Abstract
Congenital neutropenia comprises a variety of genetically heterogeneous phenotypic traits. Molecular elucidation of the underlying genetic defects has yielded important insights into the physiology of neutrophil differentiation and function. Non-syndromic variants of congenital neutropenia are caused by mutations in ELA2, HAX1, GFI1, or WAS. Syndromic variants of congenital neutropenia may be due to mutations in genes controlling glucose metabolism (SLC37A4, G6PC3) or lysosomal function (LYST, RAB27A, ROBLD3/p14, AP3B1, VPS13B). Furthermore, defects in genes encoding ribosomal proteins (SBDS, RMRP) and mitochondrial proteins (AK2, TAZ) are associated with congenital neutropenia syndromes. Despite remarkable progress in the field, many patients with congenital neutropenia cannot yet definitively be classified by genetic terms. This review addresses diagnostic and therapeutic aspects of congenital neutropenia and covers recent molecular and pathophysiological insights of selected congenital neutropenia syndromes.
Definition and Clinical Findings
Historically, severe congenital neutropenia is the first inborn error of immunity recognized. In 1950, the pediatrician Rolf Kostmann described the first patients with absence of neutrophil granulocytes in consanguineous families in northern Sweden.1,2 Half a century later, the genetic basis of this heterogeneous condition has begun to be revealed. These genetic studies provided clues to the pathophysiology of a heterogeneous group of disorders and the development of novel therapeutic approaches.
Neutropenia is commonly defined as a peripheral absolute neutrophil count (ANC) of less than 1500 cells/μL blood. Further categories involve the terms of “mild” (ANC 1000–1500), moderate (ANC 500–1000), severe (ANC 200–500), and very severe (ANC < 200) neutropenia. Patients with congenital neutropenia are prone to severe and recurrent bacterial infections such as otitis media, bronchitis, pneumonia, osteomyelitis, or cellulitis. Long-term neutropenic states also predispose to fungal infections. A characteristic feature is the absence of pus. Many patients suffer from chronic gingivitis and tooth decay. In addition, decreased bone mineral density, leading to ostopenia or osteoporosis and increased propensity to fractionation, is a common clinical problem. In contrast to congenital neutropenia, cyclic neutropenia is characterized by an oscillating neutrophil count usually following a 21-day period. As a consequence, patients develop low neutrophil counts for several days, putting them at risk for severe bacterial infections.
When evaluating normal or aberrant peripheral neutrophil counts, the ethnic background is of considerable importance. Individuals of African descent and some ethnic groups in the Middle East have lower neutrophil counts without predisposition to bacterial infections. This condition, termed “benign ethnic neutropenia,” represents the most common variant of low neutrophil counts throughout the world. Recent genetic studies in people of African descent have highlighted the role of a polymorphism in the gene encoding the Duffy antigen receptor for chemokines (DARC).3 The Duffy Null polymorphism (SNPrs2814778), associated with protection against Plasmodium vivax malaria, is strongly associated with the phenotype of ethnic neutropenia, but the molecular mechanism remains completely unknown.
In addition to quantitative abnormalities, many patients with congenital neutropenia show qualitative neutrophil aberrations. A prominent finding in severe congenital neutropenia (SCN) is the so-called “maturation arrest” in neutrophil differentiation. Bone marrow smears typically reveal an absence of mature neutrophil granulocytes while promyelocytes are present. Morphological aberrations include prominent vacuolization and occasionally aberrations of azurophilic granules. Functional abnormalities include defective migration, bacterial killing, or increased apoptosis. However, none of these findings is specific. Since most early studies could not take the underlying genetic mutation into account, the interpretation of these results faces limitations.
From a clinical perspective, congenital neutropenia may be an isolated hematologic finding or may be a feature associated with involvement of other organs, such as the skin, the brain, the heart, the pancreas, or the urogenital tract. The clinical presentation may offer important clues to the genetic diagnosis (Table 1 ). Even in the era of molecular medicine, the importance of the clinical approach and systematic physical exam cannot be underestimated.
