Abstract
Allogeneic hematopoietic stem cell transplantation is a life-saving procedure for hematopoietic malignancies, marrow failure syndromes, and hereditary immunodeficiency disorders. However, wide application of this procedure is limited by availability of suitably HLA-matched adult donors. Umbilical cord blood (UCB) has being increasingly used as an alternative hematopoietic stem cell source for these patients. To date, over 6000 UCB transplant procedures in children and adults have been performed worldwide using UCB donors. Broader use of UCB for adult patients is however limited by the available infused cell dose. This has prompted intensive research on ex vivo expansion of UCB stem cells and UCB graft-engineering including accessory cells able to improve UCB engraftment and reconstitution and for tissue regenerative potential. Recently, two large European and North American retrospective studies demonstrated that UCB is an acceptable alternative source of hematopoietic stem cells for adult recipients who lack HLA-matched adult donors. UCB is anticipated to address needs in both transplantation and regenerative medicine fields. It has advantages of easy procurement, no risk to donors, low risk of transmitting infections, immediate availability and immune tolerance allowing successful transplantation despite HLA disparity.
Introduction: Possibilities and Limitations of Umbilical Cord Blood as a New Stem Cell Source
Allogeneic blood and bone marrow stem cell transplantation has been used successfully to treat children and adults with high-risk or relapsed hematopoietic malignancies, marrow failure syndromes, and hereditary immunodeficiency disorders. Its use is limited by the availability of suitably HLA-matched donors. Only 30% of patients have HLA-identical sibling donors and through the National Marrow Donors Program (NMDP) and other registries worldwide nearly 75% of Caucasians, but far fewer racial minorities find suitably HLA-matched donors.
In 1988 umbilical cord blood (UCB) hematopoietic stem cells (HSC) from a related sibling were transplanted successfully into a 5-year-old child with Fanconi anemia by Gluckman and colleagues.1 Subsequently, over 6000 UCB transplant procedures have been performed worldwide using UCB from related and unrelated donors into pediatric2–8 and adult patients.5,9–12 UCB offers the advantages of easy procurement, no risk to donors, the reduced risk of transmitting infections, immediate availability of cryopreserved units, and acceptable partial HLA mismatches. Nearly all patients can find at least one potential 4 of 6 HLA-matched UCB units through either Netcord, New York Blood Center, and NMDP Registry or other banks (www.marrow.org/PHYSICIAN/likelihood_of_finding.html).
UCB Graft Characteristics, Engraftment and Outcome
Transplantation of unrelated UCB permits a greater degree of HLA mismatching without an unacceptably high incidence of graft-versus-host disease (GVHD). Graft characteristics known to allow rapid donor engraftment in recipients of conventional allografts include: cell dose, CD34 content, and HLA matching. The higher primary graft failure rates and delayed donor myeloid recovery in UCB recipients are due to the low graft HSC dose, which include up to 10-fold fewer nucleated and CD34 cells compared with adult donor grafts.13 UCB graft variables that have predictive value for time to donor myeloid engraftment include cryopreserved and re-infused total nucleated graft cell content, CD34 content and infused colony-forming units (CFU).2,5,8,9
The rate of donor hematopoietic reconstitution is lower and kinetics of engraftment are delayed using UCB compared to bone marrow grafts.11,12,14 UCB characteristics predictive of engraftment include nucleated graft cell dose and HLA matching, though graft failure is still observed. Minor histocompatibility disparity in unrelated allogeneic transplantation may contribute to graft rejection and to graft-versus-leukemia effects.17 Effects of the number and type of graft HLA disparities on UCB donor engraftment have not been fully studied. HLA class I mismatching including HLA-C, with NK epitope mismatching in the rejection direction are associated with higher rates of graft rejection and severe acute GVHD after unrelated donor transplantation.15,16 Allele matching for adult unrelated blood and marrow grafting has improved rates of engraftment and GVHD. High-resolution HLA matching for UCB graft selection might improve successful engraftment, though most patients will not have allele-matched UCB donors available.
