Abstract
Patients with multiple myeloma (MM) are at an increased risk of venous and arterial thrombosis. The pathogenesis remains unclear, but probably involves several factors such as activation of procoagulant factors, acquired activated protein C resistance, and inflammation. In addition to general risk factors for venous thromboembolism, such as older age, immobility, surgery, and inherited thrombophilia, there are some MM-specific and treatment-related factors that contribute to the increased risk. The risk for venous thromboembolism is high when patients are treated with thalidomide or lenalidomide in combination with dexamethasone or multi-agent chemotherapy. Thromboprophylaxis should be given in these settings. Which agent is the most appropriate is a matter of debate, but aspirin, low-molecular-weight heparin, and warfarin all seem to be effective. This review discusses risk factors for thromboembolism in MM and general, disease-specific and treatment-related mechanisms for thrombosis. Recommendations for thromboprophylaxis are described and treatment choices for venous thrombosis in MM patients are reviewed.
Introduction
It has been known for more than four decades that patients with multiple myeloma (MM) have an increased risk of venous thromboembolism (VTE).1 During the past decade, the introduction of the oral immunomodulatory drugs (IMiDs) thalidomide and lenalidomide has improved the clinical outcome of patients diagnosed with MM.2–3 However, a high rate of thromboembolic complications has been observed when using these agents, especially in combination with chemotherapy and high-dose corticosteroids,2,4–6 which has led to increased clinical awareness of VTE and research focus on the topic.
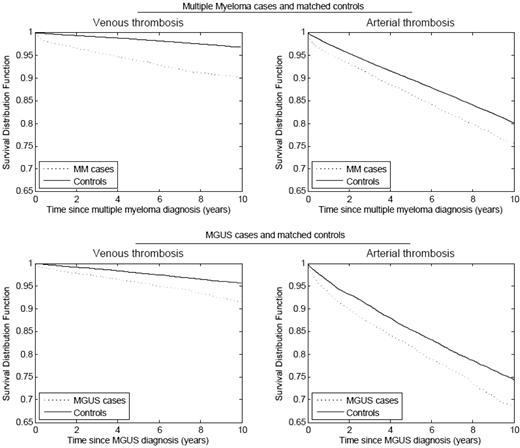
We performed two population-based studies on the risk of thrombosis in MM patients. In one study, based on more than 4 million military veterans in the United States, we identified 6192 patients with MM, of whom 2.4% developed deep-vein thrombosis (DVT). MM patients demonstrated a 9.2-fold increase in DVT risk compared with all other patients in the database. The greatest risk was observed during the first year following diagnosis.7 In a study from Sweden, including 18,627 MM patients and 70,991 matched controls, the risk for VTE was found to be 7.5-fold after 1 year of follow-up and 4.1-fold after 10 years (Figure 1). Interestingly, the risk for arterial thrombosis (myocardial infarction, transient ischemic attack, ischemic stroke, and angina) was also significantly increased compared with the controls (Figure 1).8 In a recent clinical study of 195 MM patients, there was a high risk of arterial thrombosis observed both during and following induction chemotherapy, irrespective of treatment type.9
Cumulative risk of arterial and venous thrombosis in patients with multiple myeloma and MGUS compared with matched controls.8 This research was originally published in Blood. Kristinsson SY et al. Blood. 2010;115:4991–4999. © the American Society of Hematology.
Cumulative risk of arterial and venous thrombosis in patients with multiple myeloma and MGUS compared with matched controls.8 This research was originally published in Blood. Kristinsson SY et al. Blood. 2010;115:4991–4999. © the American Society of Hematology.
