Abstract
Despite the availability of safe, effective targeted therapy that controls intravascular hemolysis, the management of paroxysmal nocturnal hemoglobinuria (PNH) remains complicated because of disease heterogeneity and close association with BM failure syndromes. The purpose of this review is to provide a framework for individualizing treatment based on disease classification. According to the recommendations of the International PNH Interest Group, patients can be placed into one of the following 3 categories: (1) classic PNH, (2) PNH in the setting of another BM failure syndrome, or (3) subclinical PNH. The PNH clone in patients with subclinical disease is insufficiently large to produce even biochemical evidence of hemolysis, and consequently, patients who fit into this category require no PNH-specific therapy. Patients with PNH in the setting of another BM failure syndrome (usually aplastic anemia or low-risk myelodysplastic syndrome) have at least biochemical evidence of hemolysis, but typically the PNH clone is small (< 10%) so that hemolysis does not contribute significantly to the underlying anemia. In these cases, the focus of treatment is on the BM failure component of the disease. Intravascular hemolysis is the dominant feature of classic PNH, and this process is blocked by the complement inhibitor eculizumab. The thrombophilia of PNH also appears to be ameliorated by eculizumab, but the drug has no effect on the BM failure component of the disease. Low-grade extravascular hemolysis due to complement C3 opsonization develops in most patients treated with eculizumab, and in some cases is a cause for suboptimal response to treatment. Allogeneic BM transplantation can cure classic PNH, but treatment-related toxicity suggests caution for this approach to management.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) has a special place in the fields of hematology and complementology because identification of the molecular basis of the hemolytic anemia that is the clinical hallmark of this disease led to a remarkable number of discoveries that helped to identify and characterize the alternative pathway and define the physiology of the complement system in humans.1 The discoveries began with the seminal observations of Thomas Hale Ham in the late 1930s that suggested a novel, antibody-independent mechanism for complement activation. Subsequently, Ham's observations contributed to elucidation of the properdin pathway (now known as the alternative pathway) by Louis Pillemer while the two were on the faculty at Case Western Reserve University in the 1950s. Systematic investigation of the aberrant regulation of complement on PNH erythrocytes contributed to the identification and characterization of the complement regulatory proteins decay accelerating factor (DAF, CD55) and membrane inhibitor of reactive lysis (MIRL, CD59) in the 1970s and 1980s and ultimately led to the development of the first successful targeted therapy for a complement-mediated disease when eculizumab was approved for treatment of PNH in 2007.2
In contrast to all other intrinsic abnormalities of the erythrocyte, PNH is an acquired disorder; and although the focus of this review is on the complement-mediated hemolytic anemia component of the disorder, PNH is actually a disease of the hematopoietic stem cell. PNH arises as a result of the nonmalignant clonal expansion of one or several hematopoietic stem cells that have acquired a somatic mutation of the X-chromosome gene PIGA that is required for synthesis of the glycosyl phosphatidylinositol (GPI) moiety that anchors some proteins to the cell surface. As a consequence of mutant PIGA, the progeny of affected stem cells (erythrocytes, granulocytes, monocytes, platelets, and lymphocytes) are deficient in all GPI-anchored proteins (GPI-APs) that are normally expressed on hematopoietic cells (and all GPI-APs that are normally expressed on hematopoietic cells are deficient on the progeny of PIGA mutant stem cells). Among the GPI-APs that are deficient in PNH are DAF (CD55) and MIRL (CD59), the 2 primary erythrocyte membrane regulators of complement. Deficiency of CD55 and CD59 accounts for the complement-mediated intravascular hemolysis that is the hallmark of the disease. The clinical manifestations of PNH are hemolytic anemia, thrombophilia, and BM failure, but only the hemolytic anemia is unequivocally a direct consequence of somatic mutation of PIGA.