Although delayed or failed engraftment may be attributable to the lower nucleated UCB graft cell dose, other characteristics of UCB progenitor cells may affect homing and maturation in the recipient and confound the correlations with engraftment. Adhesion molecule expression on UCB HSC,18 their homing characteristics,19,20 the maturational stage of UCB progenitor cells, and/or altered allo-reactivity between UCB graft lymphocytes and recipient antigen-presenting cells may modulate UCB engraftment. Graft facilitating activity of donor lymphocytes contained in the UCB transplant may inhibit or eliminate residual recipient immune cells capable of graft rejection.21 These graft HSC and immune factors may facilitate engraftment in adults transplanted with UCB and those receiving low graft CD34 and nucleated cell doses.
CD34 quantification in UCB has not been consistently predictive of time to donor hematopoietic engraftment. The poor correlation between CD34 content of infused UCB grafts and time to hematopoietic engraftment may be confounded by quantification of CD34 in UCB grafts pre-freezing versus post thaw and by reduced surface epitope density of CD34 on UCB progenitor cells.22 In vitro analyses of UCB CD34 progenitors point to a less mature phenotype compared to adult marrow and peripheral blood grafts.23 The frequency of early HSC is similar in adult marrow, mobilized peripheral blood, and UCB, but the proliferative potential of UCB is significantly higher.24 Cobblestone area-forming cell (CAFC) assays show that UCB CD34+ cells contain the highest frequency of CAFC (wk 6) (3.6- to 10-fold higher than BM CD34+ cells and peripheral blood stem cells [PBSC], respectively), and the engraftment capacity in vivo by nonobese diabetic/severe combined immunodeficiency (NOD/SCID) repopulation assay is also significantly greater than BM CD34+ cells.25 These unique characteristics of UCB allow durable engraftment despite reduced graft cellular content. After UCB transplantation, late graft failure has been uncommon.6
Clinical Reports: UCB Pediatric and Adult Recipients
Published reports have summarized transplant outcomes for approximately 1240 patients undergoing UCB related and unrelated allogeneic transplantation.2,3,6–9,11,12,26–29
These studies focus primarily on pediatric recipients with high risk or recurrent hematologic malignancies and a smaller proportion with non-malignant hematologic disorders. Myeloablative preparative regimens have been either total body irradiation-based or busulfan-based, plus infusion of anti-lymphocyte globulin for further immunosuppression. Recently, fludarabine has been introduced to provide immunosuppression and avoid use of antithymocyte globulin (ATG) in the non-ablative setting.26 Donor myeloid engraftment is delayed compared to conventional allogeneic grafts, and ranges from 22 to 30 days. The probability of donor engraftment ranges from 65% to 88%. The majority of patients received filgrastim daily from day 0 until durable neutrophil recovery, though the utility of single or combination hematopoietic growth factors after UCB transplantation is uncertain. Acute GVHD grades II–IV has ranged from 35% to 40%, despite most receiving grafts disparate at two or more HLA loci. Event-free survival falls in a broad range (22%–62%), reflective of patient heterogeneity in these early, mostly single institution pilot trials. High peri-transplant mortality is attributed in part to delayed donor myeloid recovery. Transplant outcomes for patients allografted with related UCB has been similar or slightly better compared to unrelated UCB grafts.5
Data regarding cord-blood transplantation in adults are limited. The minimum numbers of HSC in UCB units required to provide durable engraftment in ablated adult recipients is not firmly established. Initially, a minimum cryopreserved UCB cell dose of 1 × 107 nucleated cells/kg recipient body weight was suggested but a high (> 40%) day 100 mortality9 led to a higher minimum dose > 2 × 107/kg. The majority of adult UCB recipients have received grafts mismatched at two or more HLA loci. Importantly, adult recipients have generally been high-risk patients appropriate for phase I studies and their poor survival may not be fully attributable to the UCB graft infused.29 Multi-institutional prospective phase II studies are awaited.
