Abstract
Chronic myeloid leukemia (CML) was the first human malignancy to be associated with a specific genetic lesion, the Philadelphia chromosome, harboring the BCR-ABL oncogene. Since then, it has become a paradigm for the discovery of molecular mechanisms and targeted therapeutic approaches in the field of hematologic neoplasias. The past 5 years or so have been particularly fruitful in the dissection of the signal transduction pathways abnormally activated in CML and in the translation of this knowledge to clinical practice. In this report, we discuss the biological basis for such translation and highlight the current and potential tools for the effective treatment of CML patients. The first part presents a review of the basic concepts on the biology of CML and their application to the design of targeted therapy. The mechanisms of action of the molecular-specific drugs currently used in clinical trials are discussed, with emphasis on the description of the most promising new compounds that are enhancing the potential for effective alternative or combination chemotherapy in CML. In the following section, we explain how molecular monitoring of response to imatinib mesylate in patients with CML can be used as a guide to clinical management. In particular, we discuss the relative value of regular quantitative RT/PCR and cytogenetic analyses, how responding patients should be monitored and managed, and how to investigate patients who are refractory or become resistant to imatinib treatment. In the last part of this report, a discussion on the possibility of managing CML with patient-specific strategies is presented. We review the current treatment options, highlight the factors impacting on decision making, discuss the range of possibilities for future therapeutic strategies and propose a systematic approach for individualizing treatment for patients in different disease categories.
I. The Biology of CML and Targets for Molecular Therapy
Junia V. Melo, MD, PhD*
Department of Haematology, Imperial College, Hammersmith Hospital, Du Cane Road, London W12 0NN, UK
Chronic myeloid leukemia (CML) is a hematopoietic disorder characterized by the malignant expansion of bone marrow stem cells. Its cytogenetic hallmark is a reciprocal t(9;22)(q34;q11) chromosomal translocation that creates a derivative 9q+ and a small 22q–, known as the Philadelphia (Ph) chromosome.1 The latter harbors the BCR-ABL fusion gene encoding a chimeric Bcr-Abl protein with a deregulated tyrosine kinase activity (Figure 1; see Appendix, page 598), the expression of which has been shown to be necessary and sufficient for the transformed phenotype of CML cells. CML is unusual among human cancers in that a single oncogene product has been identified as having a central role in its pathology.
Through the contribution of various researchers, the past 20 years have brought us considerable knowledge on the molecular and cell biology of CML, creating the essential platform for targeted therapy to be engineered (Figure 2; see Appendix, page 599). It soon became clear that the Bcr-Abl oncoprotein itself is the best molecular target presented by CML cells because it is not expressed by normal cells. Furthermore, the dissection of the signal transduction pathways affected by the deregulated kinase activity of Bcr-Abl provided information on additional or alternative signaling steps that could be interrupted in an attempt to eliminate the oncogenic effect of Bcr-Abl. More recently, attention has also been focused on immunological means of recognizing and destroying the leukemic clone, and these approaches look promising, particularly in the context of eliminating residual disease after various sorts of “debulking” therapy.
Molecular Targeting of the BCR-ABL Gene Products
Although initial efforts in the early 1990s were directed toward attempts to inhibit BCR-ABL gene function via its RNA message, this strategy was never successfully translated into efficient forms of treatment for CML. Thus, the elucidation of the structure and mechanisms of action of the end product of the chromosomal translocation, the Bcr-Abl protein itself, shifted the focus to the investigation of small molecules that could interact with and inhibit this oncoprotein.
Bcr-Abl Oncoprotein
In CML, the mRNA molecules transcribed from the hybrid gene usually contain 1 of 2 BCR-ABL junctions, designated e13a2 (formerly b2a2) and e14a2 (b3a2), respectively. Both mRNAs are translated into an oncoprotein of 210 kDa molecular weight. The leukemogenic potential of the p210Bcr-Abl resides in the fact that the normally regulated tyrosine kinase activity of the Abl protein is constitutively activated by the juxtaposition of “alien” Bcr sequences (Figure 1; see Appendix, page 598). Bcr acts by promoting dimerization of the oncoprotein such that the 2 adjacent Bcr-Abl molecules phosphorylate their respective partners on tyrosine residues in their kinase activation loops.2 The uncontrolled kinase activity of Bcr-Abl then usurps the physiological functions of the normal Abl enzyme by interacting with a variety of effector proteins, the net result of which is deregulated cellular proliferation, decreased adherence of leukemia cells to the bone marrow stroma and reduced apoptotic response to mutagenic stimuli (Figure 1; see Appendix, page 598). The relative contributions of these effects to the phenotype of chronic phase CML is still poorly understood.3
The Bcr-Abl protein structure and the biochemical pathways in which it is involved have been extensively investigated and recently reviewed by us.4 Knowledge of the function of several structural domains ‘inherited’ from the parental Bcr and Abl proteins allows one to test for certain properties of the fusion product. Thus, the tyrosine kinase encoded by the Src-homology 1 (SH1) domain of the Abl component of Bcr-Abl is undoubtedly the most crucial for oncogenic transformation. Other important motifs in the Abl portion are the protein-interaction SH2 and the C-terminal nuclear localization signal (NLS), DNA- and actin-binding domains. On the Bcr moiety, the coiled-coil motif encoded by the first BCR exon is responsible for dimerization of the oncoprotein; a tyrosine at position 177 is crucial for the binding of adaptor proteins such as Grb-2; and amino terminal phosphoserine/phosphothreonine residues are required for interaction with SH2-containing proteins, including Abl itself (Figure 1; see Appendix, page 598).
Inhibiting the Tyrosine Kinase Activity of Bcr-Abl
Due to its essential role in the leukemogenicity of the oncoprotein, the SH1 domain of Bcr-Abl is an obvious molecular target. Early screenings of natural products for compounds capable of antagonizing this catalytic activity identified an isoflavonoid, genistein, and an antibiotic, herbimycin-A, as potential candidates. Subsequent efforts have focused on the rational design of synthetic compounds with chemical structures that are able to compete with either adenosine triphosphate (ATP) or a substrate for occupancy of the binding site in the kinase domain.
The most successful synthetic ATP-binding inhibitor that has been developed to date is the 2-phenylaminopyrimidine, imatinib mesylate, formerly known as STI571 (Glivec® or Gleevec™, from Novartis Pharma, Basel, Switzerland). Initial preclinical studies showed that imatinib was effective at inhibiting the autophosphorylation of Abl, the platelet-derived growth factor receptor (PDGFR), the Kit receptor5 and the Arg (ABL-related gene) tyrosine kinases at submicromolar concentrations.6 The most striking feature of the compound is its remarkable degree of specificity, its effect on other tyrosine kinases being negligible. The proliferation of CML progenitor cells was inhibited by treatment with the inhibitor but control normal cells were largely unaffected.7,8 Selective inhibition of growth could also be demonstrated for BCR-ABL+ cell lines both in vitro8 and in mice.7,9
The combined preclinical data suggested that imatinib might have value as a therapeutic agent, and the first clinical trials with this drug were initiated in 1998. Presently, over 60,000 patients have been treated worldwide with imatinib, and the success forecasted from those initial data has been largely achieved (see below on the clinical sections of this article). However, there remain problems associated with the clinical use of imatinib for the treatment of CML. In advanced phase disease, the responses are often short-lived and patients invariably undergo disease progression following the brief periods of respite. This results from the emergence of a leukemic clone resistant to the drug following its regular administration. The mechanisms of resistance to imatinib are still not completely known, but include the selection of cells that overexpress Bcr-Abl,10–,12 cells that express normal levels of Bcr-Abl but with mutations in the Abl kinase domain,13–,16 and cells that seem to be independent of Bcr-Abl expression, perhaps via abnormal activation of other oncogenic pathways.17–,19 Conceivably, more than 1 of these mechanisms may coexist in the same cell. Cells resistant via any of these mechanisms may be specifically selected by imatinib regardless of the fact that in Bcr-Abl-independent cells, the drug appears to be effective in inhibiting Bcr-Abl signaling.10,18 It has been argued that resistance to imatinib might be expected to develop more rapidly and more uniformly than resistance to conventional cytotoxic drugs precisely because imatinib has such a highly specific target profile.20,21
A mutant amino acid in the Abl kinase domain leads to drug resistance if it directly or indirectly obstructs the appropriate binding of imatinib to the kinase pocket, without, however, preventing the kinase from accepting ATP and thus phosphorylating the substrates that generate the CML phenotype. In these instances, it might be possible to design new analogues of imatinib. Ideally, these agents would inhibit the modified kinase configuration, and yet retain the same degree of specificity and lack of effect in normal cells. Such an agent would be a welcome addition to imatinib in the drug armamentarium for treating CML. This would allow one to combine analogues, individualizing therapy on the basis of molecular surveillance of the BCR-ABL sequences present in the tumor load. Such an approach is technically feasible and would likely represent an improvement on imatinib monotherapy. Certainly, other small-molecule inhibitors of the Bcr-Abl kinase are in development, and appear to partake in slightly different molecular interactions.22
The best example of alternative ATP competitors potentially useful for the treatment of CML is a family of pyridol[2-3-d]pyrimidine compounds originally described as potent Src inhibitors.23 Among 7 of these compounds tested recently by the Memorial Sloan-Kettering group, 2 in particular, PD173955 and PD166326, were shown to be strong suppressors of the growth of BCR-ABL-positive cells via their effects on Bcr-Abl.24 Furthermore, these pyridol-pyrimidine kinase inhibitors appear to be effective, at nanomolar concentrations, in suppressing the proliferation of some types of imatinib-resistant BCR-ABL-positive cells25 (and Tipping et al, in preparation).
