities [ORDA]), the RAC, and the role of the NIH Director. The responsibilities for local oversight by Institutional Review Boards (IRB) and Institutional Biosafety Committees (IBC) were also delineated. In addition, responsibilities of NIH-funded institutions and gene therapy investigators were defined.
Initially, the RAC reviewed every gene therapy clinical trial submitted to the NIH and made recommendations to the NIH Director regarding approval of the research. However, in 1996, after considerable public debate, the role of the RAC was revised. In order to avoid duplicative regulatory review of trials required by the FDA, the role of the RAC changed to focus on scientific, safety, ethical and public health issues as they applied to gene therapy. Today, NIH guidelines still require RAC review of all gene therapy trials that involve “novel” technologies or procedures, but neither the RAC nor the NIH Director currently grant approval to gene therapy trials.2
Since the creation of the RAC, 372 clinical gene therapy trials involving over 4,000 research participants have been registered with OBA and the RAC.5 These trials have involved the use of numerous gene delivery systems.5 Eighty-nine percent of these trials have been designed to ascertain the safety of gene transfer methodology (phase I) with 10% designed to test safety and efficacy (phase II). Only 1% of these trials have involved phase III designs.6 It is important to note that despite the fact that the vast majority of gene therapy clinical trials conducted over the past decade have been phase I toxicity trials, significant toxicities directly related to gene transfer have not yet been reported in the medical and scientific literature.
Guidelines for the safe conduct of and reporting of adverse events for human clinical trials involving gene transfer have been developed by the NIH.4 These guidelines apply to principal investigators conducting research that is supported by the NIH or research that is conducted at institutions that receive NIH funding for basic or clinical recombinant DNA research. The intent of these guidelines is to delineate the role and responsibilities for principal investigators, institutions, Institutional Biosafety Committees and the Institutional Biologic Safety Officer. Proposed changes to the NIH Guidelines and other federal regulations are based upon new scientific and medical information as well as ethical considerations. Prior to implementation, proposed changes are posted in the Federal Register with a defined comment period for public input.
As specified in the NIH Guidelines, experiments involving the deliberate transfer of DNA or RNA derived from recombinant DNA into human subjects cannot be initiated without simultaneous submission of all required documents to both the FDA and OBA (Table 1). Among its many responsibilities, OBA is the focal point for information on NIH-funded recombinant DNA activities. Once a gene therapy study is received by OBA, it is assigned a review level. Trials that use novel methods require full RAC review prior to initiation. For studies that must undergo full RAC review, RAC reviewers examine the rationale, scientific content, preliminary in vitro and in vivo safety data, information obtained from relevant animal models, and relevant social and ethical issues raised by the research. If the proposed research is not considered to be novel, full RAC review may not be required, but the proposed research, all amendments to the study, adverse events and new data from humans and animal studies must still be submitted to the OBA for registration purposes, to ensure continued public access to relevant gene therapy information and for monitoring of adverse event reporting.
Submission, review and approval requirements for gene therapy clinical trials.
| Approval Required: | Institutional Review Board (IRB) |
| Institutional Biosafety Committee (IBC) | |
| Food and Drug Administration (FDA) | |
| Corporate Partners | |
| National Gene Vector Laboratory (If site of vector production) | |
| Review Only Required: | Recombinant DNA Advisory Committee (RAC) (Only if novel methods proposed) |
| Registration Only Required: | Recombinant DNA Advisory Committee (RAC) (If novel technology is not proposed) |
| Approval Required: | Institutional Review Board (IRB) |
| Institutional Biosafety Committee (IBC) | |
| Food and Drug Administration (FDA) | |
| Corporate Partners | |
| National Gene Vector Laboratory (If site of vector production) | |
| Review Only Required: | Recombinant DNA Advisory Committee (RAC) (Only if novel methods proposed) |
| Registration Only Required: | Recombinant DNA Advisory Committee (RAC) (If novel technology is not proposed) |
Food and Drug Administration
The Food and Drug Administration (FDA) is an agency within the United States Department of Health and Human Services (DHHS) (Figure 2). The FDA has regulations for the protection of human research subjects as well as regulations governing the use of drugs, medical devices and biologic products in clinical research settings. The rules regulating clinical research are contained in the Code of Federal Regulations (CFR).