Recent studies have shown that MM is consistently preceded by the precursor condition monoclonal gammopathy of undetermined significance (MGUS).10 In the first of our two population-based studies (the U.S. study), we identified 2374 MGUS patients and found a 3-fold increased risk of DVT.7 In the second study (the Swedish study), we found a 2.1-fold increased risk for VTE after 5 and 10 years follow-up among 5326 MGUS patients compared with 20,161 matched controls (Figure 1). Interestingly, only patients with IgG and IgA, and not IgM MGUS, had an increased risk, suggesting that there might be a biological difference between IgG/IgA and IgM MGUS with regard to risk of thromboembolism.8 In both of these studies of MGUS patients, the increased risk of thrombosis was not explained by malignant transformation. These studies show that the increased risk of VTE in MM is present prior to the onset of MM, possibly due to a result of ongoing clonal plasma cell activities.
This review discusses the risk factors for VTE and the mechanisms for VTE in MM, reviews the evidence and current recommendations for thromboprophylaxis in thalidomide- and lenalidomide-treated patients, and summarizes treatment choices for VTE in MM patients.
Risk Factors for Thromboembolism
The cause of VTE is multifactorial and is often a consequence of a combination of risk factors.11 VTE is primarily a disease of the elderly; other patient-related risk factors include previous VTE, inherited thrombophilic abnormalities such as factor V Leiden, prothrombin G20210A mutation, protein C or protein S deficiency, and antithrombin deficiency. Immobilization has repeatedly been observed to be an independent risk factor, as well as trauma and several chronic diseases. Surgery is associated with a very high risk of thrombosis, and central venous catheters (CVCs) increase the risk of VTE. Treatment-specific factors include hormone therapy, chemotherapy, and erythropoietin.11
Cancer has been shown to increase the risk of thrombosis by 4- to 5-fold.11 Thrombotic complications are frequently observed in patients with solid tumors, such as cancers of the pancreas, lung, stomach, breast, ovaries, and brain. In addition, patients with an unprovoked VTE are more likely to have an underlying occult malignancy than those with a known risk factor for VTE. Recent studies suggest that patients with hematological malignancies have a similar or even higher risk of solid tumors, with the highest risk found in patients with MM, acute leukemia, and central nervous system lymphoma.12 In addition to tumor type, advanced disease is also associated with a higher VTE risk. Chemotherapy for malignancy is associated with a high risk of VTE, ranging from 7% to 11% in patients with solid tumors and up to 12% in acute leukemia in different studies.12 The risk for VTE among patients with cancer is exponentially increased with the simultaneous presence of the general risk factors mentioned above, such as in cancer patients undergoing surgery or having a CVC. The risk of recurrence after a previous VTE is higher in cancer patients than in those without malignancy.13 Despite the traditional division of risk factors for VTE versus arterial thrombosis, the two disorders have many features in common, and patients with VTE have been shown to be at an increased risk for arterial thrombosis.14
MM and Risk of Thrombosis
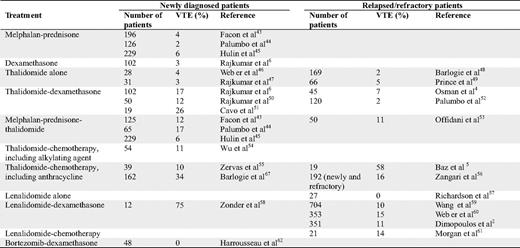
Many clinical studies have found an increased risk of VTE in patients with MM; however, most of these studies were retrospective or were not designed to evaluate the risk of VTE. The incidence of VTE in patients treated with melphalan and prednisone is 2% to 6%. MM patients treated with high-dose dexamethasone have a VTE incidence of 3% (Table 1).
