Abstract
Overt strokes, previously one of the most common neurological complications in sickle cell disease (SCD), have become far less frequent with routine transcranial Doppler (TCD) assessment followed by regular blood transfusion therapy. Nevertheless, children and adults with SCD continue to have overt strokes, and in the foreseeable future will continue to require secondary prevention of strokes. With the exception of the most recently completed “Stroke With Transfusions Changing to Hydroxyurea” Trial (SWiTCH; NCT00122980), randomized trials providing best evidence for long-term management of overt strokes in SCD is lacking. Instead of randomized clinical trials, a series of observational and single-arm studies have predominated. This review assesses the best available evidence for acute and chronic management of overt stroke and the efficacy of regular blood transfusion therapy, hydroxyurea therapy, and hematopoietic stem cell transplantation (HSCT), including matched sibling donor and unrelated HSCT.
Introduction
Before the use of transcranial Doppler (TCD) for the assessment of overt stroke risk, children with sickle cell disease (SCD) had the highest incidence rates of strokes compared with any other pediatric population.1 Arguably, the most significant recent advances in the treatment of children with SCD to reduce the occurrence of overt strokes are the use of TCD to assess risk for overt stroke and regular blood transfusions to reduce this risk. In institutions that were early adapters of this new technology, the incidence of overt strokes decreased 10-fold. Before the common use of regular TCD measurement, overt strokes occurred with an incidence of 0.67 events/100 patient-years, compared with 0.06/100 patient-years after the routine use of TCD to guide the initiation of transfusion therapy (P < .001) in a single-center study.2 Despite major advances in the primary prevention of overt strokes, strokes and their mimics remain relatively common and require prompt recognition followed by initial and long-term management. This review focuses on the best available evidence for acute management and secondary preventive strategies of overt strokes.
Definition of overt strokes in SCD
The classic definition of overt stroke and transient ischemic attack, a focal neurological deficit lasting for more or less than 24 hours, respectively,3 was developed in an era when neuroimaging and rapid therapeutic interventions were not available. The proclivity to retain this definition of overt stroke in the current management of individuals with SCD is problematic for several reasons. The 24-hour threshold to distinguish an overt stroke from a transient ischemic attack only has historical value. More specifically, cerebral infarcts can be seen in individuals who have focal neurological deficits that last < 24 hours.4 Further, individuals with SCD who present with overt strokes are often treated promptly with blood transfusion therapy that may reverse or abate the progression of the neurological findings within a 24-hour window from the acute onset of symptoms. Therefore, the absence of a focal neurological deficit on examination 24 hours after presentation does not mean that the patient has not had a cerebral infarct.
Initial imaging of focal neurological deficits
Evidence-based strategies demonstrating the optimal initial imaging sequence of the brain for individuals who present with focal neurological deficits do not exist, and the options are often limited by the local institution's resources. In an optimal situation, imaging of the brain for any patient with SCD who presents with a focal neurological deficit should include either a prompt computed tomography (CT) scan or magnetic resonance imaging (MRI) of the brain. The CT scan has typically been used to detect cerebral hemorrhage because of the unique advantage that it is ready within 30 minutes of ordering the examination and provides information primarily about cerebral hemorrhage, an event that is more common among adults compared with children with SCD. Recently, MRI sequences with similar sensitivity and specificity to CT scans have been added to detect cerebral hemorrhage.5 However, the application of MRI sequences to supplant the standard CT scan requires that hospitals have a predefined, timely MRI protocol that is instituted when a patient presents with a focal neurological deficit. Prompt diagnosis of cerebral hemorrhage is critical for potential expedient surgical evaluation.
MRI of the brain is the preferred strategy over CT scans to detect cerebral infarct.6 In addition, with the appropriate MRI sequence, diffusion-weighted image, the MRI can also identify the age of the lesion that may be present in the hyperacute period of < 6 hours7 or that may persist for up to 10 days.8–10 The ability to distinguish acute cerebral infarcts from chronic infarcts with a MRI is clinically relevant. Acute lesions strengthen the indication to perform acute exchange blood transfusion, a procedure that may require central line placement, multiple units of blood, and that can be associated with additional CNS events.