Coincident with the regulatory structure created by the NIH, the FDA also declared in 1993 its authority, according to the Public Health Service Act and the Federal Food, Drug, and Cosmetic Act, to oversee human gene therapy trials. Regulatory oversight of these trials is conducted at the FDA by the Office for Biologics Evaluation and Research (CBER) and is accomplished by using the Investigational New Drug (IND) mechanism. The IND mechanism for gene therapy trials has been identical to the mechanism used for other biologic products. The FDA has statutory oversight authority over all clinical gene therapy trials, regardless of funding source and research site. The FDA has the authority to halt research activities if violations of federal regulations are identified. The FDA also has the authority to place phase I studies on a “clinical hold” status for safety reasons. Reasons for a clinical hold are typically associated with insufficient data to assess risk (e.g. lack of product manufacturing information or lack of appropriate pharmacology/toxicology testing) or the result of concerns about the design of a clinical trial. A clinical hold is not placed upon studies secondary to concerns about potential efficacy or scientific rationale.
Office for Human Research Protection
The OHRP (formerly the Office for Protection of Research Risk within the NIH) is now an agency within DHHS. This office negotiates Assurances that are required for federally sponsored research. Assurances, whether Single Project Assurances, Multiple Project Assurances or Cooperative Project Assurances, imposes requirements on investigators and institutions for the conduct of clinical research and protection of human subjects, including the informed consent process and the establishment of an IRB. The Assurances specifies the responsibilities of the individual investigator and requires that institutions monitor research and report non-compliance with federal regulations. The OHRP can terminate or suspend the Assurances for institutions based on non-compliance with federal regulations or for compromising the protection afforded to research subjects. This means that no federally supported clinical research can be conducted at the institution until the Assurances is reinstated. DHRP also has the authority to suspend enrollment on specific studies, remove individual departments within an institution from the Assurances and/or impose sanctions upon individual researchers.7
Institutional Review Boards
Assurances mandate that all research involving human subjects undergo IRB review and approval prior to initiating research. The composition of IRB is specified in the CFR. Investigators may use the IRB at their local institution, create their own IRB, or contract with a private IRB. IRB are charged with reviewing proposed research to evaluate risks to research subjects and ensure protection of the rights of research participants. While IRB procedures may vary among institutions, IRB are required to adhere to FDA regulations set forth in the CFR. IRB must approve research protocols and informed consent documents prior to beginning a study (Table 1), must approve all amendments to the study, and must review a progress report at least yearly. Finally, both OHRP and the FDA have the authority to regulate, inspect, and close IRB.
Informed consent requirements
Adequate informed consent is critically important to the conduct of ethical clinical research. Informed consent is a process that involves verbal and written communication between investigators and research participants. This process must include question and answer sessions and an agreement to participate in the study that is documented by the research participant's signature (or that of the parents or legal guardian) on an IRB-approved informed consent document. Federal regulations require that all informed consent documents contain at a minimum the following eight requirements: a statement that the individual is a research participant; the purpose of the study; a description of what is involved by participating in the trial; potential risks; potential benefits; alternatives to participation in the research; a statement about the confidentiality of research records; a statement about compensation for injury; a list of contact persons to whom questions about the research or the individual's rights as a research participant can be directed; and finally, a statement that participation in the study is voluntary.
Institutional Biosafety Committee
The CFR mandates that an Institutional Biosafety Committee (IBC) must be comprised of at least five members who have experience and expertise in recombinant DNA technology. These individuals must be capable of ascertaining the safety of recombinant DNA research and be able to identify potential risk to the public and environment that may result from these research activities. The NIH Guidelines mandate that all gene therapy trials undergo review by the IBC and receive approval prior to initiation of the trial (Table 1). The IBC must also periodically review not only individual research protocols, but also review all recombinant DNA research at the institution to ensure compliance with NIH Guidelines. Institutions conducting large scale research, production activities involving viable organisms or recombinant DNA research at Biosafety Level 3 (BL3) or BL4 must appoint a Biological Safety Officer (BSO). Among the responsibilities of the BSO is the requirement to conduct periodic inspections and report violations of the NIH Guidelines to the IBC.
Corporate partners
As a result of the complexity and numerous technologies required for gene therapy trials, most studies involve the participation of numerous corporate partners. Each of these partners will have specific regulatory requirements that are mandated by the FDA as well as individual corporate regulations.
