Abstract
After approximately 20 years of development and after several prospective clinical trials, the detection of minimal residual disease (MRD) has emerged as part of state-of-the-art diagnostics to guide the majority of contemporary treatment programs both in pediatric and adult acute lymphoblastic leukemia (ALL). For ALL, several methods of MRD analysis are available, but 2 are widely applicable. One is based on the detection of aberrant expression of leukemia specific antigens by flow cytometry and the other one uses the specific rearrangements of the TCR or Ig genes, which can be detected by quantitative PCR in the DNA of leukemic cells. In some cases with known fusion genes such as BCR/ABL, RT-PCR can be used as a third method of identifying leukemic cells by analyzing RNA in patient samples. Clinical application of such sophisticated tools in the stratification and treatment of ALL requires reliable, reproducible, and quality-assured methods to ensure patient safety.
Introduction
Several pediatric and adult acute lymphoblastic leukemia (ALL) study groups have established informative checkpoints in their respective treatment protocols that are being used for risk stratification. Predefined levels of minimal residual disease (MRD) at these checkpoints predict the risk of relapse, but large differences in the response rates of various ALL subtypes have been found. MRD detection before and after stem cell transplantation (SCT) has also been established for individual treatment adaptation, and whether response assessment by MRD detection can be used as a surrogate clinical end point for the evaluation of single agents or combination therapy is now being investigated.
In vivo sensitivity of ALL can be defined by the early blast cell reduction in the peripheral blood (PB) or BM after exposure to one or several antileukemic agents. Systematic response assessment is regarded as a necessary tool to risk-stratify patients with ALL. Lack of adequate response, in particular at the end of remission induction, indicates poor prognosis, but this may vary significantly according to individual patient characteristics.1 Cytological response assessment early in treatment can distinguish prognostic subgroups very well if done by experienced investigators in one reference laboratory either in the PB (day 8)2 or the BM (day 15).3 Despite the clear separation of risk groups through BM cytology, two-thirds of the relapses are nevertheless comprised in patients with M1 or M2 BM on day 15 of induction.3 Therefore, advanced and highly sensitive methods for response assessment were needed and developed to detect MRD.
The choice of technique for the detection of MRD depends mainly on the aims of the clinical trial and on the availability of resources.4–7 If MRD analysis is used to identify high-risk (HR) patients, it may be sufficient to use a faster but less sensitive method.8 If the aim is also to identify “super responders,” it is necessary to seek the highest sensitivity because the lack of signal in the corresponding MRD investigation must be absolutely reliable, particularly if such patients shall be spared additional therapy or even be assigned to reduced therapy. The second most important prerequisite is the prospective analysis with the MRD method of choice to determine the prognostic significance of certain MRD levels on the background of a predefined uniform chemotherapy regimen.4,9–15 It is now widely acknowledged that MRD detection is part of state-of-the-art diagnostics and is necessary in the management of ALL.7,16–18 This short review describes the clinical utility of MRD application in the treatment of ALL, with a specific focus on pediatric ALL. The necessity to work with well-established definitions is emphasized. In addition, a few examples in which MRD was used for assessment of efficacy of novel treatment modalities, either alone or in combination with conventional end points, are briefly reviewed.
Pros and cons of commonly used techniques for MRD assessment
Table 1 illustrates some key characteristics of the methods used in ALL for MRD detection. These factors were recently reevaluated before being put into a consensus recommendation in Europe.4 Although RT-PCR may provide highly sensitive MRD detection in ALL with specific fusion genes (eg, BCR/ABL or MLL rearrangements), the main limitation is the lack of such targets in the majority of ALL cases. It is highly relevant, however, for quantitative monitoring of BCR/ABL+ ALL when being treated with novel tyrosine kinase inhibitors.4,19 Both PCR and flow cytometry (FCM) require minimum cell counts at diagnosis and during follow-up. This needs to be strictly observed because there is a risk of misinterpretation of quantitative readouts if cell counts are low.5,7 For reproducible quantification, clear guidelines are needed, especially if several reference laboratories perform the initial and follow-up diagnostics in the same clinical trial.20
Characteristics of the techniques currently used for MRD detection in ALL4

Ph+ indicates Philadelphia chromosome positive.