Given that the majority of individuals with SCD presenting with a focal neurological deficit undergo an MRI of the brain, the biggest clinical challenge is whether to perform an exchange transfusion. This decision is often based on the presence of a focal deficit on neurological examination and the presence of an acute MRI diffusion-weighted image. However, in the setting of a focal neurological deficit, explicit care must be taken to ensure that there is not overreliance on a negative MRI to forgo exchange transfusion. Several studies have documented that patients with focal neurological deficits may have a negative MRI/diffusion-weighted image scan, specifically negative diffusion-weighted images, within 24 hours of the onset of symptoms.11,12 In some patients who present with a focal neurological deficit and negative MRI, repeat MRI of the brain 30 days later may reveal the lesion corresponding to the focal deficit.12 After the exclusion of stroke mimics that are associated with a negative MRI of the brain, such as a migraine headache or a seizure that was not witnessed, the possibility of a stroke still exists. Ultimately, the diagnosis of an acute overt stroke and the decision to perform a timely exchange transfusion is multidisciplinary—a bedside decision requiring input from the neurologist, neuroradiologist, and hematologist.
If MRI changes are present, the differential diagnosis should include cerebral infarct, posterior reversible encephalopathy syndrome (PRES)13–15 and dural venous sinus thrombosis (DVST).16,17 The initial management of PRES and DVST often does not include an exchange transfusion, so neuroradiology and neurology input should be sought to distinguish these 2 clinical entities from cerebral infarct. Although evidence-based management is lacking, in some situations, patients may have either PRES or DVST with an acute cerebral infarct. In either case, treatment of the underlying etiology and a prompt exchange transfusion should be considered. Although MRI angiography images are commonly ordered with the acute presentation of focal neurological deficits in SCD, no information is obtained from them that will alter the initial management of the patient. An MRI venogram is far more helpful because a diagnosis of DVST can be excluded. Figure 1 is a schematic of the neuroimaging studies and the differential diagnosis of focal neurological deficits.
Proposed schematic of management of a patient with SCD presenting with a focal neurological deficit.
Proposed schematic of management of a patient with SCD presenting with a focal neurological deficit.
Initial therapy for the treatment of overt stroke
Evidence-based strategies for the treatment of overt stroke for SCD are lacking. The basic principle for treatment for stroke is to improve oxygen delivery to the brain,18 which can be accomplished primarily by providing supportive measures such as oxygen and blood transfusion therapy (simple or exchange).
The potential benefit of exchange transfusion therapy over simple transfusion therapy has never been and likely never will be demonstrated in a clinical trial. The best, although still weak, evidence that exchange blood transfusion therapy is a better strategy than simple transfusion therapy alone for acute presentation of strokes is from a large retrospective cohort in which the long-term outcomes of these 2 initial therapies were compared. Hulbert et al demonstrated that patients presenting with stroke who were treated with simple transfusion therapy had a 5 times greater odds (odds ratio 95%; confidence interval [CI] = 1.3-18.6) of subsequent strokes compared with children initially treated with exchange transfusion therapy.19 In select situations, exchange or simple transfusions may not be appropriate. When a patient has had an acute anemic event associated with stroke and a hemoglobin S level < 50% of their baseline, an exchange transfusion is probably not indicated. Similarly, a simple transfusion may be contraindicated, as is the case when the baseline hemoglobin is > 10.0 g/dL at presentation.