In MM patients, the VTE risk is higher at the time of diagnosis than after relapse. Before it became evident that treatment with thalidomide and lenalidomide was associated with an increased risk of VTE, investigators did not require the use of any thromboprophylaxis. Table 1 shows the incidence of VTE in MM patients treated with thalidomide or lenalidomide without thromboprophylaxis. Thalidomide alone does not seem to increase the risk for VTE when used in relapsed or refractory patients. However, the risk of VTE increases when thalidomide is given in combination with dexamethasone, alkylating agents, anthracyclines, or as multi-agent chemotherapy. The highest reported risk was observed in patients treated with the combination of thalidomide, pegylated doxorubicin, vincristine, and dexamethasone (Table 1). Similarly, the risk does not seem to be increased if thalidomide is used as a single-agent therapy in newly diagnosed patients. This risk is increased beyond that observed in relapsed/refractory patients when thalidomide is used in combination with dexamethasone, alkylating agents, as part of a three-agent therapy, and with doxorubicin. In randomized clinical trials combining thalidomide with melphalan and prednisone, the incidence of VTE was 6% to 17% when given without thromboprophylaxis. In most studies, the highest risk was observed during the first few months of therapy; however, VTE still occurs after several months of therapy. Studies on maintenance treatment with thalidomide have not found a high risk for VTE, probably due to low tumor burden.
Clinical studies on the incidence of VTE in patients treated with lenalidomide have shown that single-agent treatment in relapsed/refractory patients does not increase the risk for VTE. However, when lenalidomide was combined with dexamethasone or cyclophosphamide, the risk increased (Table 1). Few studies have been conducted with lenalidomide in newly diagnosed patients without any thromboprophylaxis. In a case-control study comparing newly diagnosed patients treated with lenalidomide + dexamethasone (n = 228) or thalidomide + dexamethasone (n = 183), no statistical difference in VTE incidence between the thalidomide- and the lenalidomide-treated patients was found.15 In two studies on lenalidomide and high-dose dexamethasone with no thromboprophylaxis, 23% to 75% of the patients were diagnosed with VTE. As for thalidomide-treated patients, most lenalidomide-related thromboses occurred within the first few months.
Treatment with bortezomib has not been associated with an increased risk of VTE, alone or in combination with dexamethasone and/or chemotherapy. When bortezomib is given in combination with thalidomide or lenalidomide, the VTE incidence is low. The possibility of a protective role of bortezomib on VTE16 has to be validated in a prospective randomized study.
In addition to the increased risk of VTE among patients treated with IMiDs in combination with dexamethasone or chemotherapy, other treatment-related factors have been described. In a study of MM patients treated with lenalidomide and dexamethasone, the addition of erythropoietin increased the incidence from 5% to 23%,17 however, this has not been supported in other studies of MM.2 The dose of dexamethasone has been shown to affect the VTE risk. In a randomized clinical trial comparing lenalidomide and low-dose dexamethasone versus high-dose dexamethasone, the incidence was 12% and 26%, respectively.18 In the Western world, approximately 25% of MM patients are treated with high-dose melphalan with stem-cell support.19 Although an increased risk of VTE has been observed in some studies, most have been CVC related.12
MM is an independent risk factor for VTE, and treatment with thalidomide and/or lenalidomide increases this risk, particularly in newly diagnosed patients and when combined with dexamethasone or chemotherapy. Furthermore, there is evidence that the higher the dexamethasone dose, the higher the risk of VTE. Erythropoietin treatment and indwelling CVC may also increase the risk for VTE. Patients with MM often have additional transient risk factors, such as infections and immobilization due to skeletal pain and during hospitalization.
Mechanisms for Thromboembolism
The mechanisms for VTE in cancer are heterogeneous, and can include hypercoagulability, vessel wall injury, and stasis. The blood coagulation system is activated in patients with cancer. The prothrombotic mechanisms often relate to the host response to the tumor, including inflammation, necrosis, and hemodynamic factors. These can also be exacerbated by chemotherapy. In addition, tumor-specific clot-promoting mechanisms, such as the expression of procoagulant and fibrinolytic activities by the tumor cells and interaction with endothelial cells and blood cells, play a role in the pathogenesis. Venous vessel wall injury may be caused by cancer surgery and by cell-to-cell interactions. Finally, venous stasis predisposes to VTE by diluting and reducing the clearance of activated coagulation factors and also may cause endothelial cell damage, increasing the risk for thrombosis.