PNH and complement
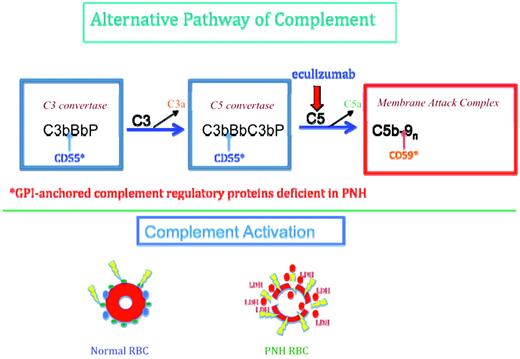
Thoughtful management of patients with PNH is facilitated by an understanding of the mechanisms involved in the activation and regulation of complement on the erythrocyte surface (Figure 1). The chronic intravascular hemolysis of PNH is mediated by the alternative pathway of complement (APC). A component of innate immunity, this ancient system evolved to protect the host against invasion by pathogenic microorganisms.3 Unlike the classical complement pathway that is part of the system of acquired immunity and requires antibody for initiation of activation, the APC is in a state of continuous activation, armed always to protect the host.4 The APC cascade can be divided into 2 functional components, the amplification C3 and C5 convertases and the cytolytic membrane attack complex (MAC) (Figure 1). The C3 and C5 convertases are enzymatic complexes that initiate and amplify the activity of the APC and ultimately generate the MAC. The MAC is the common pore-forming, cytolytic subunit of the classical and lectin complement pathways and the APC. Because the APC is always primed for attack, overlapping and redundant mechanisms for self-recognition and protection of the host against APC-mediated injury have evolved. Both fluid-phase and membrane-bound proteins are involved in these processes. Normal human erythrocytes are protected against APC-mediated cytolysis primarily by DAF (CD55)5–7 and MIRL (CD59),8 and these proteins act at different steps in the complement cascade. CD55 regulates the formation and stability of the C3 and C5 convertases, whereas CD59 blocks the formation of the MAC (Figure 1).9 Deficiency of both CD55 and CD59 is the pathophysiological basis of the Coomb-negative, intravascular hemolysis that characterizes the disease in its untreated state.9
Complement-mediated lysis of PNH erythrocytes. Top panel shows that the C3 convertase (left blue rectangle) of the APC consists of activated C3 (C3b), activated factor B (Bb, the enzymatic subunit of the complex), and factor P (a protein that stabilizes the complex, formally called properdin). The C5 convertase (right blue rectangle) has the same components as the C3 convertase except that 2 C3b molecules are required to bind and position C5 for cleavage by activated factor B (Bb). C3a and C5a are bioactive peptides that are generated by cleavage of C3 and C5, respectively, by their specific activation convertases. The C3 and C5 convertases greatly amplify complement activation by cleaving multiple substrate molecules. The MAC (red rectangle) consists of activated C5 (C5b), C6, C7, C8, and multiple molecules of C9 (C9n). The MAC is the cytolytic unit of the complement system. The GPI-anchored complement-regulatory protein CD55 restricts the formation and stability of both the C3 and the C5 amplification convertases by destabilizing the interaction between activated factor B (Bb) and C3b (indicated by the blue arrows), whereas GPI-anchored CD59 blocks formation of the MAC by inhibiting the binding of C9 to the C5b-8 complex (indicated by the brown arrow). Inhibition of MAC formation by the humanized anti-C5 mAb eculizumab (indicated by the red arrow) ameliorates the intravascular hemolysis of PNH. Bottom panel shows that normal erythrocytes (left) are protected against complement-mediated lysis (lightning bolts) primarily by CD55 (blue circles) and CD59 (green circles). Deficiency of these GPI-anchored complement-regulatory proteins results in APC activation on PNH erythrocytes (right). Consequently, MACs form pores in the red cell membrane, resulting in colloid osmotic lysis and release of hemoglobin (red circles) and other contents of the red cell, including LDH, into the intravascular space.
Complement-mediated lysis of PNH erythrocytes. Top panel shows that the C3 convertase (left blue rectangle) of the APC consists of activated C3 (C3b), activated factor B (Bb, the enzymatic subunit of the complex), and factor P (a protein that stabilizes the complex, formally called properdin). The C5 convertase (right blue rectangle) has the same components as the C3 convertase except that 2 C3b molecules are required to bind and position C5 for cleavage by activated factor B (Bb). C3a and C5a are bioactive peptides that are generated by cleavage of C3 and C5, respectively, by their specific activation convertases. The C3 and C5 convertases greatly amplify complement activation by cleaving multiple substrate molecules. The MAC (red rectangle) consists of activated C5 (C5b), C6, C7, C8, and multiple molecules of C9 (C9n). The MAC is the cytolytic unit of the complement system. The GPI-anchored complement-regulatory protein CD55 restricts the formation and stability of both the C3 and the C5 amplification convertases by destabilizing the interaction between activated factor B (Bb) and C3b (indicated by the blue arrows), whereas GPI-anchored CD59 blocks formation of the MAC by inhibiting the binding of C9 to the C5b-8 complex (indicated by the brown arrow). Inhibition of MAC formation by the humanized anti-C5 mAb eculizumab (indicated by the red arrow) ameliorates the intravascular hemolysis of PNH. Bottom panel shows that normal erythrocytes (left) are protected against complement-mediated lysis (lightning bolts) primarily by CD55 (blue circles) and CD59 (green circles). Deficiency of these GPI-anchored complement-regulatory proteins results in APC activation on PNH erythrocytes (right). Consequently, MACs form pores in the red cell membrane, resulting in colloid osmotic lysis and release of hemoglobin (red circles) and other contents of the red cell, including LDH, into the intravascular space.