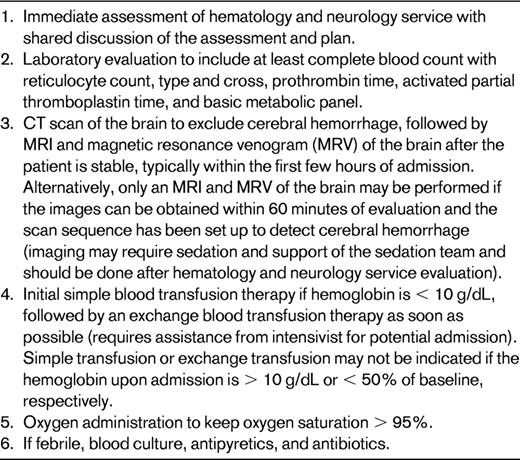
There are many limitations in using these data as the basis for the decision to choose initial exchange transfusion therapy over simple transfusion when treating an acute focal neurological deficit. These limitations include the retrospective nature of the study, the lack of a second study confirming the results, and moderately weak biological plausibility to explain the findings. Nevertheless, the magnitude of the benefit was large. Before the acknowledgement of these data, most pediatric hematologists at large academic centers (N = 14 in the study) elected to initially treat with an exchange transfusion over simple transfusion alone (Figure 2). Initial exchange transfusion for patients presenting with strokes within 24 hours is standard care. The period of time after the stroke when an exchange transfusion is no better than a simple transfusion is not known and is not likely to be determined; however, simple blood transfusions followed by exchange transfusions should be provided as soon as possible. Table 1 outlines the major steps in the management of a patient with SCD and an acute neurological deficit.
Increased use of exchange blood transfusion therapy as the predominant method of transfusion therapy compared with simple transfusion therapy for the acute presentation of overt stroke in 14 pediatric hematology centers. (Reprinted with permission from Hulbert et al, 2006.19 )
Increased use of exchange blood transfusion therapy as the predominant method of transfusion therapy compared with simple transfusion therapy for the acute presentation of overt stroke in 14 pediatric hematology centers. (Reprinted with permission from Hulbert et al, 2006.19 )
Secondary prevention of overt strokes
Regular blood transfusion therapy
No randomized trials have been conducted to determine the best treatment for the secondary prevention of strokes. Initial evidence implicating the need for secondary prevention of strokes was demonstrated by the seminal study by Powers et al showing that 50% of the individuals with SCD and overt stroke will have a second stroke within 2 years and 66% within 10 years.20 In addition, a temporal clustering occurred, with 80% of the strokes being observed within 36 months of the first stroke. Subsequently, in a landmark single-arm intervention study, Sarnaik et al demonstrated that a transfusion program that consisted of keeping the maximum hemoglobin S levels < 25% and the baseline line hemoglobin between 10 and 12 g/dL was beneficial in preventing recurrent strokes. Using a before and after study design, the intervention group of 12 patients had 1-9 CNS recurrences; however, after the start of the program, no other recurrences occurred and none of the patients demonstrated progression of neurological abnormalities. The biggest limitation of the study was the lack of a multivariable regression to control for known risk factors, the most important being the length of time after a stroke. Nevertheless, the results from this study formed the foundation of the current practice of monthly blood transfusion therapy for the secondary prevention of strokes.
Since the mid-1990s, 3 studies (2 retrospective and 1 prospective cohort) have been conducted describing the relative benefits of regular blood transfusion therapy. In a multicenter study, Pegelow et al described the efficacy of blood transfusion therapy in a retrospective cohort.21 In this study, the investigators identified 60 children with an overt stroke followed for 192 patient-years. The incidence of stroke was 4.2 events/100 patient-years (95% CI = 1.8-8.0 strokes/100 patient-years). This study provided the first systematic effort to demonstrate that progressive overt strokes are common. Subsequently, in a retrospective cohort, Scothorn et al identified 137 children with strokes who received regular blood transfusion therapy followed for 1387 patient-years.22 The incidence of stroke was 2.2 events/100 patient-years (95% CI = 1.5-3.2 strokes/100 patient-years). In the only prospective study performed to date addressing the benefit of blood transfusion therapy, Hulbert et al identified 40 participants followed for 222 patient-years, with an incidence of overt strokes of 3.2 events/100 patient-years (95% CI = 1.3-6.5 strokes/patient-years) while receiving regular blood transfusion therapy, with the average maximum hemoglobin S level < 30%.23
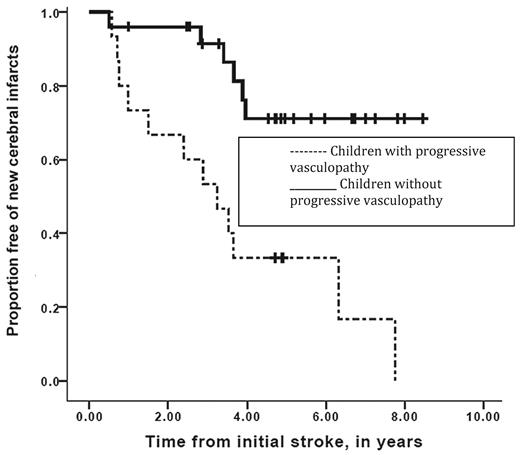