Proposed Mechanisms in MM
In addition to the general mechanisms for thrombosis observed in cancer patients, there are some specific pathogenetic issues in MM. A prothrombotic state has been observed in patients with MM. Increases in von Willebrand factor and factor VIII have been found, and were associated with a more advanced stage of disease, even before the start of treatment.20 The association between the increased level of these factors and VTE is unclear and requires larger prospective studies. The increase in the procoagulant factors can also reflect an inflammatory reaction. A higher incidence of acquired activated protein C resistance has been observed in MM patients, which disappeared during therapy and was associated with an increased risk of VTE.21 The production of paraprotein has also been suggested to have a role in thrombosis in MM patients. The suggested mechanisms are increased blood viscosity, procoagulant antibody formation, and interference with fibrin. High levels of inflammatory cytokines have also been suggested to play a role in the pathogenetic mechanism of VTE in MM, with interleukin-6, C-reactive protein, and tumor necrosis factor being the most important.16
The observed excess risk for both arterial and venous thrombosis in MM patients suggests that there might be some shared biological features, most probably involving platelet activation.8–9 Arterial thrombi consist mainly of platelets and fibrin, whereas venous thrombi consist mainly of fibrin and red blood cells. However, there are common pathophysiologic features, including activation of endothelium, platelets, leukocytes, and high levels of coagulation factors.14 This is further supported by reports suggesting that aspirin is an effective prophylactic agent in venous thrombosis in MM, as discussed below.22 Additionally, some studies have found evidence of platelet aggregation5 and activation caused by thalidomide, which is also abrogated by aspirin.23
Thalidomide and its analog lenalidomide have various mechanisms of action, including immunomodulatory, anti-angiogenic, and anti-inflammatory effects, as well as an effect on the bone marrow microenvironment. The exact mechanisms for the underlying thrombogenic effects of these agents are not known. There is evidence that the IMiDs enhance expression of tissue factor and vascular endothelial growth factor, down-regulate thrombospondin, and cause cytokine-mediated, activated protein C resistance.12,16 Thalidomide has been shown to increase the levels of von Willebrand factor and factor VIII.20 In addition, thalidomide regulates the level of the prothrombotic factor COX-2.24 Furthermore, there is some evidence to support an effect on the endothelial cells in patients treated with thalidomide and lenalidomide, possibly via tumor necrosis factor. Interestingly, a study of 1966 MM patients found evidence for an individual genetic variation in thalidomide-mediated VTE.25
The mechanism for the apparent protective effect of bortezomib on the risk of VTE is not well understood. Some authors have suggested an inhibition of platelet aggregation,26 and others a prevention of the up-regulation of prothrombotic molecules such as thrombomodulin.27 Further research is needed to explore the mechanisms for the treatment-related effects on the risk of VTE with these agents.