In the presence of an ATP-competitor such as imatinib, the SH1 domain of Bcr-Abl retains its ability to associate with substrate molecules but is unable to catalyze their phosphorylation. An alternative approach for inhibiting the tyrosine kinase activity of the oncoprotein is the use of chemical agents that inhibit or modify the binding of substrates. Under these conditions, the ATP-binding pocket of the SH1 domain is unaffected but association of the kinase with its substrates is impaired. A class of synthetic compounds generically called tyrphostins inhibits the tyrosine kinase activity of Bcr-Abl in this fashion, resulting in the loss of kinase autophosphorylation, and the subsequent degradation of Bcr-Abl. Adaphostin (NSC 680410), the adamantyl ester of the tyrphostin AG957, has a longer serum half-life than AG957 and greater in vitro potency. The compound has been reported to induce apoptosis in a dose- and time-dependent manner via the mitochondrial pathway, and continues to exert its effects following withdrawal.26 Interestingly, adaphostin appears to induce cell death without downregulating the antiapoptotic proteins (such as XIAP and Bcl-XL) that are thought to contribute a survival advantage to CML cells. In a recent study,26 the effects of adaphostin were compared with those of imatinib mesylate on K562 cells, BCR-ABL-transduced FDC-P1 cells and primary hematopoietic progenitors from CML patients. In K562, levels of p210Bcr-Abl were reduced following a 6-hour exposure to 10 mM adaphostin. Caspase activation occurred by 12 hours, and by 24 hours 90% of the K562 cells were apoptotic. In contrast, treatment of K562 with 20 mM imatinib led to rapid inhibition of Bcr-Abl autophosphorylation without degradation of the p210 protein. Adaphostin was selectively toxic for leukemic cells, inhibiting CML granulocyte colony-forming units (CFU-G) but not normal CFU-G. Importantly, imatinib-resistant K562 cells proved to be sensitive to adaphostin. This last finding is consistent with the notion that imatinib and adaphostin exert their inhibitory effects on the tyrosine kinase activity of Bcr-Abl by separate and distinct mechanisms. Treatment of K562 cells with both imatinib and adaphostin induced greater cytotoxicity than that achieved with either agent alone. Hence, adaphostin may prove to be a valuable compound for use in combination with imatinib mesylate.
Blocking Oligomerization of Bcr-Abl
The oligomerization domain of Bcr-Abl represents an attractive target for molecular therapy because several lines of evidence suggest that this domain is also essential for the transforming activity of the oncoprotein. The correlation between oligomerization and transformation has been interpreted as evidence that Bcr-Abl must undergo tetramerization in order to become activated. Thus, the formation of Bcr-Abl homotetramers would promote their intermolecular cross-phosphorylation and activation in a manner analogous to the dimerization, cross-phosphorylation and activation of growth factor receptor tyrosine kinases.2 According to this model, monomers of Bcr-Abl are non-transforming whereas the oncogenic, transforming species are Bcr-Abl tetramers. In agreement with this hypothesis is the finding that expression of a peptide consisting of the first 160 amino acid residues of Bcr, including the oligomerization domain, was able to restore growth factor dependence to a growth factor–independent, BCR-ABL+, murine hematopoietic cell line.27 Furthermore, by cotransfecting the cells with vectors encoding the BCR fragment and the full-length BCR-ABL in different stoichiometric ratios, growth factor–independent colony formation, characteristic of the transformed phenotype, could be inhibited in a dose-dependent manner. The Bcr fragment was presumed to disrupt the formation of transforming Bcr-Abl homotetramers by oligomerizing with Bcr-Abl monomers.
Although none of the currently available therapies for CML target the oligomerization domain of Bcr-Abl, this approach could be exploited for therapeutic gain. In a recent study in which mice were injected with bone marrow cells that had been retrovirally transduced with mutant BCR-ABL constructs, those animals receiving cells expressing the full-length Bcr-Abl oncoprotein developed a myeloproliferative disorder resembling human CML, whereas mice receiving cells expressing a mutant Bcr-Abl protein lacking the first 63 amino acids failed to develop this disease.28 A case could be made for the development of synthetic molecules capable of inhibiting or disrupting Bcr-Abl oligomerization. Although such inhibitors might be expected also to inhibit the oligomerization of wild-type Bcr, there is evidence that disruption of the normal functioning of this protein need not necessarily be deleterious to health.29
Destabilizing the Bcr-Abl Protein
In most cases of molecular therapies that target particular domains of Bcr-Abl in order to inhibit its function, the Bcr-Abl oncoprotein, despite being functionally inhibited, continues to be expressed by the leukemic cells. The persistence of Bcr-Abl presents particular problems for treatment with imatinib where the selection of clones with mutated amino acids or overexpression of the oncoprotein leads to the emergence of resistance. Hence, the development of therapies that aim at inhibiting expression of Bcr-Abl is particularly desirable. The stability of Bcr-Abl is dependent upon it forming a multiprotein complex with the heat shock protein (HSP) Hsp90 and a cochaperone protein, p23. Brief exposure to geldanamycin, a specific inhibitor of Hsp90, causes Bcr-Abl to dissociate from Hsp90 and p23 and to form another complex with the chaperone proteins, Hsp70 and p60Hop.30 The p210Bcr-Abl-Hsp70-p60Hop complex is less stable than the p210Bcr-Abl-Hsp90-p23 complex since increased proteasome degradation of Bcr-Abl occurs following treatment with geldanamycin.31 A recent report32 suggests that these drugs may be of particular benefit to patients who have relapsed following treatment with imatinib mesylate. Both geldanamycin and its less toxic analogue 17-allylamino-17-demethoxygeldanamycin (17-AAG) induced the degradation of wild-type p210Bcr-Abl and two mutant Bcr-Abl proteins (T315I and E255K) found in imatinib-resistant patients. Moreover, both compounds exhibited greater potency against the mutant forms than against the wild-type Bcr-Abl. Similar effects have been observed in cells rendered resistant to imatinib via Bcr-Abl overexpression (Topaly et al, submitted; Barnes et al, in preparation).
Arsenic trioxide (As2O3) downregulates Bcr-Abl protein levels by translational modulation. It has been shown by various groups to enhance the selective cytotoxic effect of imatinib on CML cells. Similarly to geldanamycin and 17-AAG, it is also effective in inhibiting the growth of cell lines resistant to imatinib.33
The BCR-ABL mRNA
In theory, BCR-ABL mRNA should be an ideal molecular target for therapy based on antisense (AS) strategies. By designing an AS oligonucleotide that is complementary to both the BCR and ABL sequences on either side of the BCR-ABL transcript junction it should be possible to generate a species that would hybridize to BCR-ABL mRNA but not to mRNA transcribed from the wild-type BCR or ABL alleles. In practice, however, conventional AS oligonucleotide approaches have failed to fulfill their promise as treatment for CML. Among the various reasons for this failure, the most important are the apparent lack of specificity of the junction AS oligos, the need to synthesize them with special chemical backbones and to introduce them into the cells via stringent permeabilization procedures, and the extremely long half-life of the Bcr-Abl protein.34
It remains to be seen whether the so-called “second generation” antisense strategies will be applicable to the treatment of CML. These include antisense molecules such as 2′-O-methoxyethyl RNA, locked nucleic acids and peptide nucleic acids, as well as more exotic chemical species such as morpholinos (nonionic DNA analogues in which the backbone linkages have been altered relative to the phosphodiester backbone of DNA).35 A promising variation on the theme of antisense is RNA interference (RNAi). Three groups have by now reported on the use of anti-BCR-ABL small interfering (si) RNA oligonucleotides in BCR-ABL-positive cells.36–,38 Significant inhibition of the BCR-ABL message and protein has been observed in cell lines and/or primary CML cells in the 3 studies. However, the growth-inhibitory and apoptosis-inducing effects of the siRNA oligonucleotides were not as powerful as those elicited by imatinib mesylate, and it is still uncertain whether anti-BCR-ABL siRNAs can synergize with imatinib.36– 38
Ribozymes are RNA molecules that are capable of associating with other RNA molecules by base-pairing and catalyzing the hydrolysis of specific phosphodiester bonds within the target RNA sequences. Different types of ribozymes such as hammerhead, RnaseP, and an artificial allosterically controllable ribozyme known as a “maxizyme,” have been used to target BCR-ABL mRNA in vitro and in murine models of leukemia.39–,41 Deoxyribozymes (DNAzymes), are smaller than ribozymes, more efficient enzymatically, less expensive to synthesize and more resistant to serum. In addition, DNAzymes may be generated to cleave the target sequence with a high degree of precision. In one study,42 where one of the DNAzymes was designed to cleave BCR-ABL mRNA at a site only 1 nucleotide away from the BCR-ABL junction, the growth of BCR-ABL+ CFU-Mix colonies was inhibited by 53%–80% when CD34+ bone marrow cells from CML patients were transfected with these molecules.
Many of the shortcomings associated with AS oligonucleotides, particularly the lack of an efficient means of delivery, also apply to ribozymes and DNAzymes. Solutions to these problems will have to be devised in order for ribozymes or DNAzymes to become clinically useful molecular therapies. Even then, systemic administration of these agents may be inappropriate for various reasons, and their use may be confined to ex vivo applications such as purging bone marrow prior to an autologous transplant.
Molecular Targeting of Downstream Pathways
Bcr-Abl is known to transduce its oncogenic signals through several pathways, which are then constitutively activated in a leukemic cell. It is thus conceivable that inhibition of these pathways as an alternative or in addition to Bcr-Abl inhibition may be a viable means of controlling leukemic cell proliferation.
Bcr-Abl is linked to Ras by a number of protein intermediates. Autophosphorylation of the tyrosine 177 residue of Bcr-Abl generates a binding site for the adapter protein, Grb2.43 Bound Grb2 associates with the son of sevenless protein, SoS, to create a complex that stimulates activation of Ras by facilitating the exchange of GTP for GDP.44 In addition, it has been demonstrated that Ras may be activated by 2 other Bcr-Abl substrates that function as adapter molecules, Shc45 and CrkL.46 It should be possible to uncouple Bcr-Abl from the Ras pathway by targeting these adapter molecules. The potential of this approach has been demonstrated in studies in which novel peptides were designed with the aim of blocking the SH3 domains of CrkL47 and Grb2.48 Both binding peptides downregulated phosphorylation of mitogen-activated protein kinase (MAPK) and significantly inhibited the proliferation of 11 of 16 cell cultures obtained from individual CML patients. Although these studies illustrate the potential of targeting the adapter molecules that link Bcr-Abl to the central Ras-MAPK mitogenic pathway, considerable efforts will have to be made to refine the peptides before they can form the basis of a molecular therapy.