Two recent large European12 and North American11 retrospective studies describe transplants of UCB or bone marrow from unrelated donors in adults with acute leukemia. The Acute Leukemia Working Party of European Blood and Marrow Transplant Group; Eurocord-Netcord Registry compared outcomes in 682 adults with acute leukemia who received HSC transplant from unrelated donors: 98 patients received UCB and 584 received bone marrow from unrelated donors from 1998 to 2002. UCB recipients were younger (median, 24.5 vs 32 years; P < 0.001), weighed less (median, 58 vs 68 kg; P < 0.001), and had more advanced disease (52% vs 33%, P < 0.001). All marrow transplants were HLA matched, whereas 94% of UCB were mismatched (P < 0.001). The median number of UCB nucleated cells infused was 0.23 × 108/kg versus 2.9 × 108/kg for bone marrow (P < 0.001). Multivariate analysis showed UCB to yield lower risks of grade II–IV acute GVHD (relative risk (RR), 0.57; 95% confidence interval (CI), 0.37 to 0.87; P = 0.01), but neutrophil recovery was significantly delayed (RR, 0.49; 95% CI, 0.41 to 0.58; P < 0.001). The incidence of chronic GVHD, transplantation-related mortality, relapse- and leukemia-free survival were not significantly different in the two groups. These investigators concluded that unrelated UCB is an acceptable alternative source of hematopoietic stem cells for adults with acute leukemia who lack an HLA-matched marrow donor.12
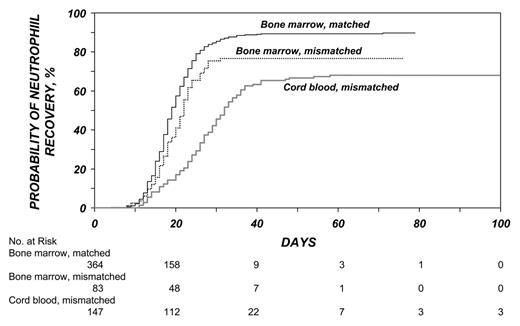
Investigators in the International Bone Marrow Transplant Registry (IBMTR) compared the outcomes of the transplantation of unrelated hematopoietic stem cells in adults with leukemia from UCB (mismatched for one HLA antigen (34 patients) or two antigens (116 patients), marrow that had one HLA mismatch (83 patients), or HLA-matched marrow (367 patients). Similar to the European patients, UCB recipients were younger, more had advanced leukemia and received lower doses of nucleated cells. Hematopoietic recovery was slower with mismatched bone marrow or UCB than with matched marrow (Figure 1 ). Acute GVHD was more likely after mismatched marrow and chronic GVHD was more likely after UCB transplantation. The rates of treatment-related mortality, treatment failure, and overall mortality were lowest following matched marrow transplants. Patients who received mismatched bone marrow or mismatched UCB had similar rates of treatment-related mortality (P = 0.96), treatment failure (P = 0.69), and overall mortality (P = 0.62) (Figure 2 ). There were no differences in the rate of recurrence of leukemia. Among UCB recipients, outcomes were similar between grafts with 1 or 2 HLA mismatches. These investigators concluded that HLA-mismatched UCB should be considered an acceptable graft for adults in the absence of an HLA-matched adult donor.11
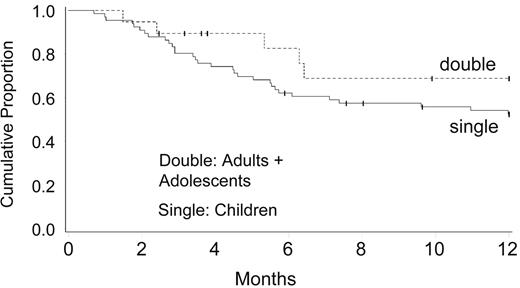
Attempts to increase UCB graft cell dose led to phase I clinical trials testing the safety of combined transplantation of 2 partially HLA-matched UCB units. Barker and co-investigators at University of Minnesota30 studied 23 patients with high-risk hematologic malignancy (median age, 24 years; range, 13–53 years) who received 2 UCB units (median infused dose, 3.5 × 107 nucleated cell [NC]/kg; range, 1.1–6.3 × 107 NC/kg) after myeloablative conditioning. All evaluable patients (n = 21) engrafted at a median of 23 days (range, 15–41 days). At day 21, engraftment was derived from both donors in 24% of patients and a single donor in 76% of patients, with 1 unit predominating in all patients by day 100. Although neither nucleated or CD34(+) cell doses nor HLA-match predicted which unit would predominate, the predominating unit had a significantly higher CD3(+) dose (P < .01). Incidences of grades II–IV and III–IV acute GVHD were 65% (95% confidence interval [CI], 42%–88%) and 13% (95% CI, 0%–26%), respectively. Disease-free survival was 57% (95% CI, 35%–79%) at 1 year, with 72% (95% CI, 49%–95%) of patients alive if they received transplants while in remission (Figure 3 ). These investigators concluded that transplantation of 2 partially HLA-matched UCB units is safe, and may overcome the cell-dose barrier that limits the use of UCB in many adults.30