Guidelines for Good Clinical Practice
All clinical research must be conducted according to a set of standards that have been formalized and agreed to by experts in clinical investigation. There is no single document that embodies all of the ethical and scientific standards that define guidelines for good clinical practice (GCP).8 GCP is instead embodied in laws, regulations and guidelines set forth in IRB and informed consent regulations,9 the Code of Federal Regulations,3 FDA Form 1572, the International Harmonization (ICH) Guidelines2 and other documents specifying the responsibility of investigators, sponsors and study monitors.5,7 These guidelines define GCP criteria that are intended primarily to assure the rights and safety of human research subjects and secondarily to ensure that data derived from these trials are accurate and credible. GCP guidelines require that clinical research be conducted for valid ethical and scientific reasons, performed in compliance with written Standard Operating Procedures (SOP), be performed by qualified investigators, initiated only after IRB approval and after valid informed consent has been obtained and documented. GCP guidelines also require periodic monitoring of the trial, audits to assess the quality of the research and integrity of the data collected, archiving of research materials and control of investigational medications and devices.8 It is the expectation and requirement of IRB, IBC, FDA, OHRP and OBA that all human gene therapy trials are developed and conducted in compliance with GCP guidelines.
Reporting Requirements for Serious Adverse Events
No area of regulation has created as much confusion over the past year as the reporting requirements for serious adverse events (SAE). The FDA and IRB and other regulatory agencies have defined an SAE as an event that prolongs hospitalization, prompted hospitalization, resulted in a persistent or significant disability, was life threatening, resulted in death or was an important medical event that required medical or surgical intervention to prevent one of the these outcomes. The FDA, IBC and IRB require prompt reporting of any SAE that is serious and unexpected and related to the study therapy with reporting of all serious events on the annual report even if expected and unrelated to the study therapy. Many investigators, however, have been confused about the reporting requirements to OBA. However, this confusion was recently clarified in the testimony of Dr. Amy Patterson, Director of OBA, before the United States Senate Subcommittee on Public Health, Committee on Health, Education, Labor and Pensions.6 It is now clear that OBA requires prompt reporting of all serious adverse events for all gene therapy trials, past, present and future, regardless of whether the SAE was expected or unexpected and whether the SAE was related or unrelated to the study therapy (Table 2).9 The NIH has not yet clarified how it intends to deal with the anticipated large volume of SAE that will likely be submitted as a result of these reporting criteria. It is anticipated that a large number of serious events will be received by OBA because many recipients of gene therapy receive toxic therapy for their underlying disease (e.g. hematopoietic stem cell transplantation) and are ill as a result of their therapy or disease. However, in this context, the numerous and frequent serious events are expected and not related to the gene therapy portion of the patient's treatment.
Criteria for immediate reporting of serious adverse events.*
| Serious**and Unexpected and Related to the Study Therapy . | Serious** . |
|---|---|
| Institutional Review Board | Office of Biotechnology Activities/Recombinant DNA Advisory Committee |
| Institutional Biosafety Committee | |
| Federal Drug Administration | |
| Corporate Partners |
| Serious**and Unexpected and Related to the Study Therapy . | Serious** . |
|---|---|
| Institutional Review Board | Office of Biotechnology Activities/Recombinant DNA Advisory Committee |
| Institutional Biosafety Committee | |
| Federal Drug Administration | |
| Corporate Partners |
All serious adverse events are reported in the annual Investigational New Drug (IND) report.
Serious defined in text.
In spite of the different reporting criteria for the FDA and OBA, SAE, study amendments and annual reports for gene therapy trials must be submitted to the IRB, IBC, FDA, NIH, OPRR, institutional Scientific Review Committee (if applicable), GCRC (if applicable) and all corporate partners.
Recent Regulatory Changes
Oversight of gene therapy trials requires continued coordination of activities between NIH, FDA and other regulatory agencies. A number of changes have been made to this process in the past year, including a requirement to file monitoring plans for gene therapy trials with the FDA. FDA has also announced its intent to increase on-site inspections to determine if the institutions monitoring plans are adequate and if investigators and institutions are in compliance with their own monitoring plans. The NIH and FDA also intend to co-sponsor symposia to educate and provide a national, public forum for discussions of scientific, technical, and ethical issues relevant to gene therapy. The NIH has also created the Advisory Committee to the Director (ACD) to further examine the roles of the RAC and NIH Guidelines. Other changes will focus on requirements for investigator training, documentation issues as timing and content of SAE reporting.