Thromboprophylaxis in MM
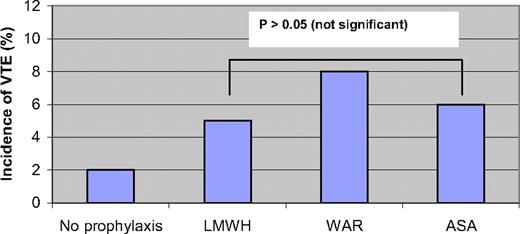
In the first prospective multicenter phase III trial to evaluate the best thromboprophylaxis in thalidomide-treated MM patients (the GIMEMA study), Palumbo et al. randomized 991 newly diagnosed MM patients to bortezomib-thalidomide-dexamethasone (VTD), thalidomide-dexamethasone (TD), bortezomib-melphalan-prednisone-thalidomide (VMPT), or bortezomib-melphalan-prednisone (VMP).28 In a sub-study, patients treated with thalidomide (VTD, TD, and VMPT) were randomized to receive enoxaparin 40 mg/d (n = 223), aspirin 100 mg/d (n = 227), or warfarin 1.25 mg/d (n = 223) for the duration of the induction therapy.28 Patients in the VMP arm (n = 257) did not receive any prophylaxis and served as controls. The results of this study are summarized in Figure 2. There was no significant difference in the incidence of VTE between the three groups, with an incidence of 5%, 6%, and 8% in the low-molecular weight heparin (LMWH), aspirin, and warfarin groups, respectively. Higher doses of thalidomide and dexamethasone were associated with a higher VTE risk. In addition, patients treated with thalidomide in combination with bortezomib had a nonsignificantly lower risk of VTE. The incidence of bleeding was not different in the three arms. The authors concluded that treatment with LMWH conferred the lowest risk of VTE, although this was not statistically different.28 Another study was performed in which 402 patients received four cycles of lenalidomide-dexamethasone as induction, and were then randomized to melphalan-prednisone-lenalidomide or tandem autologous stem-cell transplantation as well as to either aspirin or enoxaparin. In an interim analysis, the incidence of thromboembolic events was similar in both groups, 2% and 1% in the aspirin and enoxaparin groups, respectively (p = 0.42).29
A randomized study of 991 newly diagnosed MM patients treated with thalidomide, evaluating the incidence of VTE with low-molecular-weight heparin (enoxaparin 40 mg/d), fixed low-dose warfarin (1.25 mg/d), and aspirin (100 mg/d).28 Patients treated with bortezomib-melphalan-prednisone (no prophylaxis) are shown as controls. P > 0.05 (not significant).
A randomized study of 991 newly diagnosed MM patients treated with thalidomide, evaluating the incidence of VTE with low-molecular-weight heparin (enoxaparin 40 mg/d), fixed low-dose warfarin (1.25 mg/d), and aspirin (100 mg/d).28 Patients treated with bortezomib-melphalan-prednisone (no prophylaxis) are shown as controls. P > 0.05 (not significant).
All MM patients who are treated with thalidomide or lenalidomide in combination with dexamethasone and/or chemotherapy should be treated with thromboprophylaxis, with the exception of patients with a major risk of bleeding. Given the results from the two randomized clinical trials, aspirin, LMWH, and warfarin can all be regarded as effective thromboprophylaxis. However, there are still areas of uncertainty; for example, the patients were not stratified according to other known risk factors.
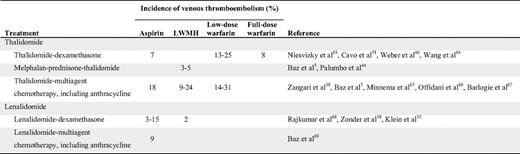
Although aspirin is inferior to LMWH in preventing VTE in general, it does have some protective effects. Earlier studies of patients undergoing orthopedic surgery showed a significant risk reduction in VTEs compared with placebo.30 In their recent guidelines, the American College of Chest Physicians recommend against the use of aspirin for any patient group.31 Conversely, the American Association of Orthopedic Surgeons recommends aspirin as an equal alternative to heparin or warfarin in standard-risk patients undergoing total hip or knee arthroplasty.32 In addition to the two randomized studies cited above,28–29 there is some evidence from before/after studies in MM indicating a protective mechanism of aspirin (Table 2). For example, in newly diagnosed patients treated with thalidomide and anthracycline, the VTE incidence was 58% without any thromboprophylaxis, but decreased to 18% when aspirin was introduced.5 Several clinical studies on MM patients treated with lenalidomide have used aspirin as thromboprophylaxis with encouraging results, with a VTE incidence of 3% to 15% (Table 2). It should be noted that these studies were not designed to evaluate the risk of VTE, and many introduced aspirin after observing a high initial incidence of VTE.