Currently, the most feasible tool for interference with the Ras pathway is represented by farnesyl transferase inhibitors (FTIs). These compounds disrupt the prenylation of Ras and other proteins that, like Ras, require this posttranslational modification to generate the functionally active, membrane associated form of the G-protein. The involvement of Ras in Bcr-Abl-induced leukemogenesis suggests that FTIs may have potential as antileukemic agents. At least 3 compounds, SCH66336 (Schering-Plough), L-744832 (Merck Research Labs), and R115777 (Janssen Research Foundation), have emerged as promising candidates for the treatment of leukemia. The largest preclinical investigations with CML cells have been done with SCH66336, which was shown to be effective against Bcr-Abl-induced acute leukemia in murine models of CML blast crisis49 and p190BCR-ABL transgenic mice,50 as well as in suppressing the in vitro growth of Bcr-Abl+ progenitor cells from CML patients, including those from individuals who were unresponsive to imatinib treatment.51 Furthermore, SCH66336 potently sensitized imatinib-resistant cells to imatinib-induced apoptosis. Taken together, these findings suggest that a combination of SCH66336 and imatinib can be a useful treatment for CML, especially in cases of imatinib resistance. Several lines of evidence suggest that the antiproliferative action of SCH66336 in Bcr-Abl+ cells must also involve protein targets other than Ras, and that the potent antitumor activity of FTIs derives from a more complex and subtle mode of action than was originally thought to be the case. In fact, even when farnesyl transferase is inhibited, Ras can be transferred to the membrane by an alternative prenylation pathway employing geranylgeranyl transferase-1 to successfully transduce Bcr-Abl signals. Therefore, bisphosphonates, developed primarily to treat bone diseases, became an attractive option as anticancer drugs for their capacity to inhibit the activation of Ras proteins through suppression of both geranylgeranylation and farnesylation. Zoledronate, a third-generation bisphosphonate, was recently shown to be a potent inhibitor of the proliferation of BCR-ABL+ cells both in vitro and in mouse models, and to augment synergistically the antileukemic activity of imatinib.52
Ras is linked to the MAPK pathway by the serine-threonine kinase, Raf-1, which activates the MAPK kinases, MEK-1/2, by catalyzing their phosphorylation. Specific inhibitors of MEK have been developed, and 3 of these compounds, PD098059, PD184352 (Pfizer), and U0126 (DuPont Merck) have been shown to inhibit the proliferation of CML cell lines and to produce a synergistic cytotoxic effect with imatinib even in some imatinib-resistant cell lines.53,54
Another signaling molecule that is activated by Bcr-Abl is phosphatidylinositol-3 (PI-3) kinase. PI-3 kinase is a heterodimer consisting of a catalytic (p110) and a regulatory (p85) subunit; Bcr-Abl associates with PI-3 kinase via the p85 subunit. Two PI-3 kinase inhibitors, wortmannin and LY294002 (Lilly), have been shown to synergize with imatinib in the suppression of in vitro growth of BCR-ABL+ cell lines and progenitor cells from CML patients.55,56 Wortmannin is not suitable for in vivo clinical applications because it is highly unstable in solution. It has yet to be established whether the synthetic PI-3 kinase inhibitors can be used as therapeutic agents for the treatment of CML; although they are more stable than wortmannin they are far less potent than this naturally occurring prototype. An alternative may be to use inhibitors of PI-3 kinase effectors, such as Bad, mTor, Fkhr, NF-κB, GSK3b, and MDM2, all of which have been shown to be associated with Bcr-Abl transformation. Thus, rapamycin, an inhibitor of mTor already in Phase I/II clinical trials for a number of cancers,57 was found to also enhance imatinib-mediated killing of CML cells, and may be effective in cases where resistance to imatinib is due to BCR-ABL overexpression.58
A final mention should be made of 2 additional compounds reported as potentially useful for the treatment of CML. The first is AG490, a Jak-2 inhibitor; the second is flavopiridol, an inhibitor of cyclin-dependent kinases. Whereas neither compound acts on pathways exclusively and directly activated by Bcr-Abl, combination of each one with imatinib produced synergistic effects on suppression of proliferation and induction of apoptosis in BCR-ABL+ cells.59,60
II. Molecular Monitoring of Response to Imatinib as a Guide to Clinical Management
Timothy P. Hughes, MD*
Institute of Medical and Veterinary Science, Division of Hematology, Frome Road, Adelaide 5000, SA, Australia
The treatment guidelines for patients with CML are undergoing substantial change61–,64 due to emerging evidence regarding the effectiveness and safety of imatinib mesylate. This drug has shown activity in all phases of CML but the most substantial and stable responses are seen in newly diagnosed patients in the chronic phase.65– 69
Despite the widespread use of imatinib in CML it is still too early to know what level of response can be used as an indication of long-term disease control and what should be regarded as treatment failure. The therapeutic targets established for interferon alpha (IFN-α) therapy are based on the results of bone marrow cytogenetic studies. Major cytogenetic response (MCR ≤ 35% Ph+ marrow metaphases) and complete cytogenetic response (CCR = no Ph+ marrow metaphases) have been verified as reliable surrogate measures for long-term survival in patients treated with IFN-α.70,71 It will be several years before we can determine whether these cytogenetic responses have equivalent long-term prognostic value for imatinib-treated patients. Current results suggest they are predictive of progression-free survival for imatinib-treated patients, at least in the short term. Chronic phase CML patients achieving MCR on imatinib have a significantly lower risk of progression in the subsequent 24 months than patients not achieving MCR.66 In a prospective multicenter randomized Phase 3 trial designated the Novartis IRIS study 1106 newly diagnosed CML patients were treated with 400 mg/day of imatinib or IFN-α plus low-dose cytarabine (Ara-C). The observed CCR rate for the imatinib arm was 76% compared to 15% for the IFN-α plus Ara-C arm (median follow-up 18 months).69 If CCR achieved on imatinib proves to be equally predictive of long-term disease control, as has been the case for IFN-α-induced CCR, then the survival advantage with imatinib will be dramatic, but this is not yet proven.
One major concern regarding imatinib therapy is the development of resistance.65,68 The commonest cause of acquired resistance appears to be mutations in the BCR-ABL kinase region.13–,16,72–,75 The risk of mutation development is particularly high in patients who are beyond chronic phase as well as those with a long duration of disease prior to imatinib therapy.75
The excellent responses seen with imatinib in newly diagnosed patients has led to a reluctance to consider allogeneic transplant before the patient has received a trial of imatinib therapy (discussed in Section III). This makes it particularly important that emerging imatinib resistance is recognized early so that transplant options can be considered before there is overt progression of the disease. Conversely, profound reductions in the leukemic cell burden are likely to predict long-term disease control, and patients who achieve this level of response need to be identified so that more hazardous therapeutic options can be avoided or deferred.
Monitoring with Quantitative RT-PCR
Competitive or real-time quantitative RT-PCR (Q-PCR) has been previously used to serially monitor response in patients receiving IFN-α and recipients of allogeneic stem cell transplants (allo-SCT).76–,82 On IFN-α therapy, the BCR-ABL transcript level in the blood correlates strongly with the percentage of Ph+ metaphases in the marrow.80 Rising levels of BCR-ABL transcripts post-allo-SCT is strongly predictive of cytogenetic and hematological relapse.83,84 Real time Q-PCR has superseded competitive Q-PCR in most laboratories. The accuracy and reliability of real-time Q-PCR as a measure of BCR-ABL transcript level is highly dependent on the quality control procedures carried out by the laboratory. Reliable and consistent Q-PCR results require thorough validation of all aspects of the procedure. The most significant factor to ensure accuracy is the normalization of the results to an appropriate control gene, in order to compensate for variations in the quality of the RNA and the efficiency of the reverse transcription reaction. The 2 control genes that have been widely assessed are BCR and ABL. Both seem to be suitable in that they are expressed at low level and have similar stability to BCR-ABL.
Correlation Between Cytogenetics and Q-PCR
Recent studies on patients treated with imatinib have shown a strong correlation between the percentage of Ph+ metaphases in the bone marrow and peripheral blood BCR-ABL levels measured by Q-PCR.85–,88 When the Q-PCR values in imatinib treated patients were grouped according to the cytogenetic response categories, very little overlap was observed in the BCR-ABL levels within the cytogenetic categories among 76 patients who had simultaneous blood Q-PCR and marrow cytogenetics.88 Patients in CCR (n = 28) had BCR-ABL/BCR values below 1% with only 1 exception whereas only 2/48 patients not in CCR had BCR-ABL/BCR values below 1%. In a UK study, 40/42 patients in CCR had BCR-ABL/ABL values less than 2%.93 Two other studies85,87 have also confirmed that the BCR-ABL/ABL ratios of patients achieving complete, partial, and minor cytogenetic responses differed significantly, but the range of BCR-ABL values for patients in CCR appeared to be less tightly clustered.
Are Regular Marrow Cytogenetic Studies Still Needed?
Additional chromosomal abnormalities in the Ph-positive cells can sometimes be observed in patients on imatinib therapy. Whether these additional chromosomal abnormalities are always clinically significant remains uncertain but in some cases they herald disease progression. Recently there has been a recognition that additional chromosomal abnormalities can also be observed in Ph-negative metaphases.89–,92 Clonal karyotypic abnormalities were found in 7/46 patients (15%) who achieved cytogenetic responses on imatinib, and 2 of these patients had clinical features of myelodysplasia.92 It is probable that Ph-negative clonal abnormalities will be less common in patients treated with imatinib in early chronic phase. However, given the current level of uncertainty, there is still a role for frequent (at least 6 monthly) monitoring of bone marrow cytogenetics, at least until we have a better understanding of the incidence and clinical significance of clonal cytogenetic abnormalities in Ph-negative cells.