Phenotypic mosaicism is characteristic of PNH
The peripheral blood of patients with PNH is a mosaic of normal and abnormal cells (Figure 2). Although PNH is a clonal disease, it is not a malignant disease and, for reasons that are unclear, the extent to which the PIGA-mutant clone expands varies widely among patients.10 As an example, in some cases, > 90% of the peripheral blood cells may be derived from the PIGA-mutant clone, whereas in others, < 10% of the circulating cells may be GPI-AP deficient. This peculiar feature (variability in the extent of mosaicism) is clinically relevant because patients with small PNH clones have minimal or no symptoms and require no PNH-specific treatment, whereas those with large clones are often debilitated by the consequences of chronic complement-mediated intravascular hemolysis and respond dramatically to complement-inhibitory therapy.
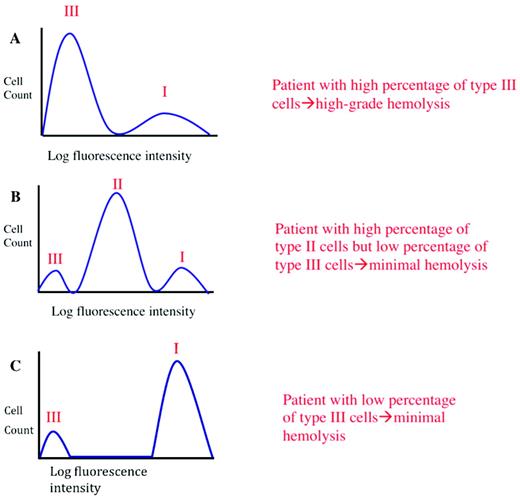
Clinical manifestations of PNH are determined by clone size and erythrocyte phenotype. Mock flow cytometry histograms of erythrocytes from hypothetical patients with PNH stained with anti-CD59 are illustrated. The proportion and type of abnormal erythrocytes varies greatly among patients with PNH, and these characteristics are important determinants of clinical manifestations. Patients with a high percentage of type III erythrocytes have clinically apparent hemolysis (A). If the erythrocytes are partially deficient (∼ 10% of normal expression) in GPI-AP (PNH II cells), hemolysis may be modest even if the percentage of the affected cells is high (B). A patient may have a diagnosis of PNH, but if the proportion of type III cells is low, only biochemical evidence of hemolysis may be observed (C).
Clinical manifestations of PNH are determined by clone size and erythrocyte phenotype. Mock flow cytometry histograms of erythrocytes from hypothetical patients with PNH stained with anti-CD59 are illustrated. The proportion and type of abnormal erythrocytes varies greatly among patients with PNH, and these characteristics are important determinants of clinical manifestations. Patients with a high percentage of type III erythrocytes have clinically apparent hemolysis (A). If the erythrocytes are partially deficient (∼ 10% of normal expression) in GPI-AP (PNH II cells), hemolysis may be modest even if the percentage of the affected cells is high (B). A patient may have a diagnosis of PNH, but if the proportion of type III cells is low, only biochemical evidence of hemolysis may be observed (C).
Another remarkable feature of PNH is phenotypic mosaicism based on PIGA genotype,11 which determines the degree of GPI-AP deficiency.10 PNH III cells are completely deficient in GPI-APs, PNH II cells are partially (∼ 90%) deficient, and PNH I cells express GPI-APs at normal density (putatively, these cells are the progeny of residual normal stem cells) (Figure 2). Phenotype varies among patients. Some patients have only type I and type III cells (the most common phenotype); some have type I, type II, and type III cells (the second most common phenotype); and some have only type I and type II cells (the least common phenotype). Further, the contribution of each phenotype to the composition of the peripheral blood varies. Phenotypic mosaicism is clinically relevant because PNH II cells are relatively resistant to spontaneous hemolysis, and patients with a high percentage of type II cells have a relatively benign clinical course (Figure 2).