MRD after 1 or 2 weeks of therapy: impact of early response assessment
In many ALL protocols, days 8 and 15 of induction therapy are considered the first checkpoints to test the in vivo sensitivity of the leukemia in the individual patient.3,21–24 The general message for the individual patient is simple: the fast reduction or even elimination of the leukemia (or its predominant clone) is highly predictive of superior relapse-free survival.9,25 Borowitz et al demonstrated that distinct levels of MRD (by FCM) in the PB at day 8 of induction therapy in a large set of precursor B-cell ALL (pcB ALL; n = 2143) patients were associated with the probability of event-free survival (pEFS). Patients with 0.01% or less MRD (called “MRD−”) had a 5-year pEFS of 90% ± 2%, whereas the worst group, with MRD at > 10%, had a pEFS of only 54% ± 7%. Interestingly, among the patients who cleared MRD in the BM by the end of induction (day 29 in that regimen), the prognosis of patients with more than 1% MRD in the PB at day 8 (18% of all) was worse than that of patients who had responded more favorably at day 8 (79% ± 4% vs 90% ± 1%, respectively).9 Another large study performed by Basso et al compared BM day 15 MRD results generated by FCM with cytomorphological response assessment and PCR analysis of MRD at the end of induction (day 33) and at the end of consolidation (day 78). Levels of MRD in the BM at day 15 were well correlated with risk of relapse: less than 0.1% of MRD conferred a cumulative incidence of relapse of 8% ± 1.7% in pcB-cell ALL and only 3.3% ± 3.3% in T-cell ALL. These fast responders comprised 43% of pcB-cell ALL patients and 34% of T-cell ALL patients, respectively. In both major subgroups of ALL, a distinct poor-risk group was identified by high levels of MRD (≥ 10%) at day 15, comprising 10% of pcB-ALL and 21% of T-ALL, respectively: the 5-year cumulative incidence of relapse was 45.5% ± 6.8% in pcB-ALL and 55.6% ± 11.7% in T-ALL.15 Investigators from Prague reported on the specific prognostic information of MRD in PB on day 15 of a Berlin-Frankfurt-Münster (BFM)–based regimen compared with MRD in the BM (both measured by PCR in PcB-ALL).26 They were able to demonstrate that 35 of 78 patients were MRD− (or low positive below 10−4) in the PB at day 15, with an excellent relapse-free survival of 100%. At day 8 of therapy, the same low level of MRD also identified a subset of patients without relapse, but that group was much smaller. Therefore, day 15 appears to be an informative MRD checkpoint, both by MRD in PB and in the BM if treatment follows the BFM regimen. This is somewhat surprising, because the first 2 weeks of this therapy comprised only a 7-day prophase with prednisone and 1 dose of intrathecal methotrexate, followed by vincristine and daunorubicin on day 8 and asparaginase on day 12. Usually, the day 15 PB and BM samples are taken before the next doses of vincristine, daunorubicin, and asparaginase are given on day 15.21
Is early MRD response assessment by FCM sufficient to identify the patient at risk of relapse and the patient who can be spared therapy?