How Should Responding Patients Be Monitored and Managed?
Definitions of molecular response categories
In the molecular analysis of the IRIS study “major molecular response” was defined as a ≥ 3 log reduction in BCR-ABL/BCR level when compared to the median pretreatment level. The latter was calculated by measuring the level of BCR-ABL/BCR in 30 patients from blood taken just prior to commencement of the study. In other studies a BCR-ABL/ABL ratio of 0.045% has been used to define a major molecular response. This is based on a study of IFN-α-treated patients who were in CCR where the median value of BCR-ABL/ABL in patients who maintained stable CCR was 0.045%.81 A 3-log reduction below baseline is in fact similar to the level defined by the Hochhaus study. The advantage of defining molecular response according to reduction from a median pretreatment level is that once a laboratory has established their median baseline level, results can be expressed on a common scale internationally. Another advantage is that molecular response can be calculated without needing to know the actual baseline level for that particular patient.
The terms “PCR negative” and “complete molecular response” should be used with caution. They imply an absolute lack of measurable leukemia, which may be misleading. There is inherent variability in the sensitivity of Q-PCR and nested PCR assays between laboratories and between samples. In addition, with technological advances, there will be further improvements in our ability to detect low numbers of BCR-ABL transcripts so that “PCR negative” will have different significance as technology advances. Using current technology, sensitivity of > 4.5 logs below baseline can usually be achieved. In the IRIS study a BCR-ABL level 4.5 logs below baseline was defined as the maximum measurable response. We have called this a “4.5 log response” so that it can be differentiated from much more substantial molecular responses that may be verifiable in future studies using improved technology.
Frequency of molecular responses
Major molecular response as defined in the IRIS study (≥ 3 logs below baseline) was achieved in 39% of newly diagnosed CML patients after 12 months of imatinib therapy compared to only 2% of patients on the IFN-α + Ara-C arm (Figure 3; see Appendix, page 599). For the IRIS study, a reduction in BCR-ABL level of ≥ 4.5 logs was detected and verified on at least 1 occasion in 3.6% of patients who were in CCR (follow-up 18 months). Given the steady downward trend in median BCR-ABL levels for patients in CCR, the number achieving a 4.5 log response will probably increase significantly with longer follow-up. Similar percentages of patients who have undetectable BCR-ABL have been reported in smaller studies.85,93 At the MD Anderson Cancer Center, 13% of chronic phase patients who received imatinib at 400 mg/day had undetectable BCR-ABL on real-time and nested PCR. Furthermore, in a pilot study of imatinib 800 mg/day for chronic phase CML patients who have failed IFN-α, 41% of patients had undetectable BCR-ABL (median follow-up 14 months).94
Prognostic Significance of Cytogenetic and Molecular Responses
Patients who achieve CCR appear to have a good prognosis. Imatinib-treated patients in the IRIS study who did not achieve CCR by 12 months had a 15% probability of progression (to acute phase or loss of complete hematologic remission or MCR) over the subsequent 12 months compared to a 3% probability of progression for patients in CCR at 12 months.
Early reduction of BCR-ABL transcript levels is predictive of subsequent cytogenetic response in chronic phase patients.85,86,88 Merx et al analyzed 364 blood samples in 106 patients. They found that the BCR-ABL level at 2 months predicted for MCR at 6 months (P = .006).85 In the IRIS study, prognosis was better for the subset of imatinib-treated IRIS patients in CCR who also achieved a ≥ 3 log reduction from baseline levels. This was observed in 58% of patients in CCR after 12 months of imatinib therapy and was associated with a 100% probability of remaining progression free over the subsequent 12 months (n = 135), whereas patients in CCR who did not achieve a ≥ 3 log reduction in BCR-ABL had a 5% probability of progression (n = 103) (Figure 4 ). In the same study, 79/83 imatinib-treated patients (95%) who achieved ≥ 3 log reductions maintained or improved the response on subsequent testing 3–12 months later.95
What Should Be the Goal of Imatinib Therapy?
While CCR or major molecular responses are useful initial goals of therapy, they may not prove to be stable in the long term. Persistent leukemia at any level may eventually be the source of resistant clones. Complete eradication of leukemia would be the optimal outcome of therapy but this is not a verifiable goal. A practical and testable treatment endpoint would be sustained absence of BCR-ABL by the most sensitive and reliable assay available. While it may be reasonable to maintain imatinib therapy at standard dose for patients in CCR, it would be valuable to formally assess higher doses of imatinib or combination therapy in patients where leukemia remains detectable at the molecular level and is not steadily falling.
If the goal of undetectable BCR-ABL is achieved, the question will arise as to when imatinib can be safely stopped. It should be remembered that a patient may still have several million leukemic cells in the bone marrow without these being detectable by the most sensitive assays currently available. If still present, these residual leukemic cells are very likely to be capable of steady expansion in the absence of imatinib. Outside of properly conducted clinical trials, imatinib should be continued long term, until more is known about the stability of molecular response off therapy. A study looking at imatinib cessation in patients with undetectable BCR-ABL for several years would determine how frequently (if ever) imatinib was able to effectively eradicate CML. While accepting that it may not often be achievable, the ultimate aim of therapy in CML should be long-term absence of measurable leukemia after cessation of therapy.
Approach for Patients with Imatinib-Resistance
Primary resistance
In the context of newly diagnosed patients, failure to achieve complete hematologic remission by 3 months, and failure to achieve MCR by 6 or CCR by 12 months can be defined as primary resistance. The frequency of primary resistance appears to be dose dependent. In the IRIS study,69 4% of previously untreated patients did not achieve complete hematologic remission on imatinib 400 mg/day. Failure to achieve MCR by 6 months was seen in 23% of newly diagnosed patients; with continuing imatinib at 400 mg/day, 40% of this group still failed to achieve MCR by 24 months. Failure to achieve CCR by 12 months was seen in 31% of imatinib treated patients in the IRIS study. Thus, overall around 20%–30% of newly diagnosed patients appear primarily resistant to imatinib at 400 mg/day. When 36 chronic phase patients who had failed IFN-α therapy were treated with imatinib 800 mg/day in an MD Anderson study,94 no cases of failure to achieve CHR were reported, and only 11% did not achieve CCR. Thus, it seems likely that that the proportion of patients with primary resistance can be reduced with the use of higher doses of imatinib. Overall, approximately 90% of newly diagnosed patients might achieve these specified response levels on higher doses of imatinib. The reason at least 10% of patients have primary resistance despite higher doses of imatinib is not clear. No mutations or polymorphisms in BCR-ABL that would explain the lack of response in these patients have been identified to date.75
Acquired resistance
This can be defined as the loss of an established hematologic, cytogenetic, or molecular response as well as progression to accelerated or blast phase. In the IRIS trial, 8% of imatinib-treated patients had acquired resistance by 18 months.69 In a recent survey of imatinib-treated chronic phase patients, the incidence of acquired resistance in early and late chronic phase patients was 15% and 25% respectively (median follow-up 14 months).75
Loss of response to imatinib could be due either to the expansion of resistant CML cells that are not dependent on BCR-ABL for their transforming activity, or to the selection and expansion of CML cells that have by various means excessively activated Bcr-Abl kinase activity despite imatinib. Most studies in patients with acquired resistance have shown the appearance of a clone of cells with fully active Bcr-Abl kinase.13,16,17 This can be achieved by increasing the expression of BCR-ABL by chromosome or gene amplification. This appears to be the commonest mechanism in experimentally induced imatinib resistance,10,11,96 but is less frequently observed in the clinical setting. In a German survey of resistant cases 19/36 patients had additional chromosomal changes (8 involved additional copies of the Ph chromosome), and genomic amplification was demonstrated by FISH in 2/32 patients.16 In this survey, 23/66 patients (34%) had point mutations in BCR-ABL that led to increased kinase activity. Other studies have found that mutations in the kinase region of BCR-ABL are the commonest cause of acquired resistance14,15,75 (Table 1 ).
Incidence and Significance of BCR-ABL Mutations
In an Australian survey, 144 CML patients from all phases of the disease who had received at least 6 months of imatinib were tested for BCR-ABL kinase domain mutations by direct sequencing, regardless of their response to therapy. Mutations were detected in 27 patients at 17 different residues, 13/40 patients (33%) in accelerated phase, 14/64 patients (22%) in late-chronic phase (> 12 months from diagnosis until imatinib started), and 0/40 patients in early-chronic phase. Acquired resistance was evident in 24/27 (89%) patients with mutations. This tight correlation between mutation development and onset of resistance strongly suggests a causal role for the mutations in acquired resistance to imatinib. Patients who commenced imatinib more than 4 years from diagnosis had a significantly higher incidence of mutations (18/44; 41%) compared to those treated within 4 years (9/100; 9%) (P < .0001). This supports the concept that the leukemic clone accumulates sequence errors during DNA replication, some of which affect BCR-ABL. The probability of developing subclones that are imatinib resistant would relate to the duration of disease and the size of the stem cell pool at risk. Gradually, a pool of BCR-ABL mutants would be generated which, if they have a lower affinity for imatinib, would selectively expand in the setting of imatinib treatment.
Screening for BCR-ABL Mutations
The emergence of clones with mutated BCR-ABL is a common cause of resistance in patients with advanced phase CML and those in late chronic phase (Table 1 ).13,14,16,72–,75,97 It is too early to determine whether mutations will emerge as a common problem for patients treated in early chronic phase.