LMWH and fondaparinux are considered the optimal thromboprophylaxis according to clinical guidelines.31 Randomized clinical trials have consistently shown LMWH to be superior to placebo in high-risk patients. Compared with unfractionated heparin, these agents show similar efficacy, but a lower risk of bleeding.31 Studies of MM patients treated with thalidomide or lenalidomide have shown promising results. In the GIMEMA study, the incidence of VTE was 5% in thalidomide-treated patients receiving enoxaparin, and in other studies the incidence was approximately 3% to 15%. In newly diagnosed patients treated with LMWH, the incidence of VTE has varied between 3% and 24% (Table 2).
The data on warfarin as a thromboprophylaxis is controversial. Some studies have used fixed, low-dose warfarin, whereas others have used full-dose warfarin. Using these approaches, the incidence of VTE has been shown to vary from 8% to 31%, with higher risk among patients treated with a fixed, low dose of warfarin (Table 2). In one study, a similar effect with both strategies was found, but a higher risk of bleeding in patients treated with the full-dose warfarin was noted.33
An expert panel reported on consensus guidelines22 before the data from the GIMEMA study28 were available. The panel recommended the use of LMWH in MM patients treated with IMiDs with high-dose dexamethasone or doxorubicin, or when more than one risk factor for VTE was present (Table 3).22 Aspirin was recommended when one or no other risk factors were present. Additionally, full-dose warfarin was suggested as an alternative to LMWH, despite the lack of corroborating data. Because the risk of VTE in cancer patients with a previous VTE is very high, LMWH should probably be used in MM patients with a previous VTE, despite lack of additional risk factors. Because most VTE events in clinical studies have occurred during the first months, it is reasonable to continue prophylaxis for at least 4 to 6 months. The duration of the thromboprophylaxis should, however, depend on the length of treatment and the number of VTE risk factors.
Risk-assessment model for the management of VTE in MM patients treated with thalidomide or lenalidomide according to consensus recommendations22
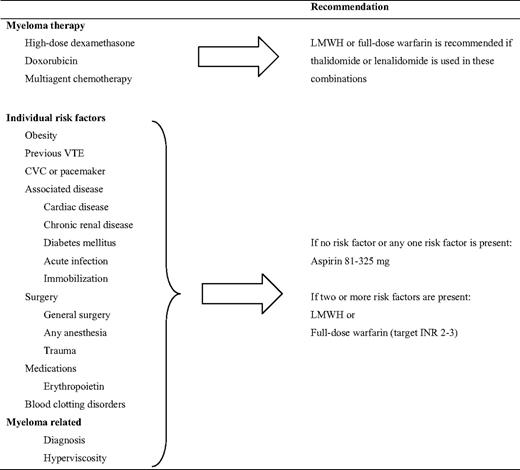
Despite evidence of an increased risk of VTE among patients with MGUS, there is no evidence to support giving thromboprophylaxis at this point. The same thromboprophylactic strategy should be applied in MGUS patients as in other patients, in accordance with clinical guidelines.31,34
All MM patients who do not have excess risk of bleeding and are treated with thalidomide or lenalidomide in combination with dexamethasone or chemotherapy, should receive thromboprophylaxis. In light of the recent randomized clinical trials, aspirin, LMWH, and fixed, low-dose warfarin seem to be effective in thalidomide-treated patients. Despite this, an individual risk-based strategy needs to be applied. Patients with MM often have additional transient risk factors, such as immobilization, infections, and surgery. The most appropriate thromboprophylaxis needs to be re-evaluated in these situations. Because 90% of all MM patients have an additional risk factor,35 most patients should be treated with LMWH. Aspirin should be used in patients with no or only one additional risk factor, as outlined in Table 3.