Further attempts to increase UCB graft cell dose has included laboratory and phase I clinical trials focused on ex vivo expansion of UCB grafts. Although early clinical trials reported thus far do not demonstrate more rapid hematopoietic recovery in UCB recipients, the laboratory studies have promise. Cord blood contains hematopoietic progenitor cells at a higher frequency than adult marrow, and these UCB progenitors have a higher proliferative capacity. Short-term in vitro cultures of UCB CD34+ progenitor cells in the presence of cytokines generates a two- to threefold expansion of these progenitors.31 UCB may contain immature progenitors that have limited proliferative response to cytokines,32 thereby maintaining the stem cell component in expanded UCB grafts. Concerns that ex vivo expansion of UCB may result in differentiation of primitive stem cells thereby increasing the risk of late graft failure, have also prompted strategies testing non-hematopoietic cell platforms (e.g., mesenchymal cells) during cytokine-driven expansion.33,34 These preclinical studies identify further UCB graft engineering questions to investigate the role of accessory lymphoid populations in ex vivo expanded allogeneic grafts, as well as the role of stromal elements in maintaining immature stem cells with self-renewal capacity during cytokine-based liquid culture expansion. These graft-engineering studies may improve UCB graft cell populations and facilitate allogeneic engraftment, thereby reducing day 100 mortality rates.
Immunobiology of UCB Stem Cell Grafts Impacting Immune Recovery and GVHD
HLA disparity between the donor and recipient in allogeneic transplantation is an important determinant of acute and subsequent chronic GVHD. Nevertheless, a higher incidence of acute and chronic GVHD has been observed in patients transplanted with HLA-matched unrelated grafts when compared with histocompatible sibling grafts, possibly attributable to reactivity of donor T cells with recipient minor histocompatibility antigens. Minor histocompatibility antigen disparity is expectedly greater between unrelated individuals.
Despite infusion of HLA class I and II disparate grafts, the incidence and severity of acute GVHD observed in pediatric and adult recipients of UCB grafts is lower when compared to recipients of unrelated adult donor grafts.2,4,10–12,28 UCB T lymphocytes are typically CD45RA+ and express low levels of activation markers, both of which are consistent with a naïve Th0 phenotype.35 Multiple factors may contribute to the reduced GVHD observed after UCB transplantation including: reduced graft lymphocyte numbers, altered recognition of recipient self antigens by UCB donor T cells interacting with antigen-presenting cells (APC), and/or limited response of these naïve donor T cells activated by recipient alloantigen; thereby limiting the cytokine and cellular cascade necessary to amplify donor alloreactivity to recipient antigens.34 Alternatively, the low incidence of GVHD observed in recipients of HLA mismatched UCB might be related to the added immunosuppression provided by ATG or fludarabine included in myeloablative preparative regimens provided to ensure donor engraftment.
Several in vitro studies point to the inherent lack of full expression of immunomodulatory cytokines by alloreactive T cells contained in UCB grafts.35,36 In primary mixed lymphocyte culture UCB T cells demonstrate proliferative responses to allogeneic stimulation, but less cytotoxic effector function, less proliferation and greater activation-induced cell death (AICD). Further mechanisms potentially underlying UCB immune tolerance includes altered toll-like receptors and adhesion molecule expression on donor graft antigen-presenting cells.37 Early recovery of NK cells able to activate the granzyme/perforin lytic pathway and Fas/Fas ligand (FasL) activity has also been proposed as contributing to the low incidence of acute GVHD observed after UCB transplantation.38 The reduced GVHD summarized in clinical reports after UCB may be related to these in vitro observations that immunologically competent cells contained in an UCB graft although capable of recognizing non-inherited antigens, respond less fully than as alloreactive lymphocytes. Reduced expression of nuclear factor of activated T cells-1 (NFAT1) may be one important molecular mechanism underlying reduced cytokine production by UCB graft T cells.36
UCB graft lymphocyte and antigen-presenting cell characteristics that may underlie the low incidence of acute GVHD may also contribute to infection risk and/or delayed immune reconstitution. The incidence and risk factors for bacterial, fungal, and viral infections after allogeneic transplantation have been shown to correlate with kinetics of immune reconstitution and serve as a guide for appropriate antimicrobial prophylaxis for allogeneic transplant patients.