Despite the initial promise that regular blood transfusion therapy was a reasonable therapy for the secondary prevention of strokes, there is overwhelming evidence that when placed on blood transfusion therapy, a significant number of children with SCD will continue to have overt strokes. In both the Scothorn22 and the Hulbert23 cohorts, patients were identified as having strokes at times when their hemoglobin S levels were < 20% and in several cases < 10%. Further, in the Scothorn cohort, individuals were followed for a mean and median of 10 years, with approximately 30% of the participants who had a second stroke going on to have a third stroke.22 In addition, when surveillance MRI of the brain is done in regular intervals to assess the efficacy of transfusion therapy, the most common etiology of new or progressive infarcts is silent cerebral infarct and not overt strokes. Among the cohort of 40 participants, 11 of 40 had progressive new silent cerebral infarcts, whereas 7 of 40 had new overt strokes; therefore, 45% of the patients had progressive cerebral infarcts over a mean of 5.2 years of follow-up (Figure 3).23
Event-free interval of new overt or silent cerebral infarcts in children with SCD while on transfusion therapy for secondary stroke prevention. Participants with progressive overt and silent cerebral infarction were stratified for the absence (solid line) or the presence (dashed line) of progressive cerebral vasculopathy during chronic blood transfusion therapy. Vertical lines represent censored cases. Median event-free interval for new silent or overt infarction was 3.2 years for the group with progressive vasculopathy and was not reached in the group without progressive vasculopathy (Mantel-Cox log-rank, P = .001). (Reprinted with permission from Hulbert et al, 2011.23 )
Event-free interval of new overt or silent cerebral infarcts in children with SCD while on transfusion therapy for secondary stroke prevention. Participants with progressive overt and silent cerebral infarction were stratified for the absence (solid line) or the presence (dashed line) of progressive cerebral vasculopathy during chronic blood transfusion therapy. Vertical lines represent censored cases. Median event-free interval for new silent or overt infarction was 3.2 years for the group with progressive vasculopathy and was not reached in the group without progressive vasculopathy (Mantel-Cox log-rank, P = .001). (Reprinted with permission from Hulbert et al, 2011.23 )
Hydroxyurea therapy
In a single-institutional study, Ware et al were the first to systematically use hydroxyurea for the secondary prevention of strokes and to decrease the iron burden. These investigators treated 35 children with hydroxyurea for approximately 123 years (35 children treated for a mean of 3.5 years). The overall progression of stroke was 5.7 events/100 patient-years. For children receiving overlapping hydroxyurea and blood transfusion therapy, the progression of strokes was 3.6 events/100 patient-years. Most importantly, as part of the treatment regimen, ferritin levels, a measure of excessive iron stores, decreased from a median of 2722 to 298 ng/mL. These data were promising, although there was some concern about the strength of evidence to support a randomized clinical trial.24 Nevertheless, the results of the study, along with the risks of transfusion-related complications, provided evidence to support a multi-institutional clinical trial funded by the National Heart, Lung and Blood Institute, referred to as Stroke With Transfusions Changing to Hydroxyurea (SWiTCH; Clinical Trials.Gov NCT00122980). Subsequent follow-up of the single-arm study revealed that the stroke recurrence rate was 4.6/100 patient-years, with 30 patients followed for an aggregate of 219 patient-years.25
The incidence rates for stroke recurrence among children who receive hydroxyurea are similar to blood transfusion therapy, suggesting but not confirming that hydroxyurea will not be as effective as blood transfusion therapy. Regardless of whether patients receive blood transfusion therapy, all large case series in patients with strokes have demonstrated that the 2- to 3-year window after an initial stroke has the highest incidence rate for stroke recurrence.20,22,23 In the largest hydroxyurea trial for secondary prevention of strokes, hydroxyurea was started well after the highest stroke recurrence risk period, with a mean interval of approximately 5 years after the initial stroke. Reliable comparison of incidence rates from different observational studies for secondary prevention of strokes cannot be done unless the time after the initial stroke is considered in the estimate of the recurrence incidence rate. To date, no such analysis has been done. Ultimately, only a randomized clinical trial can determine whether the 2 treatment strategies are equivalent.