The anemia of PNH is multifactorial
The anemia of PNH is multifactorial because an element of BM failure is present in all patients, although the degree of dysfunction is variable.12,13 In some patients, PNH arises in the setting of aplastic anemia. In this case, BM failure is the dominant cause of anemia. In other patients with PNH, evidence of BM dysfunction may be subtle (eg, an inappropriately low reticulocyte count), with the degree of anemia being determined primarily by the rate of hemolysis, which is determined by the PNH clone size and erythrocyte phenotype (Figure 2).
Diagnosis of PNH
Once suspected, diagnosing PNH is straightforward because a deficiency of GPI-APs on peripheral blood cells can be readily demonstrated by flow cytometry.14 Analysis of both RBCs and peripheral mononuclear cells is warranted, because clone size will be underestimated if only RBCs are examined due to the fact that GPI-AP–deficient red cells are selectively destroyed by complement. Recent transfusion will also affect the estimate of clone size if only RBCs are analyzed, but delineation of PNH phenotypes (ie, the percentage of type I, type II, and type III cells) requires flow cytometric analysis of the erythrocyte population (Figure 2).
In addition to flow cytometric analysis, the basic initial evaluation of a patient with PNH should include: complete blood count to assess the effects of the disease on the production of leukocytes, platelets, and erythrocytes; measurement of serum concentration of lactate dehydrogenase (LDH), bilirubin (fractionated), and haptoglobin, which are biochemical markers of hemolysis; determination of iron stores; BM aspirate and biopsy; and cytogenetics. These diagnostic studies will allow classification into 1 of 3 groups based on the recommendation of the International PNH Interest Group (Table 1).13
Classification of clinical PNH*

PMNs indicates polymorphonuclear cells.
Based on the recommendations of the International PNH Interest Group.13
†Based on macroscopic hemoglobinuria, serum LDH concentration, and reticulocyte count.
‡Karyotypic abnormalities are uncommon.
§Aplastic anemia or low-risk MDS.
¶Analysis of PMNs is more informative than analysis of RBCs due to selective destruction GPI-AP–deficient RBCs.
In patients with classic PNH, the leukocyte and platelet counts are usually normal or nearly normal, whereas leukopenia, thrombocytopenia, or both invariably accompany PNH in the setting of another BM failure syndrome. The reticulocyte count is needed to assess the ongoing capacity of the BM to respond to the anemia. Although the reticulocyte count is elevated in patients with classic PNH, as noted above, it may be inappropriately low for the degree of anemia, reflecting underlying relative insufficiency of hematopoiesis that is characteristic of the disease. Serum LDH is always markedly elevated in classic PNH. The degree of serum LDH elevation is variable in patients with PNH in the setting of another BM failure syndrome (determined by the size of the PNH clone); however, in a large majority of patients with PNH/BM failure, the clone size is < 10%, with < 10% of patients with PNH/BM failure having a clone size of > 50% (Table 1).15,16
By definition, patients with subclinical PNH have neither clinical nor biochemical evidence of hemolysis (Table 1). Patients with classic PNH may be iron deficient due to chronic hemoglobinuria and hemosiderinuria. BM aspirate and biopsy are needed to distinguish classic PNH from PNH in the setting of another BM abnormality. Nonrandom cytogenetic abnormalities are rare in classic PNH.17
Management of PNH based on classification
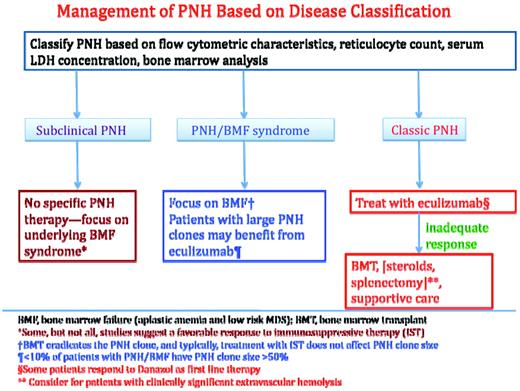
Completing the recommended diagnostic evaluation will allow the development of a systematic treatment plan (Figure 3) based on disease classification (Table 1).
Treatment algorithm based on disease classification. Disease classification is based on the recommendations of the International PNH Interest Group.13
Treatment algorithm based on disease classification. Disease classification is based on the recommendations of the International PNH Interest Group.13
Subclinical PNH
A close association exists between PNH and aplastic anemia and, to a lesser extent, between PNH and low-risk myelodysplastic syndrome (MDS). Using high-sensitivity flow cytometry, approximately 60% of patients with aplastic anemia and 20% of patients with low-risk MDS have been found to have a detectable population of GPI-AP–deficient erythrocytes and granulocytes.18–20 In ∼ 80% of these cases, the proportion of GPI-AP–deficient cells is < 1.0% of the total. These patients (designated subclinical PNH patients) with very small populations of GPI-AP–deficient erythrocytes have no clinical or biochemical evidence of hemolysis and require no specific treatment for PNH. However, finding a population of GPI-AP–deficient erythrocytes in patients with aplastic anemia may be clinically relevant, because some,19,20 but not all,21 studies suggest that these patients have a particularly high probability of responding to immunosuppressive therapy with a more rapid rate of onset of response compared with patients with aplastic anemia without a population of GPI-AP–deficient erythrocytes.