Thromboembolism in Patients with Renal Dysfunction and Thrombocytopenia
No data are available on thromboprophylaxis in MM patients with renal dysfunction. Extrapolating data from other studies, the recommendation for these patients would be LMWH with dose adjustments for patients with creatinine clearance below 30 mL/min. For example, enoxaparin can be given at the dose of 30 mg/d instead of 40 mg/d. In addition, anti-Xa should be monitored closely to minimize risk of over-anticoagulation. Another option is to use warfarin if renal dysfunction is present.31
MM patients with thrombocytopenia due to tumor burden or treatment are at an increased risk of bleeding from thromboprophylaxis. The risk is highest among patients treated with full-dose warfarin. Low-dose aspirin has been shown to be safe in this setting.5 Furthermore, LMWH has been used without major bleeding risks in patients with thrombocytopenia after stem-cell transplantation, and is thus an alternative agent.36
Screening for Inherited Thrombophilia in Multiple Myeloma
Cancer patients who carry factor V Leiden or prothrombin G20210A mutations have a high risk of VTE. Analyses by the Multiple Environmental and Genetic Assessment study showed that testing for inherited thrombophilia did not reduce the recurrence of venous thrombosis.37 Some studies have observed a high prevalence of inherited thrombophilia among MM patients; however, no large study has evaluated its association with VTE incidence. Currently, there is no role for screening for inherited thrombophilia in MM patients treated with thalidomide or lenalidomide.
Treatment of Thalidomide- and Lenalidomide-Related VTE
If a patient with MM is being treated with thalidomide or lenalidomide and is subsequently diagnosed with VTE, then lenalidomide/thalidomide treatment should be withheld until full anticoagulation has been established. This usually takes a few days, and then thalidomide or lenalidomide can be reintroduced.38 In patients with cancer, LMWH has been shown to be superior to warfarin in terms of efficacy, safety, and possibly survival.39 However, this issue has not been addressed in randomized studies of patients with MM. LMWH can be considered an appropriate choice in patients with MM, due to lower risk of bleeding compared with warfarin. MM patients with a low risk of thrombocytopenia or bleeding can also be treated with oral warfarin. LMWH should be reduced if thrombocytopenia occurs; a 50% reduction can be performed with platelet counts below 50,000/μL, and a discontinuation of LMWH if the platelet count is less than 20,000 /μL. Patients should continue treatment as long as they are being treated with thalidomide or lenalidomide, or for a minimum of 6 months.12
The occurrence of DVT or pulmonary embolism in the general population is associated with an inferior survival rate. Additionally, patients with cancer have a 2-fold higher mortality if diagnosed with VTE. Two independent studies have been published on this topic in MM. In a retrospective study on 668 MM patients treated with or without thalidomide, patients with VTE had similar survival rates as those without MM.40 In a study on 353 MM patients treated with lenalidomide, no survival difference was observed in patients with a thromboembolic episode.41 The reason for this finding is not clear. It is possible that the occurrence of thrombosis is partly related to the response to the treatment, and that patients with VTE have a better response than others. This could partly explain why newly diagnosed patients have the highest risk, because they usually have the most therapy-sensitive tumors. This has to be studied in a large prospective study.
Summary and Future Perspectives
MM patients have an increased risk of thrombosis, particularly when treated with thalidomide and lenalidomide in combination with other agents. The two available randomized clinical trials suggest that aspirin, LMWH, and warfarin are effective in lowering the VTE risk. Individually based risk stratification should be taken into account when choosing the agent. Future studies will hopefully help us to gain more insight into the pathogenesis of thromboembolism in MM. Also, new, orally available anticoagulants, such as thrombin inhibitors and FXa inhibitors, have shown efficacy in preventing VTE.42 In addition to the advantage of being taken orally, these agents have predictable effects, which eliminates the need for monitoring; however, they need to be tested in MM patients.
Disclosure
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Sigurdur Yngvi Kristinsson, MD, PhD, Department of Medicine, Division of Hematology, Karolinska University Hospital Solna, SE-171 76 Stockholm, Sweden; Phone: 46–7-36696116; Fax: 46–8-318264; e-mail: sigurdur.kristinsson@karolinska.se