To this end, the analysis by Basso et al reveals that 41% of the patients who are classified as low risk by FCM (< 0.1%) on day 15 of induction therapy according to protocol Italian Association of Pediatric Hematology and Oncology (AIEOP)-BFM ALL 2000 are not MRD− by PCR at the end of induction (day 33). The rate of relapses was very low among FCM day 15 good responders (< 0.1%), but 12 of 19 recurrences within that group were observed in the subset of patients with any level of MRD positivity by PCR at day 33.15 Another large study comparing MRD results obtained in the same protocol by several reference laboratories also revealed that concordance, sensitivity, and specificity of MRD analysis by FCM and PCR largely depend on the time in therapy and on the cell material used.27 Both studies reflect the practical realities of large clinical study groups, and these comparisons reveal that both techniques, FCM on day 15 and PCR at end of induction and at additional later time points, may be complementary in optimizing the treatment stratification. Rapid clearance of blasts as measured by PCR-based MRD detection is also associated with superior prognosis in standard-risk (SR) adult ALL. Approximately 10% of the patients have undetectable or low positive (< 10−4) MRD at days 11 and 24 of induction. Their prognosis is excellent, with 3-year disease-free and overall survival of 100%.28
Prognostic information based on MRD at end of induction
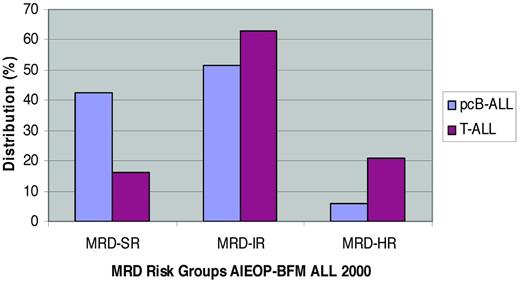
In the largest study for de novo ALL published so far, the AIEOP and BFM study groups used MRD by PCR for risk stratification in a total of 3184 pcB-ALL and 464 T-ALL patients. All patients were treated by identical chemotherapy in the first 9 weeks of therapy. Large differences in pEFS between MRD-defined subgroups were found.29,30 MRD was analyzed in the BM at the end of induction (day 33) and at the end of induction consolidation (day 78) and revealed major differences in the kinetics of the MRD response between pcB-ALL and T-ALL (Figure 1) and large similarities in the pEFS of all 3 MRD risk groups: In pcB-ALL, the pEFS at 5 years was 92.3% ± 0.9% for MRD-SR, 77.6% ± 1.3% for MRD-intermediate risk (MRD-IR), and 50.1% ± 4.1% for MRD-HR. The corresponding results in T-ALL were 93.0% ± 3.0% (5-year pEFS for MRD-SR), 80.6% ± 2.3% (MRD-IR), and 49.8% ± 5.1% (MRD-HR), respectively. Interestingly, the AIEOP-BFM trial showed that patients with the unfavorable subtype of early T-precursor ALL31 were also more frequently slow MRD responders, which resulted in treatment in the most intensive treatment group. Within pcB-ALL, the prognostic impact of MRD was maintained, even in the 2 large subgroups of TEL/AML1+ and hyperdiploid ALL.29 Two major differences between pcB-ALL and T-ALL can be found: (1) MRD at the end of induction (day 33) is more informative in pcB-ALL, whereas MRD at the end of consolidation (day 78) is more informative in T-ALL, and (2) MRD levels in pcB-ALL are correlated with risk of systemic relapse, whereas MRD in T-ALL is predictive of both systemic and extramedullary relapse. The results obtained here, in combination with the results obtained by FCM15 in the same trial, extend the current risk group definition for MRD-HR patients. Any patient who has > 10% leukemic blasts by FCM on day 15 is enrolled into the MRD-HR group; any patient with pcB-ALL who has MRD > 10−3 (0.1%) at day 33 and is still MRD+ at day 78 is stratified into the MRD-HR group; any pcB-ALL patient with MRD < 0.1% at day 15 is eligible for being considered MRD-SR if the PCR results (with at least one highly sensitive molecular target, quantitative range up to 10−4) at days 33 and 78 are also negative.
pcB- and T-ALL in AIEOP-BFM ALL 2000. Shown is the distribution of patients in the different MRD risk groups according to immunophenotype.29,37 MRD-SR indicates no MRD detectable at days 33 and 78 from diagnosis, with a sensitivity for the 2 targets of at least 10−4; MRD-HR indicates that the MRD level at day 78 is ≥ 10−3; and MRD-IR indicates all other constellations of MRD.
pcB- and T-ALL in AIEOP-BFM ALL 2000. Shown is the distribution of patients in the different MRD risk groups according to immunophenotype.29,37 MRD-SR indicates no MRD detectable at days 33 and 78 from diagnosis, with a sensitivity for the 2 targets of at least 10−4; MRD-HR indicates that the MRD level at day 78 is ≥ 10−3; and MRD-IR indicates all other constellations of MRD.