The presence of PNH cells has also been observed in patients with MDS.19,20,22,23 The association between PNH and MDS appears to be confined to low-risk categories of MDS, particularly the refractory anemia (RA) variant.18–20,22 Using high-sensitivity flow cytometry in which ≥ 0.003% of GPI-AP–deficient RBCs or peripheral mononuclear cells was classified as abnormal, Wang et al reported that 21 of 119 (18%) patients with RA MDS had a population of PNH cells, whereas GPI-AP–deficient cells were not detected in patients with RA with ringed sideroblasts, RA with excess of blasts, or RA with excess of blasts in transformation.20 Compared with patients with RA without a population of PNH cells, RA patients with a population of PNH cells had a distinct clinical profile characterized by the following features: (1) less pronounced morphological abnormalities of the blood cells, (2) more severe thrombocytopenia, (3) a lower rate of karyotypic abnormalities, (4) a higher incidence of HLA-DR15, (5) a lower rate of progression to acute leukemia, and (6) a higher probability of response to cyclosporine therapy. More recently, the findings by Wang et al that a population of PNH cells was associated only with low-risk MDS variants in Japanese patients were confirmed in a North-American study of 137 patients classified by World Health Organization criteria.22
When combined with evidence of polyclonal hematopoiesis (based on the pattern of X-chromosome inactivation in female patients), the presence of a population of PNH cells in patients with MDS predicts a relatively benign clinical course and high probability of response to immunosuppressive therapy.18 A relatively good response to immunosuppressive therapy for patients with MDS and aplastic anemia was also predicted by expression of HLA-DR15 in studies of both North American and Japanese patients.24,25 These observations support the hypothesis that aplastic anemia and a subgroup of low-risk MDS are immune-mediated diseases, and that the immune pathophysiological process provides the selection pressure that favors the outgrowth of PIGA mutant, GPI-AP–deficient stem cells.
PNH in the setting of another BM failure syndrome
Patients with a BM failure syndrome (aplastic anemia or MDS) and a PNH clone with clinical/biochemical evidence of hemolysis are classified as PNH in the setting of another BM failure syndrome (Table 1). In these patients, BM failure dominates the clinical picture and hemolysis is primarily an incidental finding.15,16,19 The large majority of patients with PNH/AA and PNH/MDS have relatively small PNH clones (< 10%) and require no specific PNH therapy; in these cases, treatment should focus on the underlying BM failure syndrome (Table 1 and Figure 3).
The PNH clone will be eradicated by the conditioning regimen in combination with the GVH effect in patients undergoing allogeneic transplantation for aplastic anemia or MDS. In most cases, the size of the PNH clone is unaffected by treatment with immunosuppressive therapy, and the presence of a PNH clone should not deter immunosuppressive therapy if that approach to treatment of the underlying BM failure syndrome is considered appropriate.15,16 In the uncommon cases in which, after immunosuppressive therapy, the size of the PNH clone is sufficiently large to produce clinical symptoms, the patient can be managed using the same approach as for patients with classic PNH.
Classic PNH
Patients with classic PNH have a large clone (> 50%), and consequently this disease subcategory is characterized by florid intravascular hemolysis as indicated by a markedly elevated serum LDH (Table 1). Patients may complain of episodic hemoglobinuria, and most experience ongoing constitutional symptoms dominated by lethargy, malaise, and asthenia that can be debilitating. The complement-mediated intravascular hemolysis of PNH can be inhibited by blocking formation of the MAC (Figure 1). The MAC consists of complement components C5b, C6, C7, C8, and multiple molecules of C9. Eculizumab (Soliris; Alexion Pharmaceutics) is a humanized mAb that binds complement C5, preventing its activation to C5b by the APC C5 convertase and thereby inhibiting MAC formation (Figure 1).2 In 2007, eculizumab was approved by both the US Food and Drug Administration and the European Union Commission for the treatment of the hemolysis of PNH. Treatment of classic PNH patients with eculizumab reduces transfusion requirements, ameliorates the anemia of PNH, and improves quality of life by resolving the debilitating constitutional symptoms associated with chronic complement-mediated intravascular hemolysis (Figure 3).26–28 After treatment, serum LDH concentration returns to normal or near normal, with approximately one-half to two-thirds of patients achieving transfusion independence26,27,29 ; however, mild to moderate anemia, hyperbilirubinemia, and reticulocytosis persist in essentially all treated patients.