Recent reports identify that the rate of infections during the early post-transplantation period is higher in adult patients transplanted with HLA mismatched unrelated UCB, while overall rates of infections at later time points are similar to that observed in unrelated adult donor transplant recipients.14 This higher incidence of early bacterial infections in the UCB patients may be related to the prolonged duration of neutropenia and lymphopenia after infusion of smaller numbers of total graft nucleated cells and CD34+ cells. A second factor may be selection of high-risk adult patients who are heavily treated before transplantation. UCB patients generally have a longer time interval between diagnosis and transplantation, and a higher proportion of these patients are considered intermediate-high risk hematological malignancy.9 Saavedra reported a high incidence of bacteremia (55%) in 27 adults at early time points after UCB transplant. Ten patients (37%) died prior to day 100. Infection was a direct cause of death in 4 patients.39 Tomonari reported cytomegalovirus (CMV) infection following UCB in 28 adults compared with sibling matched (R-BMT) and URD BM recipients. CMV antigenemia was observed in 19 (79%) of UCB patients at median 42 days. A higher proportion of UCB patients treated with preemptive gancyclovir therapy required a second course of treatment compared with R-BMT and URD BM patients, suggesting that CMV-specific immunity after UCB may be delayed.40 These higher infection rates, however, are not observed after pediatric UCB transplantation and are comparable to those observed in children transplanted with marrow from adult unrelated donors.41
Lower incidence of acute GVHD in UCB transplant recipients would be expected to be associated with higher rates of malignancy relapse, particularly since UCB has been tested as a new allogeneic stem cell source in high-risk patients. However, relapse rates after UCB transplant remain low, and the mechanisms underlying the strong graft-versus-leukemia (GVL) effects mediated by UCB have not been clearly delineated. Clinical reports of allogeneic UCB recipients have not identified increased relapse rates, despite the majority of patients having advanced disease at the time of transplant, and many pediatric UCB recipients having acute lymphocytic leukemia, which has lower sensitivity to allogeneic GVL. UCB grafts are unique in that despite HLA disparity, transplant outcomes are acceptable and graft manipulation to deplete T cells is therefore not required. These UCB graft immunologic features may facilitate elimination of HLA disparate malignant and non-malignant host hematopoietic cells and thereby effectively facilitate engraftment despite low CD34 stem cell content. This may, in part, underlie the observed potent GVL accompanying UCB allografts.
UCB and Regenerative Medicine
Although clinical experience to date with UCB has focused on hematology applications, recent preclinical work has identified a rare population of pluripotent, CD45− cells from UCB which grows adherently and can be expanded to 1015 cells without loss of pluripotency.42 These CD45− cells from UCB show differentiation into osteoblasts, chondroblasts, adipocytes, and hematopoietic and neural cells including astrocytes and neurons that express neurofilament, sodium channel protein, and neurotransmitter phenotypes. Stereo-tactic implantation of these pluripotent UCB-derived CD45− cells into intact adult rat brain reveal that human Tau-positive cells persisted for up to 3 months and showed migratory activity and a typical neuron-like morphology. In vivo differentiation along mesodermal and endodermal pathways has also been demonstrated in animal models. Bony reconstitution was observed after transplantation of calcium phosphate cylinders loaded with pluripotent UCB-derived cells in nude rat femurs. Chondrogenesis occurred after transplanting cell-loaded gelfoam sponges into nude mice. Transplantation in a non-injury model, the pre-immune fetal sheep, revealed up to 5% human hematopoietic engraftment. More than 20% albumin-producing human parenchymal hepatic cells and substantial numbers of human cardiomyocytes in both atria and ventricles of the sheep heart were detected many months after transplantation of these pluripotent, CD45− cells from UCB. No tumor formation was observed in any of these models. Additional studies have examined UCB cells cultured from CD34+ endothelial precursor cells (EPC), which can be expanded in vitro to clinically relevant numbers. In vivo, these cells proliferate, form vascular structures, and improve left ventricular function after experimental myocardial infarction. UCB cells migrate to infarcted, not to normal myocardium, where they engraft, participate in neoangiogenesis, and beneficially influence remodeling processes.43 Similar findings in myocardial infarction induced in Wistar rats by coronary artery ligation confirm efficacy of UCB CD34(+) cells which have been noted to significantly improve ventricular function compared to cytokine control animals.44 UCB therefore may potentially be useful for cell therapy of is-chemic vascular disease.