So far no preimatinib screening test has been developed that has demonstrated predictive value for subsequent resistance. Even though it is likely that in many cases the mutant clone is present prior to imatinib therapy, direct sequencing of the BCR-ABL kinase region prior to imatinib therapy does not appear to indicate patients at risk.75 This may be because the sensitivity of direct sequencing will not generally allow detection of a mutant population which is less than 10–20% of the total population of leukemic cells. At present, (1) phase of disease, (2) duration of CML prior to imatinib therapy, and (3) initial response to imatinib provide the best indication of the risk of mutation development. Serial mutation screening once imatinib is commenced may be valuable in high-risk cases, but in nearly all patients a significant rise in BCR-ABL levels is seen around the time of mutation detection and may be used as an indication of the need for mutation screening.
Significance of emerging mutations
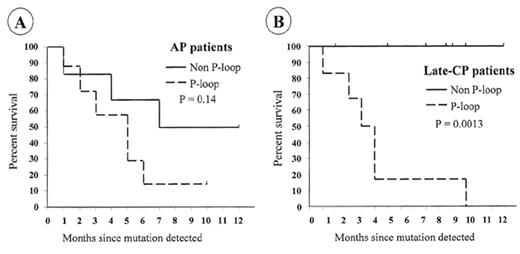
For patients who develop resistance, a search should be made for the cause. Direct sequencing of the BCR-ABL kinase domain should reveal any emerging mutant clones. If mutations are identified, the appropriate response will depend on the actual amino-acid change. Nearly all mutations that become detectable by direct sequencing are associated with evidence of loss of molecular response. The commonest pattern when first detected is a mix of mutant and wild type BCR-ABL cells, but with continued imatinib therapy the mutant clone usually predominates.75 Detection of mutations that completely prevent imatinib binding (e.g., T315I) should probably lead to cessation of imatinib and the use of other therapy. Patients with mutations that have less marked effects on imatinib binding (e.g., M244V, F311L, M351T, F317L, E355G, F359V)14,99,100 may respond to increased doses of imatinib.75 The ATP phosphate binding loop (P-loop) is a highly conserved region of the kinase domain involved in ATP binding and is a frequent site of mutations. Mutations in the P-loop (amino acids 250–255) may have a particularly poor prognosis.75 In the Australian survey, 12/13 patients (92%) with mutations in the P-loop died, with a median survival of 4.5 months after the mutation was detected. In contrast, only 3/14 patients (21%) with mutations outside the P-loop died (median follow-up of 11 months) (Figure 5 ). Patients with no evidence of mutation had a 3% mortality with a similar follow-up.
Conclusions
Based on the accuracy and sensitivity of Q-PCR for measuring BCR-ABL levels in peripheral blood, this should become the method of choice for monitoring patients on imatinib and will facilitate comparisons of different imatinib-based treatment strategies. At the individual patient level, Q-PCR studies can identify degrees of molecular response that predict long-term stability, as well as patterns of response that provide an early indication of relapse and imatinib resistance. For patients with acquired resistance, a search for BCR-ABL mutations is warranted both to guide future therapy and to further our understanding of the biological and clinical impact of these emerging mutant clones.
III. Managing CML with Patient-Specific Strategies
Jane F. Apperley, MBChB*
Imperial College of Medicine, Hammersmith Hospital, Department of Haematology, Du Cane Road, London W12 0NN, UK
It is highly unusual that the introduction of novel, effective, and safe therapies for any malignancy results in controversy regarding the optimal management of the disease. However, this seems to be the current situation for CML. The development of imatinib has the potential to revolutionize the management of CML and other disorders resulting from the deregulation of 1 of the tyrosine kinase targets of this drug. However, the long-term outcome for patients with CML treated with imatinib alone is unknown—hence the dilemma. In advocating imatinib as the treatment of choice for all patients with CML we may deny individual patients the chance of cure by a long-standing and universally accepted therapy, namely allogenice stem cell transplantation (allo-SCT). As new information becomes available from patients treated with imatinib from diagnosis, we must try to develop a wide range of prognostic indicators to help distinguish patients for whom the apparently safe option is not necessarily the correct option, from those destined to have prolonged survival with targeted drug therapy.
Furthermore, the introduction of imatinib into clinical practice has raised a number of other questions, including the optimal use of this drug in terms of dose, dosing schedules, duration of treatment, definitions of response and conversely failure, and the continuing management of patients who fail to achieve molecular responses. The answers to these dilemmas are not yet available and the future of management of CML will depend on the rapid accumulation of data from clinical studies and their objective interpretation. In this section we will attempt to set out these questions and begin to suggest tools that might be used to determine the answers.
How Should the Newly Diagnosed Patient Be Managed?
In the future, randomized trials for the use of imatinib in higher doses or in combination with other agents will be available. Small Phase II studies of the safety and feasibility of giving imatinib in combination with interferon (IFN)-α or cytosine arabinoside (Ara-C) have now been completed and form the basis of a large multinational study, known as SPIRIT. The full protocol will have 4 arms, comparing imatinib 400 mg/day with imatinib 600–800 mg/day, imatinib in combination with pegylated IFN-α, and imatinib in combination with Ara-C. The study has commenced in France and hopefully will begin recruiting patients in the United Kingdom in the latter part of 2003. A similar four-arm study will soon be activated in the US.
In the meantime, decisions must be made concerning the management of newly diagnosed patients. At the present time there are broadly two approaches to the management of the newly diagnosed patient. The first is to offer all patients a trial of imatinib and offer transplant only to those patients who fail treatment according to a preestablished set of guidelines for lack or loss of response. The second is to continue to discuss up-front allogeneic transplant with selected groups of individuals for whom more aggressive therapy is deemed appropriate.
Should Every Patient Have a Trial of Imatinib?
The advantages of recommending imatinib as first-line therapy for all newly diagnosed patients are clear (Table 2 ). The results of the IRIS study of imatinib alone versus IFN-α plus Ara-C69 showed that the chance of remaining progression free at 18 months was 97% for patients receiving imatinib alone versus 93% for those given IFN-α plus Ara-C. This was associated with a considerable increase in the proportion of patients achieving MCR and CCR at 87% and 76% for imatinib alone versus 34% and 15% for IFN-α plus Ara-C (P < .001). By giving all newly diagnosed patients imatinib, the immediate morbidity and mortality of allo-SCT will be avoided, perhaps indefinitely, for some patients.
The disadvantages are also apparent. Despite the encouraging results of the IRIS study, 20–30% of newly diagnosed patients fail to achieve such a good response.69 Furthermore, a number of questions concerning imatinib remain outstanding, not least of which is whether the occurrence of MCR and CCR will translate into a survival advantage. As mentioned earlier, although the majority of patients achieve hematologic and cytogenetic remission, a very small proportion achieve reverse transcriptase polymerase chain reaction (RT-PCR) negativity for BCR-ABL.95 This is in contrast to RT-PCR results in long-term survivors of allo-SCT,101,102 and raises the possibility that the effects of imatinib may not be durable. Alongside these considerations are concerns regarding the development of resistance to imatinib.13–,16,75
In pursuing a strategy of offering a trial of imatinib to all patients it is important to have clear definitions of both “response to” and “failure of” treatment (Table 3 ), and a plan for managing both of these situations. There are some patterns of patient response (or lack of response) that can be considered failure of treatment without controversy. Any patient who has achieved a degree of response, hematologic, cytogenetic, or molecular, and then loses this response while still on treatment, should be considered at risk of disease progression. An individual who fails to achieve hematologic control is also unlikely to do well in the long term. However, patients who have partial and/or gradual changes in their cytogenetic responses are more difficult to classify. Previously, we arbitrarily decided that those patients who had not achieved an MCR at 12 months, were imatinib “failures.”103 More recently we have altered our definition of treatment failure to a lack of CCR after 12 months of treatment at 400 mg/day. This change has been prompted by the relatively small increase in the proportion of patients achieving CCR at 18 months (76%) compared to 12 months (69%) in the IRIS study and by the knowledge that the best outcome of allo-SCT is found in patients transplanted within 1 year of diagnosis.104,105 It would, therefore, be preferable to reduce the duration of an imatinib trial to less than 12 months. Furthermore, there are data to suggest that the rapidity of response may in itself be a prognostic indicator of the likelihood of achievement of MCR and therefore possibly of progression-free survival.85,86 In the future, there may be additional features of response to treatment in newly diagnosed patients that might be used to guide management. For instance, we have studied the outcome of patients treated with imatinib after ‘failure’ of IFN-α at our own institution, and feel that for this particular group, we can identify relatively early in their treatment, patients who are unlikely to achieve MCR or CCR. The 2 most important predictive factors appeared to be a failure to obtain at least a minor cytogenetic remission (≥ 35% Ph-negativity) after 3 months of treatment, and the development of neutropenia during this time.106 These 2 features were used to develop a prognostic score in which patients could score 2 (high risk), 1 (intermediate risk), or 0 (low risk) points. At 18 months, the probabilities according to risk group were 100%, 90% (CI 79%–95%) and 53% (CI 44%–63%) (P = .0002) for survival and 100%, 75% (CI 62%–85%) and 30% (CI 20%–44%) (P < .0001) for progression-free survival, respectively. Although highly predictive in these individuals, we have no information as to whether such a prognostic score will be applicable to newly diagnosed patients.
Is There a Role for Up-Front Transplant?
A logical approach to this question would be to restrict up-front transplantation to those patients likely to do extremely well with allo-SCT (and for whom it might be misguided to offer them an alternative therapy) and those likely to do poorly with imatinib. The question remains as to whether these two groups can be accurately identified.
The factors influencing the outcome of allo-SCT are well recognized. Age, disease status, disease duration, recipient-donor gender combinations, and the source of the transplant product have all been identified as significantly influencing long-term survival.105 In recipients of unrelated transplant, cytomegalovirus (CMV) serostatus is also influential.107 Young patients with CML in chronic phase within the first year of diagnosis who have HLA-identical donors should continue to be offered allo-SCT. The area of debate must be the definition of “young.” For recipients of sibling transplant, this might be up to 45 years of age, and up to 35 years for patients with unrelated donors. If the Sokal or Hasford scores108,109 at diagnosis suggest a good prognosis, then the ages might be reduced. In contrast, for unrelated transplant if both donor and recipient are CMV negative and HLA-matched by high resolution typing, the definition of “young” might be more liberal.