Molecular Genetics
Severe Congenital Neutropenia and Mutations in ELANE/ELA2
Marshall Horwitz, David Dale, and colleagues were the first to identify heterozygous mutations in ELANE/ELA2 (elastase, neutrophil expressed), the gene encoding neutrophil elastase, in patients with cyclic neutropenia4 and subsequently in severe congenital neutropenia.5 NE is a myeloid cell–specific serine protease exhibiting pleiotropic functions by cleaving a wide range of bacterial and host proteins.6 More than 50 mutations have been identified in patients with SCN or cyclic neutropenia6; they are either transmitted in an autosomal dominant pattern of inheritance or spontaneously acquired.6,7 Approximately 50% of cases of congenital neutropenia have mutations in ELANE/ELA2. Recently, the concept of the “unfolded protein response” has been invoked to explain the pathomechanism of ELA2 mutations.8 The “unfolded protein response” has evolved to protect cells against damaging effects of improperly folded proteins. Nascent proteins destined for secretory vesicles are directed to the endoplasmic reticulum where protein folding takes place.9 The unfolded protein response signal cascade is initiated by three ER-localized protein sensors: IRE1alpha (inositol-requiring 1alpha), PERK (double-stranded RNA-dependent protein kinase [PKR]–like ER kinase), and activating transcription factor 6 (ATF6). In cases of ER stress, these sensors are activated and trigger a complex series of events destined to maintain the homeoastasis of the ER and to promote protein folding, maturation, secretion, and ER-associated protein degradation. If these rescue mechanisms fail, the unfolded protein response initiates apoptosis to protect cells and the organism from dysfunctional or toxic proteins.10 Cells expressing mutated neutrophil elastase show increased biochemical evidence of ER stress such as upregulation of Bip and cleavage of XBP-1,11,12 suggesting that the unfolded protein response is at least partially involved in increased apoptosis. Consistent with this hypothesis, neutrophils from patients with ELA2 mutations show decreased transcription and translation of neutrophil elastase and other host constitutive neutrophil granule proteins.13
Severe Congenital Neutropenia and Mutations in HAX1
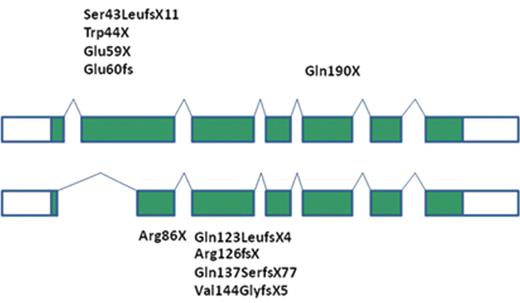
In his seminal historic paper, Rolf Kostmann identified an autosomal-recessive mode of inheritance, yet the molecular defect has remained unknown for more than 50 years. To investigate the molecular etiology of autosomal-recessive SCN, we have undertaken a genome-wide linkage and candidate sequencing approach in three unrelated Turkish families. We identified homozygous mutations in HAX1, an antiapoptotic protein localized primarily in mitochondria.14 HAX1-deficient cells are characterized by a lower threshold to dissipate their inner mitochondrial membrane potential, defining a point of no return for the apoptotic pathway triggering activation of Bax, release of cytochrome c and activation of death-inducing caspases. Thus, HAX1 has emerged as a major control factor governing the life and death of myeloid cells. Independent studies have shown that HAX1 may interact with multiple viral proteins (such as HIV-rev, HIV-vpr, EBV nuclear antigen 5, EBV nuclear antigen leader protein, HHV8-K15 protein), suggesting that viruses may have developed strategies to hijack cellular proteins as a survival strategy. Recently, HAX1 has been shown to be involved in membrane dynamics. HAX1-deficient cells show increased morphological and biochemical features of autophagy, a cellular process known to optimize energy consumption (unpublished observations). Important insights into the biology of HAX1 came from a murine model system.15 Murine hax1 controls parl-dependent conformational changes in htra2/omi, suggesting that hax1 plays a role in presenting htra2 to a proteolytic processing machinery. In contrast to human patients, hax1-deficient mice die shortly after reaching adolescence of neuronal degeneration.15 This observation has triggered a series of neurological investigations in HAX1-deficient patients. Swedish patients originating from the families described by Rolf Kostmann suffer from cognitive disorders.16 Furthermore, a number of SCN patients could be identified that associate congenital neutropenia with various levels of neurological impairment, ranging from mild developmental delay to severe epilepsy. A comprehensive review revealed a surprising genotype-phenotype correlation.17 Two human splice variants are known, yielding isoform A and isoform B. Isoform B is characterized by a splice event removing part of exon 2 and is preferentially expressed in neuronal cells. Mutations affecting only isoform 1 (ie, mutations in exon 2) give rise to a phenotype restricted to congenital neutropenia, while mutations affecting both isoform 1 and isoform 2 are associated with a phenotype of congenital neutropenia and variable degrees of neurological impairment (Figure 1 ).18,19
Severe Congenital Neutropenia and Mutations in Adenylate Kinase-2 (AK2) (Reticular Dysgenesis)
Reticular dysgenesis (RD) is the most severe variant of severe combined immunodeficiency (SCID), associating an early differentiation arrest in the myeloid lineage associated with severe lymphopenia due to impaired lymphoid development. Furthermore, affected patients suffer from sensorineural hearing loss. Using a genome linkage analysis and candidate gene sequencing approach, biallelic mutations in adenylate kinase 2 (AK2) have recently been identified by two independent groups.20,21 Like HAX1, AK2 is localized in the mitochondrial intermembrane space and may be important in mitochondrial energy metabolism and control of apoptosis via FADD and caspase 10.22
Of note, other variants of primary immunodeficiencies such as Hyper IgM syndrome may also present with congenital neutropenia.