The primary aim of SWiTCH was to compare 30 months of hydroxyurea and phlebotomy with standard secondary prevention of stroke therapy, regular blood transfusions, and chelation therapy.26 Participants were enrolled during October 2006 and April 2009. The primary end point was composite and included both stroke recurrence rate and iron burden. Similar to the earlier single-arm study, a transfusion overlap period in the hydroxyurea arm was used. A total of 133 children with hemoglobin SS and overt strokes were randomly allocated and received study treatment or the standard arm. A scheduled interim data analysis was performed and the liver iron content was compared between the arms and deemed not statistically different. Accordingly, the Data Safety and Monitoring Board determined that the increased stroke risk in the hydroxyurea arm did not justify the evidence that there was an observed decrease in the liver iron burden, and the study was therefore stopped earlier than planned in May of 2010. Only the participants with strokes in each group were provided and not the incidence rates. In the hydroxyurea group, 7 of 67 recurrent strokes occurred, whereas in the standard-therapy arm of regular blood transfusion therapy, no stroke occurred in 66 participants. The full details of the trial have not been published, so we await more information as to the incidence rates of recurrent strokes and other components of the trial.
Despite the premature cessation of the SWITCH Trial coupled with the evidence that hydroxyurea therapy may be less efficacious than regular blood transfusion therapy for secondary prevention of strokes,24,25 many practitioners continue to use hydroxyurea for this purpose. In a survey of over 350 pediatric hematologists in the American Society of Pediatric Hematology/Oncology, 36% elected to use hydroxyurea for the secondary prevention of strokes.27 Undoubtedly, in some cases, hydroxyurea has been selected because regular blood transfusion therapy is not possible due to poor access or extensive red blood cell allo-immunization. However, in other cases in which the patient could receive regular blood transfusion therapy, the parents and the health care provider presumably have made an explicit and informed decision to select the convenience of hydroxyurea over the inconvenience of blood transfusion therapy with chelation therapy. Implicit in their decision is the potential incremental increased risk of overt strokes with hydroxyurea therapy that outweighs the burden of regular blood transfusion therapy with chelation (ie, excessive iron stores, red blood cell allo-immunization, and inconvenience). Unfortunately, no study has been done comparing the parents' and the patients' ability to understand the known and postulated benefits and risks of hydroxyurea compared with regular blood transfusion therapy for the secondary prevention of strokes.
HSCT
The definitive treatment and the first option for secondary prevention of overt stroke is HSCT with an HLA-matched, sibling-identical donor. However, most children with SCD and strokes do not have a sibling identical donor, making this option unavailable. Nevertheless, recent evidence from Walters et al strongly suggests that, when available, matched-sibling donor HSCT should be the first option for the secondary prevention of strokes.28 In the most comprehensive study to date, which was performed from 1991-2000, none of the 28 patients with engraftment experienced recurrent strokes or had evidence of silent cerebral infarct events after HSCT, with a mean follow-up of 3.6 years (range 0.6-7.3 years).