The early identification of patients likely to do badly with imatinib is more problematic and probably impossible at this time. It is possible that Sokal and/or Hasford scores will be found to correlate with outcome of treatment with imatinib, and could be used to distinguish groups of patients at diagnosis. Huntly et al have previously reported that the presence of derivative chromosome 9q+ deletions at diagnosis is associated with adverse outcome on conventional therapy.110 More recently this group was unable to document a difference in survival after treatment with imatinib for patients with or without a deletion. However, a decrease in the proportion of patients achieving hematologic and cytogenetic responses and an increased rate of progression on imatinib were observed in the presence of the deletion suggesting that, with time, a difference in survival might become apparent.111
With the advent of gene expression profiling it might be possible to identify a pattern associated with early disease progression on imatinib. However, if this were related to the presence of very small numbers of resistant cells with point mutations in the kinase domain of BCR-ABL, the nature of the micro-array assays would preclude their detection.
What Is the Place of Reduced Intensity Transplant Procedures?
Recent developments in reduced intensity conditioning transplantation (RICT) procedures also cloud the issue of early transplant. In theory, the concept of the gradual ablation of host hematopoiesis by donor T cells present in the original graft, or arising in the posttransplant period, or by additional lymphocyte infusions, should have its optimal outcome in CML. Evidence to support this has been somewhat delayed, probably due to the introduction of imatinib into clinical practice, and a reluctance to subject these patients to an experimental technique. Recently however, Ori et al112 have reported remarkable results in 24 patients with CML transplanted with reduced intensity regimens (fludarabine, melphalan). The overall and disease-free survival at 5 years was 85%, with all evaluated patients being free of leukemia by RT-PCR.
In an attempt to confirm these remarkable results, the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT-CLWP) have recently conducted a retrospective study of the outcome of reduced intensity transplant procedures for CML.113 They identified 223 patients with a median age of 49 years who had received RICT using a variety of conditioning regimens. Transplant-related mortality at 1 year was acceptable at 18% for patients in first chronic phase and 31% for more advanced phase disease. Acute graft-versus-host disease (GvHD) > Grade I occurred in 29% of patients, and 26% of evaluable patients experienced extensive chronic GvHD. Factors predictive of improved outcome included transplant in chronic phase and transplant within 1 year of diagnosis. It would appear that patients should be offered RICT based on the same criteria that are applied to conventional allo-SCT. This group of patients was highly heterogeneous with respect to their disease status, types of donor, prior therapy, and conditioning regimens. A clearer picture of the benefits of RICT and imatinib in the management of patients with CML will only emerge from well-conducted prospective studies.
Can Imatinib and Allo-SCT Be Combined to Optimize Management of Newly Diagnosed Patients?
With the exception of a small proportion of patients on IFN-α, we have never previously had a chemotherapeutic agent capable of inducing cytogenetic remissions of reasonable duration. The use of imatinib prior to transplantation would permit transplant in “complete remission,” and might therefore reduce the risk of disease recurrence. This approach could then be reasonably combined with a RICT to minimize transplantrelated mortality. The risk of this strategy is a possible harmful effect of prior therapy with imatinib on the outcome of transplant, akin to that observed by some groups with IFN-α.114,115
Imatinib has already been widely and effectively used to restore remission in patients relapsing after allo-SCT.116 An alternative to the above approach would be to perform RICT soon after diagnosis without prior imatinib. The kinase inhibitor would be introduced shortly after transplantation prophylactically or preemptively to control minimal residual disease, thereby delaying administration of donor lymphocytes to beyond 1 year of transplant, and thus reducing the risk of GvHD and cytopenias.117
How Should the Patient in Continuing Chronic Phase Be Managed?
We have defined a response to imatinib as the achievement of a CCR within 12 months of the onset of therapy. Patients who fail to respond or who lose their response, even though they remain in chronic phase, should be offered an allogeneic transplant (conventional or RICT) as soon as possible if they are of a suitable age and performance status and have an available donor. However, allo-SCT will be unsuitable for some of these patients and other therapies should be investigated. Various possibilities include: (1) continuing imatinib at 400 mg/day; (2) increasing the dose of imatinib to 600 or 800 mg/day; (3) adding another agent such as IFN-α, Ara-C, hydroxyurea, decitabine, or homoharringtonine; (4) changing treatment to one or more of these agents; and (5) considering an autologous transplant.
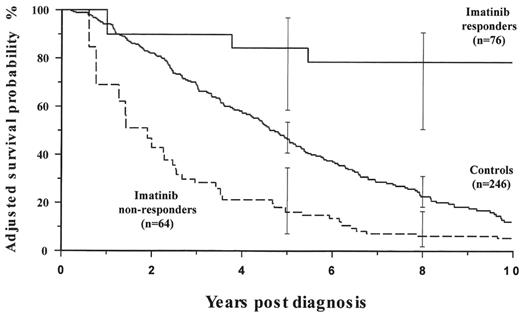
We have recently completed a case matched study of patients who received IFN-α during the Medical Research Council (MRC) CML III trial,118 who were unable to receive imatinib as their disease predated its introduction, with patients who received IFN-α as standard therapy, failed treatment, and then received imatinib. Patients treated with imatinib after IFN-α failure who achieved at least a minor cytogenetic response had a much improved outcome compared to the historical group. However patients who received imatinib and failed to get a minor cytogenetic response had a considerably worse outcome than the controls (Figure 6 ). This might suggest that, for some patients, continuing imatinib in the absence of a minor cytogenetic response might actually be harmful.119
Kantarjian et al recently reported that 19 (56%) of the 34 patients classified as cytogenetically resistant on imatinib at 400 mg/day subsequently improved cytogenetically when imatinib was increased to 600 or 800 mg/day.120 Our own experience has been less favorable. Of 36 consecutive patients with CML in chronic phase in complete hematologic response whose imatinib dosage was increased when they failed to achieve a CCR on 400 mg/day, 14 (39%) improved their cytogenetic responses and 7 (19%) achieved CCR. Unfortunately, many of these responses were short-lasting and at the latest follow-up 6 (43%) of the 14 had lost their best response. Similarly disappointing results have been reported by Zonder et al.121
Homoharringtonine (HHT) is a cephalotaxine alkaloid that has both in vitro and in vivo activity against CML. O’Brien et al122 treated 71 patients in late chronic phase with HHT by continuous infusions over 7–14 days. Of these, 72% of the patients achieved complete hematologic remission with 15% developing MCR. Laboratory data suggested that the effects of HHT in combination with IFN-α or Ara-C would be synergistic123 and this was subsequently confirmed in a further cohort of 37 patients in early chronic phase who received HHT and IFN-α. The rates of complete hematologic and cytogenetic responses were higher at 84% and 52%, respectively, compared to historical groups treated with either agent alone.124 Further in vitro data using CML cell lines and primary material suggest that the combination of imatinib and HHT may also be additive.125,126
Autologous transplantation has been widely used in the management of CML but its exact role has never been clearly defined. There is a suggestion that autografting may prolong the chronic phase for some patients127 and, in addition, that it may restore sensitivity to IFN-α in patients who have lost prior cytogenetic responses.128 Autologous transplant may therefore have a role to play in both improving the degree of cytogenetic and molecular responses to imatinib and in restoring responses. Furthermore, patients who achieve CCR may be offered autologous transplantation with Ph-negative stem cells either to consolidate their remission or to prolong the chronic phase at the time of loss of response or progression to advanced phase. These patients should therefore be offered the opportunity to cryopreserve a population of Ph-negative stem cells at the time of CCR. However, the attempts at harvesting stem cells under these circumstances have met with mixed success. Some groups have attempted stem cell collection while the patients remained on imatinib. This appears to be successful in approximately 40–50% of patients in CCR129 (and Drummond et al, submitted). In Drummond et al’s study, shorter duration of the chronic phase prior to commencing imatinib was associated with successful collection. In contrast, Hui et al found no correlation between the duration of CML or of treatment with imatinib and the cell yield. This was, however, improved when imatinib was withheld for 3 days prior to the start of growth factor administration.128 To date there have not been any reports of the use of cells mobilized from imatinib responders in autologous transplant procedures.
The management of patients who achieve CCR but who continue to have molecular evidence of disease is highly controversial. In this context, managing a clear “failure” might be considerably easier than managing a partial success. These patients are not “cured” of their disease and are therefore at risk of disease progression. The simplest approach is to continue treatment with regular molecular monitoring by quantitative RT-PCR. If levels of residual disease show a sustained increase, the patients can be offered transplant if possible. Alternatively they, like patients with cytogenetic responses other than complete, might be suitable candidates for alternative strategies within carefully conducted Phase II/III studies. Such strategies include those listed above for imatinib nonresponders with the additional choices of conventional or reduced intensity allo-SCT where possible and adoptive immunotherapy.
Adoptive immunotherapy is designed to enhance the autologous or allogeneic immune response to the leukemia. CML is known to respond to immune-mediated therapies, in particular the use of donor lymphocyte infusions to treat relapse occurring after allo-SCT.130 To date, a consistent target antigen for the immune response has not been identified. Several groups have confirmed the immunogenicity of peptides derived from the junctional region of the e14a2 fusion protein in that lysis of BCR-ABL-expressing leukemic cells by HLA-restricted cytotoxic T cells (CTL) can be achieved in vitro (reviewed by Schwartz et al131). However, lysis is relatively inefficient and this was attributed to poor expression of peptides on the cell surface. More recently, Clark et al132 have shown the presence of both cell surface Bcr-Abl peptides presented by HLA A0301 and circulating peptide-restricted T cells. Attempts to use e14a2-derived peptides as vaccines have met with limited success.133 Cathcart et al134 treated 12 patients by vaccination with a combination of peptides over a 10-week period. Proliferative responses to autologous cells were demonstrated in 11 individuals. Three patients achieved an increase in the proportion of Ph-negative metaphases in their bone marrow and 2 patients treated for disease recurrence after allo-SCT, became RT-PCR negative for BCR-ABL transcripts.