Congenital Neutropenia and Mutations in GFI1
GFI1 (growth factor independence-1) is a zinc finger transcription factor controlling hematopoietic stem cell differentiation by controlling multiple target genes and regulatory microRNAs. Person et al described two patients with heterozygous mutations in GFI1 (GFI1N382S) affecting the DNA-interacting domains and rendering GFI1 as a dominant negative protein.23 Affected patients showed a myeloid maturation arrest, a paucity of mature neutrophils and propensity to recurrent infections. Recently, a murine model of this particular mutation has been studied in greater detail, confirming a dominant negative block in granulopoiesis associated with dysregulation of CSF1 and its receptor.24
Congenital Neutropenia and Mutations in WASP
Whereas “loss of function” WASp mutants cause the primary immunodeficiency Wiskott-Aldrich syndrome, “gain of function” mutants have been associated with a variant of congenital neutropenia.25 Affected patients carry mutations that affect the autoinhibitory structure of WASp, leading to conformational changes that induce increased actin polymerization. In addition to neutropenia, patients showed variable degrees of lymphopenia, reduced lymphocyte proliferation, and abrogated phagocyte activity.26 A large pedigree with multiple affected members has highlighted the clinical variability of patients harboring the same mutation.27 Interestingly, activating WASP mutations may also lead to myelodysplasia.26 A similar phenotype could be reproduced in vitro using retroviral gene transfer of the mutant WASp (I294T) allele into hematopoietic stem cells.28 This caused enhanced and delocalized actin polymerization throughout the cell, decreased proliferation, and increased apoptosis. Cells became binucleated, suggesting a failure of cytokinesis, and micronuclei were formed, indicative of genomic instability.28 These elegant studies provided an interesting link between WASP as a regulator of the cytoskeleton and the control of cell division.
Congenital Neutropenia and Disorders of Glycogen Metabolism
Glycogenosis type 1 is a metabolic disorder leading to glycogen storage and hypoglycemia due to mutations in glucose-6-phosphate (G6PC1). In contrast, glycogenosis type 1b, caused by a mutation in the transporter for glucose-6-phosphate (G6PT–SLC37A4 ), is characterized not only by signs of glycogen storage but also by congenital neutropenia. In addition, a number of systemic complications such as liver adenomas, nephropathy, bone mineral density defect, polycystic ovaries, short stature, Crohn-like inflammatory bowel disease and hypothyroidism have been described.29 Recently, a novel genetic defect in the glucose-6-phosphate pathway has been discovered.30 Patients with G6PC3-deficiency present with congenital neutropenia and variable developmental disorders affecting the cardiovascular and/or urogenital system. Patients may suffer from atrial septal defects or other structural heart aberrations. Urogenital disorders include urachal fistulations and cryptorchidism. Some patients show a peculiar visibility of subcutaneous veins, potentially secondary to decreased subcutaneous fat tissue. This disorder has been found in multiple ethnic groups. G6PC3, a homolog of glucose-6-phosphatase (G6PC1), is a ubiquitously expressed protein localized in the endoplasmic reticulum. In G6PC3-deficient cells, increased ER-stress leads to the activation of the “unfolded protein response” and an increased susceptibility to apoptosis via decreased enzymatic activity of GSK3beta.30
Syndromes that Combine SCN or Neutrophil Dysfunction with Hypopigmentation
Neutrophils contain secretory vesicles, serving to sequester, transport, and secrete agents such as microbicidal peptides and proteins. Defects in secretory lysosomes may result in clinical phenotypes associating hypopigmentation and immunodeficiency: Hermansky-Pudlak syndrome (HPS), type 2; Griscelli syndrome (GS), type 2; Chediak-Higashi syndrome (CHS); and p14 (ROBLD3/MAPBPIP) deficiency. Congenital neutropenia is consistently seen in HPS type 2 and p14 deficiency, whereas in GS type 2 and CHS, neutropenia may be intermittent. HPS type 2 is caused by a mutation in the gene AP3B131 and is characterized by congenital neutropenia and defective function of NKT cells and dendritic cells. A rare syndrome combining neutropenia, lymphoid immunodeficiency and hypopigmentation has been described, caused by a homozygous point mutation in the 3′ untranslated region of the gene that encodes p14 (ROBLD3, MAPBPIP).32 Additional features of p14 deficiency include short stature, hypogammaglobulinemia (in particular low IgM serum levels) and reduced numbers of B cell subsets, and defective function of cytotoxic T cells. ROBLD3/P41 is an endosomal adaptor protein that acts as a scaffold when binding to MP1, enabling MP1 to participate in the ERK signaling cascade. In contrast to the phenotype of myeloid maturation arrest seen in patients with mutations in ELA2, HAX1, or G6PC3, ROBLD3/p14- and AP3-deficient patients show myeloid maturation in the bone marrow.