Eculizumab appears to reduce the risk of thromboembolic complications.30 For patients being treated with eculizumab who have no prior history of thromboembolic complications, prophylactic anticoagulation may be unnecessary. Because PNH patients with prior thrombosis are at higher risk for recurrent thrombosis, anticoagulation for eculizumab-treated patients who experienced a prior thromboembolic event should be continued.29
Eculizumab is expensive (∼ $400 000/year in the United States) and has no effect on either the underlying stem cell abnormality or on the associated BM failure. Consequently, treatment must continue indefinitely and leukopenia, thrombocytopenia, and reticulocytopenia, if present, persist. Treatment with eculizumab appears to have a favorable impact on survival,31 because a recent study of 79 patients treated between 2002 and 2010 showed the same survival rates as those of age- and sex-matched controls from the general population.29 The contribution of eculizumab to survival cannot be quantified accurately, however, because a control patient group was not included in that study.
Reasons for suboptimal response to treatment with eculizumab
The recommended maintenance dose of eculizumab is fixed (900 mg every 2 weeks ± 2 days) rather than being based on weight or body surface area. Some patients may show evidence of breakthrough intravascular hemolysis (ie, increases in LDH and development of constitutional symptoms) near the end of a treatment cycle. In these cases, breakthrough hemolysis can be ameliorated by reducing the length of the treatment cycle from 14 days to 13 or 12 days, and in some cases, the maintenance dose of eculizumab may also have to be increased.
All patients with PNH have an element of BM failure, and patients treated with eculizumab who have higher degrees of relative reticulocytopenia may remain anemic or even transfusion dependent despite excellent control of intravascular hemolysis. Iron stores and serum erythropoietin concentration should be quantified in these patients, and if iron stores are adequate and serum erythropoietin concentration is inappropriately low, a trial of recombinant erythropoietin is warranted in patients who have symptomatic anemia or who are transfusion dependent.
After treatment with eculizumab, serum LDH returns to normal or near normal, but mild to moderate anemia and laboratory evidence of hemolysis persist in essentially all treated patients.26,27 A small subgroup of eculizumab-treated patients experiences little or no improvement in either anemia or constitutional symptoms. In these patients, hemolysis is mediated by opsonization of the PNH erythrocytes by activation and degradation products of complement C3, which, when tested, are found to be Coomb-positive for C3 but not IgG.32–34 The known pathophysiology of the PNH predicts that CD55 deficiency would result in ongoing extravascular hemolysis of PNH erythrocytes as a consequence of C3 opsonization (Figure 4) because eculizumab does not block the activity of the APC C3 convertase that is unregulated because of DAF deficiency (Figure 1). Support for this hypothesis is provided by the studies of Risitano et al, who showed that in patients treated with eculizumab, a portion of the PNH erythrocytes (ie, the CD59-deficient population) had complement C3 bound.34 Those studies also confirmed the Coomb-negative designation of PNH: no C3 was found bound to PNH erythrocytes before initiation of treatment with eculizumab, implying that PNH erythrocytes upon which complement has been activated are destroyed directly as a consequence of MAC-mediated cytolysis. These studies provide a plausible explanation for the persistent hemolytic anemia observed in PNH patients treated with eculizumab. By inhibiting the formation of the MAC, eculizumab prevents direct cytolysis of PNH erythrocytes, allowing the manifestations of DAF deficiency to become apparent in the form of aberrant regulation of the APC C3 convertase and the consequent deposition of activated C3 on the cell surface (Figures 1 and 4).4 Covalently bound activation and degradation products of C3 then serve as opsonins that are recognized by specific receptors on reticuloendothelial cells, resulting in extravascular hemolysis (Figure 4).