Others have utilized alternative target antigens such as proteinase 3, Wilms tumor protein (WT1) and minor histocompatibility antigens. Proteinase 3, normally found in myeloid granules, is overexpressed in myeloid leukemias. HLA-restricted T cells specific for a nonpolymorphic peptide (PR1) were capable of inhibiting the growth of CML progenitors.135 Furthermore, high avidity PR1-specific T cells are absent from the blood of newly diagnosed patients with CML but can be found in cytogenetic responders. These high avidity T cells underwent apoptosis when exposed to leukemic cells overexpressing proteinase 3 suggesting that they are preferentially deleted during the course of the disease.136 Phase I/II studies of the efficacy of PR1 peptide vaccination have now been initiated.
WT1 is also overexpressed in leukemic cells and is currently used as a marker of minimal residual disease. Several groups have generated WT1-derived peptide-specific T cells that can lyse autologous targets and preferentially inhibit the growth of CML progenitors (reviewed by Schwartz et al131). There is also evidence of a humoral response to WT1 with a high incidence of WT1-specific antibodies in patients with CML. Antibodies were found in 19% of affected patients compared to 2% of normal individuals.137 It is highly likely that studies of both WT1 cytotoxic T cells and peptide vaccination will be tested clinically in the near future.
HSP-peptide complexes contain a broad array of peptides tightly but noncovalently bound to the HSP molecules. It is the peptide component that is patient-specific and most importantly, immunogenic. Udono and Srivastava have shown that Hsp70 derived from tumor cells elicits tumor-specific immunity while Hsp70 derived from normal cells does not.138 Preliminary results of a phase I study using Hsp70-peptide derived from autologous leukocytes of patients with CML suggest that the vaccine is safe and a phase II study of efficacy is currently under way.
In the context of allogeneic transplantation, differences in the expression of minor histocompatibility antigens (mH), might be suitable for exploitation for immunotherapy. Goulmy’s group has identified HLA-restricted mH antigen-specific CTL in recipients of allogeneic transplantation.139 In particular, a patient with CML in accelerated phase was successfully treated with leukemia-specific CTLs.140 More recently, this group has been able to characterize a number of hematopoietic-specific mH (i.e., HA-1 and HA-2) and has subsequently generated HA-1 and HA-2 specific CTL.141 They suggest that these CTL might be used clinically to eradicate leukemia without the complication of GvHD, which still presents an obstacle to the successful use of bulk populations of donor lymphocytes.
The future of CML management must lie in devising individual patient-specific strategies. At the present time, the long-term outcome of treatment with imatinib remains uncertain. Patients must be evaluated for adverse features at the time of diagnosis and repeatedly throughout the course of their disease. Those who are responding suboptimally to their current therapy should be offered alternative approaches in the context of well-designed and well-recorded clinical studies. The possibilities for management are numerous and exciting. However, our enthusiasm must be tempered with an objective approach to the problem that CML is a heterogeneous disease and, therefore, the various subpopulations of patients differ from each other in the nature of their response to individual therapeutic regimens.
Point-mutations in the BCR-ABL kinase domain associated with imatinib resistance in chronic myeloid leukemia (CML) and Ph+ acute lymphoblastic leukemia (ALL) patients.
| Nucleotide Change* . | Amino Acid Change† . | Proposed Mechanism of Resistance . | No. of Cases (Detected/Tested)‡ . | Refs. . |
|---|---|---|---|---|
| * Positions according to GenBank no. M14752. | ||||
| † Positions according to GenBank no. AAB60394 for ABL type 1a, and in brackets the corresponding position for Abl type 1b (where the N-terminal domain is 19-amino acids longer). | ||||
| ‡ Among patients who developed resistance or never responded to imatinib. The table collates data from 8 independent series where patients were screened for DNA mutations in the tyrosine kinase region of Bcr-Abl. A total of 149 mutational events were reported in these 206 patients (with a few cases exhibiting more than 1 mutation in the resistant clone). This indicates that point mutations in the kinase domain account for approximately 72% of the cases where lack or loss of response to imatinib is observed in the clinic. No mutations were yet detected in primary or acquired resistance of patients in early chronic phase. | ||||
| A1094G | M244V (M263V) | Impairs conformational change (?) | 1/32 | 14 |
| 1/66 | 16 | |||
| 1/27 | 75 | |||
| C1106G | L248V (L267V) | Impairs conformational change | 1/27 | 75 |
| G1113A | G250E (G269E) | Impairs conformational change | 2/28 | 15 |
| 2/32 | 14 | |||
| 2/27 | 75 | |||
| A1119G | Q252R (Q271R) | Impairs conformational change | 1/32 | 14 |
| G1120C/T | Q252H (Q271H) | Impairs conformational change | 6/32 | 14 |
| 1/66 | 16 | |||
| 5/27 | 75 | |||
| T1121C | Y253H (Y272H) | Impairs conformational change | 2/8 | 72 |
| 1/28 | 15 | |||
| 2/32 | 14 | |||
| 4/66 | 16 | |||
| A1122T | Y253F (Y272F) | Impairs conformational change | 3/32 | 14 |
| 1/66 | 16 | |||
| 2/27 | 75 | |||
| G1127A | E255K (E274K) | Impairs conformational change | 1/12 | 97 |
| 2/8 | 72 | |||
| 4/28 | 15 | |||
| 6/9 | 142 | |||
| 10/32 | 14 | |||
| 3/66 | 16 | |||
| 2/27 | 75 | |||
| A1128T | E255V (E274V) | Impairs conformational change | 1/8 | 72 |
| 1/66 | 16 | |||
| 1/27 | 75 | |||
| T1495C | F311L (F330L) | Unknown | 1/24 | 73 |
| C1308T | T315I (T334I) | Affects imatinib binding | 2/8 | 72 |
| 10/32 | 14 | |||
| 3/28 | 15 | |||
| 1/9 | 141 | |||
| 3/24 | 73 | |||
| 6/66 | 16 | |||
| 2/27 | 75 | |||
| C1315G | F317L (F336L) | Affects imatinib binding | 1/28 | 15 |
| 3/32 | 14 | |||
| T1392C | M343T (M362T) | Unknown | 1/32 | 14 |
| T1416C | M351T (M370T) | Impairs conformational change | 2/28 | 15 |
| 10/32 | 14 | |||
| 1/24 | 73 | |||
| 4/66 | 16 | |||
| 7/27 | 75 | |||
| A1428G | E355G (E374G) | Impairs conformational change | 1/32 | 14 |
| 1/66 | 16 | |||
| 2/27 | 75 | |||
| T1439G | F359V F378V) | Affects imatinib binding (?) | 2/32 | 14 |
| 2/27 | 75 | |||
| G1499A | V379I (V398I) | Impairs conformational change (?) | 1/32 | 14 |
| T1508C | F382L (F401L) | Unknown | 1/32 | 14 |
| T1523A | L387M (L406M) | Impairs conformational change (?) | 1/32 | 14 |
| A1551G | H396R (H415R) | Impairs conformational change (?) | 3/32 | 14 |
| 1/66 | 16 | |||
| 1/27 | 75 | |||
| C1614A | S417Y (S436Y) | Unknown | 1/27 | 75 |
| G1739A | E459K (E478K) | Unknown | 1/27 | 75 |
| T1821C | F486S (F505S) | Unknown | 1/27 | 75 |
| Nucleotide Change* . | Amino Acid Change† . | Proposed Mechanism of Resistance . | No. of Cases (Detected/Tested)‡ . | Refs. . |
|---|---|---|---|---|
| * Positions according to GenBank no. M14752. | ||||
| † Positions according to GenBank no. AAB60394 for ABL type 1a, and in brackets the corresponding position for Abl type 1b (where the N-terminal domain is 19-amino acids longer). | ||||
| ‡ Among patients who developed resistance or never responded to imatinib. The table collates data from 8 independent series where patients were screened for DNA mutations in the tyrosine kinase region of Bcr-Abl. A total of 149 mutational events were reported in these 206 patients (with a few cases exhibiting more than 1 mutation in the resistant clone). This indicates that point mutations in the kinase domain account for approximately 72% of the cases where lack or loss of response to imatinib is observed in the clinic. No mutations were yet detected in primary or acquired resistance of patients in early chronic phase. | ||||
| A1094G | M244V (M263V) | Impairs conformational change (?) | 1/32 | 14 |
| 1/66 | 16 | |||
| 1/27 | 75 | |||
| C1106G | L248V (L267V) | Impairs conformational change | 1/27 | 75 |
| G1113A | G250E (G269E) | Impairs conformational change | 2/28 | 15 |
| 2/32 | 14 | |||
| 2/27 | 75 | |||
| A1119G | Q252R (Q271R) | Impairs conformational change | 1/32 | 14 |
| G1120C/T | Q252H (Q271H) | Impairs conformational change | 6/32 | 14 |
| 1/66 | 16 | |||
| 5/27 | 75 | |||
| T1121C | Y253H (Y272H) | Impairs conformational change | 2/8 | 72 |
| 1/28 | 15 | |||
| 2/32 | 14 | |||
| 4/66 | 16 | |||
| A1122T | Y253F (Y272F) | Impairs conformational change | 3/32 | 14 |
| 1/66 | 16 | |||
| 2/27 | 75 | |||
| G1127A | E255K (E274K) | Impairs conformational change | 1/12 | 97 |
| 2/8 | 72 | |||
| 4/28 | 15 | |||
| 6/9 | 142 | |||
| 10/32 | 14 | |||
| 3/66 | 16 | |||
| 2/27 | 75 | |||
| A1128T | E255V (E274V) | Impairs conformational change | 1/8 | 72 |
| 1/66 | 16 | |||
| 1/27 | 75 | |||
| T1495C | F311L (F330L) | Unknown | 1/24 | 73 |
| C1308T | T315I (T334I) | Affects imatinib binding | 2/8 | 72 |
| 10/32 | 14 | |||
| 3/28 | 15 | |||
| 1/9 | 141 | |||
| 3/24 | 73 | |||
| 6/66 | 16 | |||
| 2/27 | 75 | |||
| C1315G | F317L (F336L) | Affects imatinib binding | 1/28 | 15 |
| 3/32 | 14 | |||
| T1392C | M343T (M362T) | Unknown | 1/32 | 14 |
| T1416C | M351T (M370T) | Impairs conformational change | 2/28 | 15 |
| 10/32 | 14 | |||
| 1/24 | 73 | |||
| 4/66 | 16 | |||
| 7/27 | 75 | |||
| A1428G | E355G (E374G) | Impairs conformational change | 1/32 | 14 |
| 1/66 | 16 | |||
| 2/27 | 75 | |||
| T1439G | F359V F378V) | Affects imatinib binding (?) | 2/32 | 14 |
| 2/27 | 75 | |||
| G1499A | V379I (V398I) | Impairs conformational change (?) | 1/32 | 14 |
| T1508C | F382L (F401L) | Unknown | 1/32 | 14 |
| T1523A | L387M (L406M) | Impairs conformational change (?) | 1/32 | 14 |
| A1551G | H396R (H415R) | Impairs conformational change (?) | 3/32 | 14 |
| 1/66 | 16 | |||
| 1/27 | 75 | |||
| C1614A | S417Y (S436Y) | Unknown | 1/27 | 75 |
| G1739A | E459K (E478K) | Unknown | 1/27 | 75 |
| T1821C | F486S (F505S) | Unknown | 1/27 | 75 |
Advantages and disadvantages of two up-front therapeutic approaches to chronic myeloid leukemia (CML).