Pathophysiology
In spite of striking clinical and morphological similarities between defined variants of SCN, the pathophysiology of congenital neutropenia may be as heterogeneous as the underlying genetic defects. Recent insights into the genetic etiology have shed light on very diverse pathways involved in aberrant neutrophil development and function. A common denominator in many SCN variants, such as in mutations in ELA2, HAX1, GFI1, WAS, SLC37A4, and G6PC3, is increased susceptibility of neutrophil granulocytes and their precursors to undergo apoptosis. However, pathways of apoptosis are very complex, and it remains unclear whether and how defective signals relay to increased apoptosis in distinct genetic mutations. For example, apoptosis-inducing signals in ELA2- and G6PC3-mutant cells appear to originate from the endoplasmic reticulum and do not involve decreased mitochondrial membrane potential, whereas HAX1 deficiency and AK2 deficiency primarily manifest by mitochondrial dysfunction.
Skokowa et al reported that myeloid progenitor cells from patients with SCN have reduced expression of lymphoid enhancer-binding factor 1 (LEF-1), resulting in defective expression of the LEF-1 target genes such as CCND1, MYC, BIRC5, c-Myc and surviving.33 Thus, altered control of differentiation pathways in myeloid cell development may also play a role for defective neutrophil counts.
Severe congenital neutropenia is a premalignant condition with an increased risk of clonal hematopoietic diseases such as MDS and leukemia.34 However, the specific risk of defined genetic subgroups remains unknown. Preliminary data suggest that the risk of patients with ELA2 mutation and patients with HAX1 deficiency developing MDS/AML may be the same.35 Various acquired somatic mutations have been identified in hematopoietic cells of patients with SCN. Of note, monoallelic mutations in the G-CSF receptor, encoded by the gene CSF3R, appear to play a key role in driving myelopoiesis. Nonsense mutations in CSF3R, truncating the distal cytoplasmic portion of the G-CSF receptor, are present in up to 40% of SCN patients and strongly associated with the development of MDS/AML.19 Truncated mutants of the G-CSF receptor lack responsiveness to negative feedback loops. For example, deficient binding of SOCS3 may lead to misrouting of the G-CSF receptor and consecutively increased activation of STAT3/ STAT5.36,37 Myeloid cells expressing mutant G-CSF receptors show an advantage over wildtype cells, as shown both in murine38 and human models.39
Mutations in CSF3R may be a first molecular signature preceding the development of a clonal disorder, but do not justify the clinical diagnosis of MDS/AML. Additional molecular and cytogenetic aberrations commonly involve partial or complete losses of chromosome 7, activating RAS mutations, or abnormalities of chromosome 21, a pattern distinct from common mutations seen in de novo AML.40
Therapy
The most imminent therapeutic goal in patients with severe congenital neutropenia is the reconstitution of adequate antibacterial host defence by neutrophil granulocytes. Recombinant human G-CSF is the first-line therapy of patients with SCN. rhG-CSF, usually given in a dose of 3–5 microgram/kg/d subcutaneously, induces differentiation of neutrophil granulocytes and reduces apoptosis via multiple molecular effectors, including nicotinamide phosphoribosyltransferase (NAMPT) and NAD(+)-dependent sirtuin-1 activation.41 Since the individual response to rh-G-CSF is quite variable, the dose should be titered to achieve a protective neutrophil count (usually > 1000/μL). Patients not responding to even high-dose rh-G-CSF therapy (up to 50–100 μg/kg) are considered poor responders. These individuals are candidates for allogeneic hematopoietic stem cell transplantation (HSCT) as a curative therapeutic strategy. Allogeneic HSCT is also indicated in patients developing MDS or AML. To optimize surveillance for the onset of clonal aberrations, yearly bone marrow analysis including cytogenetic studies and molecular studies to detect somatic mutations in the G-CSF-receptor gene (CSF3R) are recommended. Transplantation protocols are currently available for these patients at several experienced centers. The molecular identification of novel genetic defects may also open horizons for specific therapeutic approaches using gene transfer. Similar to other primary immunodeficiency syndromes that can successfully be treated by transplanting autologous gene-corrected hematopoietic stem cells, there is hope that congenital neutropenia syndromes may be amenable to low-toxicity and efficacious gene therapy approaches in the future.