Generation of C3 opsonins on PNH erythrocytes in patients treated with eculizumab. Deficiency of DAF on PNH cells results in activation of the APC on PNH erythrocytes. Eculizumab blocks MAC-mediated complement lysis, allowing accumulation of C3 opsonins on PNH cells. The opsonized erythrocytes are recognized by reticuloendothelial cells of the spleen and liver that express receptors (primarily CR2 for C3dg and CR3 for iC3b), resulting in extravascular hemolysis. The figure illustrates covalent binding of activated C3 (C3b) to glycophorin A on the erythrocyte membrane surface. The bound C3 serves as the nidus for formation of the APC C3 convertase (C3b, activated factor B [Bb], and factor P) that enzymatically activates many molecules of C3 to C3b, which then bind covalently via an exposed thioester bond to carbohydrate residues on glycophorin A. Supported by interaction with sialic acid residues on glycophorin A, the plasma protein factor H binds to C3b and serves as a cofactor for degradation of C3b to iC3b by the plasma protein factor I. CR1 also binds to C3b and to iC3b and serves as a cofactor for the degradation of C3b to iC3b and then C3dg by factor I.
Generation of C3 opsonins on PNH erythrocytes in patients treated with eculizumab. Deficiency of DAF on PNH cells results in activation of the APC on PNH erythrocytes. Eculizumab blocks MAC-mediated complement lysis, allowing accumulation of C3 opsonins on PNH cells. The opsonized erythrocytes are recognized by reticuloendothelial cells of the spleen and liver that express receptors (primarily CR2 for C3dg and CR3 for iC3b), resulting in extravascular hemolysis. The figure illustrates covalent binding of activated C3 (C3b) to glycophorin A on the erythrocyte membrane surface. The bound C3 serves as the nidus for formation of the APC C3 convertase (C3b, activated factor B [Bb], and factor P) that enzymatically activates many molecules of C3 to C3b, which then bind covalently via an exposed thioester bond to carbohydrate residues on glycophorin A. Supported by interaction with sialic acid residues on glycophorin A, the plasma protein factor H binds to C3b and serves as a cofactor for degradation of C3b to iC3b by the plasma protein factor I. CR1 also binds to C3b and to iC3b and serves as a cofactor for the degradation of C3b to iC3b and then C3dg by factor I.
The extravascular hemolysis of patients with PNH receiving eculizumab does not require treatment in the absence of constitutional symptoms, symptoms of anemia, or transfusion dependence. Because the process is extravascular, splenectomy or corticosteroids may ameliorate the hemolysis in symptomatic or transfusion-dependent patients by removing or inhibiting the function of phagocytic cells (Figure 3).35 Long-term use of corticosteroids is associated with significant toxicity, however, and concerns about both postoperative and late complications temper enthusiasm for splenectomy. It is also conceivable that the primary site of phagocytosis is hepatic rather than splenic. In such cases, response to splenectomy would likely be inadequate. Based on experience in the treatment of refractory autoimmune hemolytic anemia, a trial of Danazol can be considered; however, Rituxan is not indicated because the process is mediated by C3 opsonization rather than opsonization by IgG antibody.
Hematopoietic stem cell transplantation for PNH
Before the availability of eculizumab, the primary indications for transplantation for PNH were bone failure, recurrent life-threatening thrombosis, and uncontrollable hemolysis.13 The latter process can be eliminated by treatment with eculizumab and the thrombophilia of PNH may also respond to inhibition of intravascular hemolysis by eculizumab.30 Nonetheless, transplantation is the only curative therapy for PNH, and the availability of molecularly defined, matched unrelated donors; less toxic conditioning regimens; reductions in transplantation-related morbidity and mortality; and improvements in posttransplantation supportive care make this option a viable alternative to medical management. The decision of who should receive a transplantation and when it should be performed is complex, however, and requires an understanding of the unique pathobiology of PNH and the input of physicians experienced in transplantation and medical management of PNH.36 The recent studies of Kelly et al29 showing normal survival for patients with PNH treated with eculizumab make the decision concerning medical management versus transplantation even more challenging.
Other treatments for PNH
Based on anecdotal experience, a portion of patients with classic PNH responds to Danazol as first-line therapy.37,38 The basis of this response is unknown but likely involves complement inhibition because reduction in hemolysis is observed quickly (within a few days) after initiation of therapy and PNH WBC clone size does not change during treatment (C.J.P., unpublished observation). Why some patients respond dramatically to Danazol whereas others do not is unknown, but it seems plausible to suggest that responders produce a metabolite that inhibits complement whereas nonresponders do not (or they produce a subtherapeutic concentration of the putative inhibitory metabolite).
Although hemolysis is ameliorated in some patients by treatment with glucocorticoids, the harm that can accrue from long-term use of prednisone cannot be overemphasized.13 Although their use in the management of PNH is a matter of ongoing debate, the main value of glucocorticoids may be in attenuating acute hemolytic exacerbations. Under these circumstances, brief pulses of prednisone may reduce the severity and duration of the crisis while avoiding the untoward consequences associated with long-term use.