The NIH has also sent a letter to all study sponsors to ensure that they are following current CBER recommendations for product characterization as well as are compliant with regulations requiring quality assurance programs and clinical monitoring of gene therapy trials.10
Incentives for Compliance with Regulatory Requirements
Every physician-scientist involved in clinical research should have as goals the protection of research participants, ensuring the accuracy and validity of research data and the identification of better treatments that will help to alleviate suffering. Regulatory guidelines and requirements are ideally designed to help investigators achieve these goals.
Barriers to Compliance with Regulatory Requirements
There is little doubt that compliance with all regulatory guidelines and requirements is costly in terms of personnel, time and financial resources. The local institution infrastructure required for the safe conduct of gene therapy trials is considerable. In general, these trials require specific expertise by the principal investigator, co-investigators, scientists (basic science, vector design and production, stem cell procurement, processing, transduction, and safety and efficacy assays), research nurses, computer and informatics support, clinical research assistants, regulatory personnel, quality assurance and improvement personnel, biostatisticians, clinical trials coordinators, financial administrators and administrative support. The added burden of additional regulatory requirements may further limit the number of institutions with the personnel and financial resources to conduct these complicated trials. The ability of academic institutions to hire private clinical trials organizations to assist in the conduct of gene therapy trials has not yet been explored by most institutions.
Gene therapy trials have recently been the subject of intense scrutiny by the media and regulatory agencies. Investigators found to be in non-compliance with federal regulations face sanctions by the NIH and FDA and are potentially subject to criminal prosecution. In addition, the FDA has recently asked the United States Congress to empower the agency to fine individual investigators and institutions guilty of non-compliance. The proposed fines would range up to $250,000 for individuals and up to $1,000,000 for institutions.
The issue of real or perceived conflict of interest by individual investigators and institutions that may have a financial stake in the success of gene therapy trials and technologies is also significant. While most academic institutions have conflict of interest policies and require yearly disclosure of potential conflict of interest by all faculty, the regulatory community is currently examining regulations relevant to this issue.11
Summary
Our society allows for human experimentation as a means to advance knowledge that may lead to better treatment of disease. The ability to conduct human experimentation is a rare privilege. All clinical investigation, including gene therapy, must be done in compliance with Good Clinical Practice Guidelines and the Code of Federal Regulations. In addition to these regulations and guidelines, human gene therapy trials must also meet additional requirements delineated in the NIH Guidelines for Research Involving Recombinant DNA Molecules.
All clinical research, including gene therapy trials, involves risk to patients. Some of these risks are known and expected while others are unknown. However, no amount of regulatory oversight will eliminate all risks to research participants. Therefore, the intent of regulatory requirements is to maximize safety for human research participants within the context of a given trial. These regulations also help to ensure the integrity of data generated from research. Non-compliance with regulations will place patients at risk, compromise data and breach the public's trust in the process of clinical investigation.
Alternatively, regulatory requirements that are overly burdensome have the potential to overwhelm investigators and regulatory agencies. In fact, there has already been significant concern expressed about the ability of local and federal regulatory agencies to handle an increased regulatory demand. Additionally, excessive regulatory requirements may inhibit scientific progress and further delay the development of safer and more effective therapies. The impact of punitive damages, if instituted, on clinical investigators' willingness to pursue this area of investigation is unclear. Therefore, the challenge now before the medical, scientific, regulatory, ethics and legal communities is to develop regulatory strategies that provide effective protection of research participants without making clinical investigation so cumbersome that development of innovative treatments are stifled. The NIH must also recognize the increasing demands of clinical trials review, oversight and compliance and provide funds to investigators and institutions if investigator-initiated research is to continue.
While regulatory agencies endeavor to provide oversight of clinical investigation and protect human research participants, it still remains the ultimate responsibility of the principal investigator to ensure that clinical research is conducted in an ethical manner. This requires adherence to the approved protocol, approval of the research by all regulatory agencies, adequate monitoring of the trial, ensuring the integrity of collected data, obtaining valid informed consent prior to initiating the research and most importantly, safeguarding the rights of research participants and the public's trust.
I. Gene Transfer into Hematopoietic Stem Cells
II. Vector Designs for Stem Cell Expression
III. Regulatory Oversight of Gene Therapy Clinical Trials
Author notes
Howard Hughes Medical Institute, Indiana University School of Medicine, Indianapolis IN 46202