| Approach . | Advantages . | Disadvantages . |
|---|---|---|
| Abbreviations: Allo-SCT, allogeneic stem cell transplantation; RICT, reduced intensity conditioning transplantation; DLI, donor lymphocyte infusion | ||
| Allo-SCT for selected patients | Proven curative ability | Transplant-related mortality |
| Decreased risk of progression of disease | Transplant-related morbidity | |
| Ability to integrate imatinib, RICT and DLI | Chronic graft-versus-host disease | |
| Psychological (some patients) | Late effects (e.g., secondary malignancy, endocrine imbalance, cataract formation) | |
| Trial of imatinib for all patients | Avoids transplant-related mortality and morbidity for some | No long-term survival data |
| Curative ability unknown | ||
| Psychological (some patients) | Emerging resistance | |
| Delaying curative therapy | ||
| Risk of progression | ||
| Allo-SCT more likely to be performed for advanced phase disease | ||
| Approach . | Advantages . | Disadvantages . |
|---|---|---|
| Abbreviations: Allo-SCT, allogeneic stem cell transplantation; RICT, reduced intensity conditioning transplantation; DLI, donor lymphocyte infusion | ||
| Allo-SCT for selected patients | Proven curative ability | Transplant-related mortality |
| Decreased risk of progression of disease | Transplant-related morbidity | |
| Ability to integrate imatinib, RICT and DLI | Chronic graft-versus-host disease | |
| Psychological (some patients) | Late effects (e.g., secondary malignancy, endocrine imbalance, cataract formation) | |
| Trial of imatinib for all patients | Avoids transplant-related mortality and morbidity for some | No long-term survival data |
| Curative ability unknown | ||
| Psychological (some patients) | Emerging resistance | |
| Delaying curative therapy | ||
| Risk of progression | ||
| Allo-SCT more likely to be performed for advanced phase disease | ||
Clinical definitions of resistance.
| Primary . | Acquired . |
|---|---|
| Absence of a hematologic response within 3 months of starting treatment with at least 300 mg/day | Loss of hematologic response |
| Failure to achieve at least a minor cytogenetic response (> 35% Ph-negativity) after 3 months’ treatment with at least 400 mg/day | Loss of a complete cytogenetic response |
| Failure to achieve a major cytogenetic response after 6 months’ treatment with at least 400 mg/day | An increase of 30% or more in the number of Ph-positive bone marrow metaphases examined at intervals of 3 months or longer |
| Failure to achieve a complete cytogenetic response after 12 months’ treatment with at least 400 mg/day | Acquisition of new cytogenetic abnormalities in the Ph-positive clone |
| An increase in the BCR-ABL/control gene ratio of one log or more on serial testing or to the range associated with Ph-positivity |
| Primary . | Acquired . |
|---|---|
| Absence of a hematologic response within 3 months of starting treatment with at least 300 mg/day | Loss of hematologic response |
| Failure to achieve at least a minor cytogenetic response (> 35% Ph-negativity) after 3 months’ treatment with at least 400 mg/day | Loss of a complete cytogenetic response |
| Failure to achieve a major cytogenetic response after 6 months’ treatment with at least 400 mg/day | An increase of 30% or more in the number of Ph-positive bone marrow metaphases examined at intervals of 3 months or longer |
| Failure to achieve a complete cytogenetic response after 12 months’ treatment with at least 400 mg/day | Acquisition of new cytogenetic abnormalities in the Ph-positive clone |
| An increase in the BCR-ABL/control gene ratio of one log or more on serial testing or to the range associated with Ph-positivity |
Landmark analysis looking at the actuarial probability of disease progression (death during treatment, progression to accelerated or blast phase of chronic myeloid leukemia [CML], loss of complete hematologic or major cytogenetic response or increase in white blood cells [WBC]) according to the level of cytogenetic and molecular response after 12 months of imatinib (P < .001; log-rank test).
Abbreviations: CCyR, complete cytogenetic response
Landmark analysis looking at the actuarial probability of disease progression (death during treatment, progression to accelerated or blast phase of chronic myeloid leukemia [CML], loss of complete hematologic or major cytogenetic response or increase in white blood cells [WBC]) according to the level of cytogenetic and molecular response after 12 months of imatinib (P < .001; log-rank test).
Abbreviations: CCyR, complete cytogenetic response
Kaplan-Meier survival curves for patients with mutations.(A) There was a significant difference in the survival rate of late-CP patients with P-loop and non-P-loop mutations. (B) The difference in the survival rate for AP patients did not reach significance.
Abbreviations: AP, accelerated phase; CP, chronic phase
Kaplan-Meier survival curves for patients with mutations.(A) There was a significant difference in the survival rate of late-CP patients with P-loop and non-P-loop mutations. (B) The difference in the survival rate for AP patients did not reach significance.
Abbreviations: AP, accelerated phase; CP, chronic phase
Adjusted probabilities of survival according to cytogenetic response and treatment (from the left truncated Cox regression model).
Patients on imatinib who achieved at least a minor cytogenetic response during the first 6 months of treatment obtained benefit when compared with patients receiving conventional treatment, whereas imatinib-treated patients who failed to respond had a poorer survival than controls. Adjusted probabilities of survival at 8 years from diagnosis were 78.4% (CI 51.3–92.6) (RR: 0.13, CI: 0.046–0.39) for imatinib responders, 22.6% (CI: 17.8–29.4) for control patients and 6.2% (CI: 2.1–16.8) (RR: 1.69, CI: 1.086–2.64) for imatinib nonresponders (see text). Other variables found to be significant in the multivariate model were age ≥ 60 years (RR: 1.58, CI: 1.16–2.14), primary hematologic resistance to interferon-α (RR: 2.62, CI: 1.69–4.07), primary cytogenetic resistance to interferon-α (RR: 0.3,CI: 0.10–0.87) and Sokal high-risk group (RR: 2.0, CI: 1.22–3.28).
Adjusted probabilities of survival according to cytogenetic response and treatment (from the left truncated Cox regression model).
Patients on imatinib who achieved at least a minor cytogenetic response during the first 6 months of treatment obtained benefit when compared with patients receiving conventional treatment, whereas imatinib-treated patients who failed to respond had a poorer survival than controls. Adjusted probabilities of survival at 8 years from diagnosis were 78.4% (CI 51.3–92.6) (RR: 0.13, CI: 0.046–0.39) for imatinib responders, 22.6% (CI: 17.8–29.4) for control patients and 6.2% (CI: 2.1–16.8) (RR: 1.69, CI: 1.086–2.64) for imatinib nonresponders (see text). Other variables found to be significant in the multivariate model were age ≥ 60 years (RR: 1.58, CI: 1.16–2.14), primary hematologic resistance to interferon-α (RR: 2.62, CI: 1.69–4.07), primary cytogenetic resistance to interferon-α (RR: 0.3,CI: 0.10–0.87) and Sokal high-risk group (RR: 2.0, CI: 1.22–3.28).

![Figure 4. Landmark analysis looking at the actuarial probability of disease progression (death during treatment, progression to accelerated or blast phase of chronic myeloid leukemia [CML], loss of complete hematologic or major cytogenetic response or increase in white blood cells [WBC]) according to the level of cytogenetic and molecular response after 12 months of imatinib (P < .001; log-rank test). / Abbreviations: CCyR, complete cytogenetic response](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2003/1/10.1182_asheducation-2003.1.132/5/m_melo_fig4.jpeg?Expires=1767714291&Signature=gxgXympKQJNf0DN8GfgaDBmCXOkoiMfwDmIgGaNKlgXO98AIuHBsnnre1t4pcO4pdoROb9RPMvvHutyS362V6OnLEkhGjgTH2zAkGkL8ySt0m7B5tluvIQQFc4Y4qQeIQXlB4UpW~A1Oy0as0jXROiCH8IJH0L6j7ZDONwy2hTEtc5BfvTB4g59U-Ilem2wgLDBfVh7H3qp-hPq8DwB5mprzcSDizl7euCemgqncpuT9KiNeBcWHKYSHNwBL-FModPYFoxwLuRy4L6~90kIorf2dU5tNOg2geUymkGNvzgFzfhb0IguK84mXMqPZa9z2dkuGH17OX6qzmGWsiN-Y8g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)