Taken together, these early preclinical studies support the hypothesis that multipotential stem cells derived from UCB exhibit functional characteristics similar to that observed in adult marrow-derived stem cells in mediating vascular and potential organ regenerative capabilities.
Summary
Banked unrelated umbilical cord blood (UCB) has emerged as an alternative allogeneic stem cell source, providing available and suitably HLA-matched donors for patients requiring allogeneic transplantation. Early clinical reports of UCB transplantation, in pediatric and adult recipients, show slower rates of hematopoietic engraftment, higher rates of infection, yet importantly, a low incidence of severe (grade III/IV) acute GVHD, even when HLA-disparate grafts are infused. Cellular and molecular mechanisms of reduced incidence of severe GVHD and graft-versus-malignancy effects in UCB grafting need further study. Preliminary observations suggest that UCB from unrelated donors is a feasible alternative source of stem cells for transplantation in adults, resulting in durable although delayed hematopoietic reconstitution, with low incidence and severity of acute GVHD. Preliminary work examining functionality of UCB-derived CD45+ and CD45− stem cells in regenerative medicine applications are intriguing. Strategies to improve kinetics of hematopoietic recovery after UCB grafting in children and adults are warranted.
Advantages of umbilical cord blood (UCB) as hematopoietic stem cells for allogeneic transplantation.
|
|
Disadvantages of umbilical cord blood (UCB) as hematopoietic stem cells (HSC) for allogeneic transplantation.
|
|
Cumulative incidence of neutrophil recovery after bone marrow and cord-blood transplantation. Despite early differences, the cumulative incidence of neutrophil recovery at day 100 was similar after the transplantation of mismatched bone marrow and of cord blood. The corresponding cumulative incidence after transplantation of HLA-matched bone marrow was significantly higher.11
Cumulative incidence of neutrophil recovery after bone marrow and cord-blood transplantation. Despite early differences, the cumulative incidence of neutrophil recovery at day 100 was similar after the transplantation of mismatched bone marrow and of cord blood. The corresponding cumulative incidence after transplantation of HLA-matched bone marrow was significantly higher.11
Adjusted probability of leukemia-free and overall survival after bone marrow and cord-blood transplantation. The adjusted probability of 3-year survival without a recurrence of leukemia was 19% for recipients of mismatched marrow, 23% for recipients of cord blood, and 33% for recipients of HLA-matched marrow. Probabilities were adjusted for age, disease status at transplantation, and positivity for cytomegalovirus in the donor, recipient, or both.11
Adjusted probability of leukemia-free and overall survival after bone marrow and cord-blood transplantation. The adjusted probability of 3-year survival without a recurrence of leukemia was 19% for recipients of mismatched marrow, 23% for recipients of cord blood, and 33% for recipients of HLA-matched marrow. Probabilities were adjusted for age, disease status at transplantation, and positivity for cytomegalovirus in the donor, recipient, or both.11
Disease-free survival (DFS) for acute leukemia in complete response (CR) single versus double umbilical cord blood transplantation (UCBT).
The probability of one-year survival without leukemia recurrence was 62% for recipients of two HLA-mismatched UCB, and 52% for recipients of one UCB unit. All patients received full myeloablative conditioning incorporating cyclophosphamide 60 mg/kg and total body irradiation 1320 cGy.30
Disease-free survival (DFS) for acute leukemia in complete response (CR) single versus double umbilical cord blood transplantation (UCBT).
The probability of one-year survival without leukemia recurrence was 62% for recipients of two HLA-mismatched UCB, and 52% for recipients of one UCB unit. All patients received full myeloablative conditioning incorporating cyclophosphamide 60 mg/kg and total body irradiation 1320 cGy.30
Department of Medicine, Case Western Reserve University, School of Medicine, University Hospitals Ireland Cancer Center, Cleveland, Ohio