Because hemolysis is a consequence of a defect intrinsic to a patient's erythrocytes, the anemia of PNH responds to transfusion. In addition to increasing the hemoglobin concentration, transfusion may lessen hemolysis by suppressing erythropoiesis. Concerns about inducing a hemolytic exacerbation as a consequence of infusion of small amounts of donor plasma that may contaminate red cell preparations appear unwarranted.39
Patients with classic PNH frequently become iron deficient as a result of renal loss (both hemoglobinuria and hemosiderinuria).38 Clinically important iron loss from hemosiderinuria can occur even in the absence of gross hemoglobinuria. Concern for inducing a hemolytic exacerbation should not deter iron repletion, because iron deficiency not only limits erythropoiesis but also exacerbates the hemolysis of PNH.38 If hemolytic exacerbation occurs in the setting of iron repletion, the episode can be controlled by treatment with corticosteroids or by suppression of erythropoiesis by transfusion. There is no concern about iron replacement therapy inducing a hemolytic exacerbation in patients being treated with eculizumab because the drug inhibits hemolysis. Patients treated with eculizumab should not become iron deficient because treatment will resolve hemoglobinuria and hemosiderinuria by blocking intravascular hemolysis.
Conclusions and future directions
Systematic investigation of the molecular basis of PNH has provided a framework for management based on an understanding of disease pathophysiology and has led to development of targeted therapy that has improved the lives of patients and changed the natural history of the disease. Nonetheless, continued investigation of new approaches to therapy aimed at obviating the extravascular hemolysis that limits eculizumab efficacy in some patients is warranted.4 A better understanding of the pathobiology that underlies the thrombophilia of PNH is needed, and defining the complex relationship between PNH and BM failure syndromes that determine clonal selection and clonal expansion40 may lead ultimately to therapy that targets the disease at the level of the hematopoietic stem cell. In particular, an understanding of the molecular basis of clonal expansion will be facilitated by the availability of next-generation sequencing that will allow comparison between the genomes of GPI-AP–positive and GPI-AP–negative cells from individual patients with PNH.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Charles J. Parker, Hematology Division, Department of Internal Medicine, The University of Utah School of Medicine, 30 N 1900 E, Room 5C402, Salt Lake City, UT 84132; Phone: (801) 585-3229; Fax: (801) 585-0309; e-mail: charles.parker@hsc.utah.edu.

![Figure 4. Generation of C3 opsonins on PNH erythrocytes in patients treated with eculizumab. Deficiency of DAF on PNH cells results in activation of the APC on PNH erythrocytes. Eculizumab blocks MAC-mediated complement lysis, allowing accumulation of C3 opsonins on PNH cells. The opsonized erythrocytes are recognized by reticuloendothelial cells of the spleen and liver that express receptors (primarily CR2 for C3dg and CR3 for iC3b), resulting in extravascular hemolysis. The figure illustrates covalent binding of activated C3 (C3b) to glycophorin A on the erythrocyte membrane surface. The bound C3 serves as the nidus for formation of the APC C3 convertase (C3b, activated factor B [Bb], and factor P) that enzymatically activates many molecules of C3 to C3b, which then bind covalently via an exposed thioester bond to carbohydrate residues on glycophorin A. Supported by interaction with sialic acid residues on glycophorin A, the plasma protein factor H binds to C3b and serves as a cofactor for degradation of C3b to iC3b by the plasma protein factor I. CR1 also binds to C3b and to iC3b and serves as a cofactor for the degradation of C3b to iC3b and then C3dg by factor I.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2011/1/10.1182_asheducation-2011.1.21/5/m_bep0011101250004.jpeg?Expires=1767708431&Signature=m0MFqpPnHnHcVl0xXR46UoiKEo48R48Ha6HYzPrKdYHCB70X0orMaigKw30Pn8Hz1qnavuTw1~632mTfmBXnfuPF01rtvSU1zskVHLNXbsmyJ1c7gqzeySfC2VXVnptNZ8PPGPehpq3dR5w~XkSjAH-mPqzFxofFett8u7QxH0vd21TCZpyuB6ptMwnjTGgs5xn7Yr8SMQLu2ljBfDXWUc99BmDBkfyBaLXndpDn6FKMe4oqtvpq-312qWl0gv32ky0VOhEWebXiVzrzg~-2OZqjOQvnD3UGF6QPCsLEJs6hVWYaFLsKFvUvimzn66ijhE9J5gbWJkQkijy4PbAapQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)