Organ involvement in congenital neutropenia (CN) syndromes.
| CN variant . | Congenital neutropenia . | Osteopenia . | Skeletal system (growth delay/ dysmorphic features) . | Skin/hair . | Neurological system . | Cardiovascular system . | Urogenital system . | Gastrointestinal system . | Endocrine system . | Adaptive immune system . | Mutated gene . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCN-ELA2 | ▪ | ▪ | ELA2 | ||||||||
| SCN-GFI1 | ▪ | ▪ | ▪ | GFI1 | |||||||
| SCN-WAS | ▪ | ▪ | WAS | ||||||||
| SCN-HAX1 | ▪ | ▪ | ▪ | HAX1 | |||||||
| SCN-AK2 | ▪ | ▪ | ▪ | AK2 | |||||||
| Glycogenosis Ib | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | SLC37A4 | ||||
| G6PC3 deficiency | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | G6PC3 | ||||
| Barth syndrome | ▪ | ▪ | TAZ | ||||||||
| SBDS | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | SBDS | |||
| CHH | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | RBDS | |||
| CHS | ▪ | ▪ | ▪ | ▪ | ▪ | LYST | |||||
| GS type II | ▪ | ▪ | ▪ | RAB27A | |||||||
| HPS II | ▪ | ▪ | ▪ | ▪ | AP3B1 | ||||||
| P14-deficiency | ▪ | ▪ | ▪ | ▪ | ROBL3 | ||||||
| Cohen syndrome | ▪ | ▪ | ▪ | COH1 | |||||||
| Poikiloderma with neutropenia | ▪ | ▪ | ▪ | unknown | |||||||
| Neutropenia-CMT-II | ▪ | ▪ | DNM2 | ||||||||
| Pearson syndrome | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
| CN variant . | Congenital neutropenia . | Osteopenia . | Skeletal system (growth delay/ dysmorphic features) . | Skin/hair . | Neurological system . | Cardiovascular system . | Urogenital system . | Gastrointestinal system . | Endocrine system . | Adaptive immune system . | Mutated gene . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCN-ELA2 | ▪ | ▪ | ELA2 | ||||||||
| SCN-GFI1 | ▪ | ▪ | ▪ | GFI1 | |||||||
| SCN-WAS | ▪ | ▪ | WAS | ||||||||
| SCN-HAX1 | ▪ | ▪ | ▪ | HAX1 | |||||||
| SCN-AK2 | ▪ | ▪ | ▪ | AK2 | |||||||
| Glycogenosis Ib | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | SLC37A4 | ||||
| G6PC3 deficiency | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | G6PC3 | ||||
| Barth syndrome | ▪ | ▪ | TAZ | ||||||||
| SBDS | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | SBDS | |||
| CHH | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | RBDS | |||
| CHS | ▪ | ▪ | ▪ | ▪ | ▪ | LYST | |||||
| GS type II | ▪ | ▪ | ▪ | RAB27A | |||||||
| HPS II | ▪ | ▪ | ▪ | ▪ | AP3B1 | ||||||
| P14-deficiency | ▪ | ▪ | ▪ | ▪ | ROBL3 | ||||||
| Cohen syndrome | ▪ | ▪ | ▪ | COH1 | |||||||
| Poikiloderma with neutropenia | ▪ | ▪ | ▪ | unknown | |||||||
| Neutropenia-CMT-II | ▪ | ▪ | DNM2 | ||||||||
| Pearson syndrome | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Acknowledgments
The joint work of two international registries, situated in Seattle (USA) and Hannover (Germany), has greatly facilitated a systematic scientific approach to severe congenital neutropenia with respect to clinical long-term follow-up and basic genetic studies. I thank the registry teams for their good collaboration, particularly Karl Welte and Conni Zeidler. Furthermore, I thank the patients, their families, and all clinicians and scientists around the world who contribute to our research activities. This work was sponsored by the Deutsche Forschungsgemeinschaft (DFG-SFB) and the BMBF (PID-NET).
References
Author notes
Department of Pediatric Hematology/Oncology, Medical School of Hannover, Hannover, Germany