The optimal timing of when a sibling-matched HSCT should be performed after a stroke is not known. When choosing the timing for this procedure, the risk of neurological complications temporally related to the HSCT, commonly a seizure, must be balanced against the risk of having a progressive infarct when the patient is receiving blood transfusion therapy and has an available genetically matched-sibling donor. In my opinion, the earlier the sibling-matched HSCT occurs after the patient is neurologically stable, the better. The rational for performing HSCT earlier is based on the observation that the most vulnerable period for progressive infarcts (overt or silent) occurs in the first several years after the stroke,20,22,23 and the primary goal of the HSCT is to prevent further injury to the brain. Alternatively, the HSCT may be performed beyond the window of highest risk of recurrence, when based only on speculation, the brain is less susceptible to transplant-related neurological injury. Regardless of the timing of the HSCT, there is small, but finite risk of death and transplanted-related complications that may tip the balance of the decision of the patient and family to forgo the option of a sibling-matched HSCT. Ultimately, the patient, parents, and the health care team should carefully weigh the pros and cons of the available therapeutic options.
Given the recent evidence that secondary prevention of strokes with rigorous blood transfusion therapy is associated with approximately a 45% chance of having new or progressive overt and silent cerebral infarcts,23 coupled with the well-established observation that successful HSCT halts the progression of strokes, careful consideration should be given to alternative HSCT options. Among children who have strokes and who are receiving regular blood transfusion therapy, those with progressive vasculopathy, which is associated with a 12 times greater risk of new cerebral infarction (95% CI = 2.65-60.5, P = .001),23 are more likely to benefit than those without this radiographic feature (Figure 3).
As listed in clinicaltrials.gov, multiple alternative HSCT options include nonmyeloablative and haploidentical HSCT. However, such therapy should only be offered in a rigorous clinical trial setting with evidence that a sufficient number of patients may be enrolled in a timely fashion to answer the primary objectives of the trial.
Secondary prevention of strokes in adults with SCD
Limited data exist to guide the hematologists providing medical care for adults for secondary stroke prevention. In pediatric SCD stroke studies with some adults included, no subgroup analyses have been provided to suggest that significant differences occur in the clinical history of an adult with SCD compared with a child with SCD.20,22
Secondary prevention of stroke in adults with SCD should include blood transfusion therapy. The decision not to transfuse an adult patient with an overt stroke puts the patient at significant risk for stroke progression, including silent cerebral infarct. The alternative use of hydroxyurea or HSCT should only be considered in a clinical trial setting with a high probability of answering the study question. Although no trial has been done in children or adults, the cessation of blood transfusion therapy for the secondary prevention of strokes in adults is not recommended. Patients are likely to have progression of at least overt strokes,29,30 and most likely both overt and silent cerebral infarct, because new or recurrent infarcts occur even in the setting of regular blood transfusion therapy.23
Summary
TCD measurement has become routine preventive care for children with SCD, resulting in a dramatic decrease in the incidence rate of new overt strokes. Despite this major advancement, children and adults continue to present with focal neurological deficits that require prompt examination, neurological imaging assessment, and therapy tailored to the underlying diagnosis. The major consideration for both children and adults with focal neurological deficits is prompt neuroimaging and medical evaluation for a definitive diagnosis and therapy. The differential diagnosis is the same in all age groups, but the relative probability of each diagnosis is different. Regardless of age, the prevalence of etiologies with different optimal management strategies is sufficiently high to warrant a full evaluation in any individual who presents with a focal neurological deficit. New evidence indicates that regular blood transfusion therapy aimed at keeping the maximum hemoglobin S levels < 30% is moderately effective in preventing progressive overt strokes and silent cerebral infarcts. Hydroxyurea is significantly less effective than regular blood transfusion therapy; however, some providers and parents have elected to start this therapy instead of regular blood transfusions. HSCT offers the option for definitive secondary prevention of strokes, but is limited because of the small number of HLA-identical donors. Alternative HSCT is a promising option, but must be done in a rigorous clinical trial setting with multiple sites, because one site will not likely have a sufficient number of patients to complete the trial in a timely fashion.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Michael R. DeBaun, Department of Pediatrics, Vanderbilt University School of Medicine and Monroe Carell Jr Children's Hospital at Vanderbilt, Nashville, TN 37232; Phone: (615) 322-8471; (615) 875-3040; Fax: (615) 936-6852; e-mail: m.debaun@vanderbilt.edu.