When the results of Conter et al29 are compared with the large study by Borowitz et al in pcB-ALL,9 one important difference can be found: MRD negativity at end of induction is strongly associated with highly favorable outcome independent of other risk factors. In the Children's Oncology Group (COG) trials, MRD negativity at end of induction was also associated with favorable outcome, but the group was much larger and half of the events still occurred within that group. This most likely reflects the different MRD techniques and sensitivities, but the different treatment intensity applied in the COG trials may also have contributed to this observation. Other groups have also demonstrated the prognostic significance of end of induction MRD despite differences in the composition of induction therapy.4,32,33
Postremission MRD and subsequent MRD surveillance at later time points
Additional postremission MRD assessment has been performed in several clinical trials and these were reviewed by Brüggemann et al.4 In AIEOP-BFM ALL 2000, all patients with MRD at ≥ 10−3 at day 78, were stratified into the MRD-HR group and then monitored after each reconsolidation element. This strategy is now used to adjust further chemotherapy and to prepare for allogeneic SCT. Although postinduction MRD was also found to have a significant prognostic impact in relapsed ALL,14 MRD monitoring of the pre-SCT response demonstrated the necessity to optimize the quality of remission before the procedure to prevent post-SCT relapse.34,35 Another recent investigation of MRD in adult patients with HR-ALL demonstrated that high MRD at day 71 after induction was associated with only 32% ± 6% disease-free survival compared with 66% ± 8% in HR patients with molecular complete remission. Allogeneic SCT appeared to be beneficial in patients with molecular failure, because those patients who did not receive the procedure had a disease-free survival of only 6% ± 5%. In SR patients, similar but less pronounced observations were made. If MRD at week 16 was analyzed, this difference between transplanted and nontransplanted patients could also be seen.36 Therefore, allogeneic SCT should be offered rather soon because relapse will occur early in such patients.
MRD in relapse and clonal evolution
MRD monitoring in relapsed patients carries some potential pitfalls, mainly due to clonal evolution.38 Detailed analysis of all molecular markers at first diagnosis and at the time of relapse may reveal a different origin of the predominant clone.39 This implies that regular monitoring of MRD by those markers defined at first diagnosis may fail if clonal evolution occurs after first relapse.40 Genome-wide copy number profiling in pairs of primary/relapsed ALL patients demonstrated a large number of differential somatic copy number alterations.41,42
Definitions of MRD status in the management of ALL
Although there is great consensus about the definition of complete cytological remission in ALL,1 it has become more difficult to achieve common definitions for certain MRD terms. These definitions are urgently needed for comparison of results in clinical trials, but more importantly, to provide safe guidelines for patient management. The consensus proposal summarized by Brüggemann et al seems useful in providing the terms for remission assessment and postremission monitoring (Table 2).
Proposals for definitions of MRD terms in ALL4

*Treatment modifications depending on these measurements are strongly recommended to be based on at least 2 analyzed samples.
†Time points should be specified within the respective protocol and at least one relevant treatment element should be administered in between.
‡MRD reappearance can be equated to “MRD relapse” (which would then be considered “event”) if a particular ALL protocol has provided unequivocal evidence that MRD reappearance is closely associated with hematological relapse.
MRD as an end point for clinical intervention
After MRD analysis became a common diagnostic tool in most multiagent clinical trials for ALL, the question arose of whether the MRD response can also be used as a primary end point in phase 2 trials. Many scenarios can be described in which response is used for the first screening to assess the efficacy of a single agent or a drug combination. However, the MRD response is only the more sensitive further development of the cytological response. Certainly, the efficacy of a given intervention with regard to response does not need to translate into the relevant clinical end points such as event-free and overall survival. However, recent novel immunotherapeutic strategies (eg, with an anti-CD3, anti-CD19 bispecific mAb) that show strong efficacy in the MRD response may contribute to long-term remission because they may provide the necessary quality of remission needed to perform subsequent allogeneic SCT successfully.43,44 In contrast, a British study comparing reinduction therapy with mitoxantrone versus idarubicin in relapsed pediatric ALL demonstrated that the MRD response was not predictive of treatment efficacy.45 This observation indicates that MRD response may be misleading in drug evaluation as long as activity is considered to be equal to efficacy.
Molecular predictors of MRD response
An obvious disadvantage of any response assessment is the fact that the information about inadequate response to a given drug combination, such as induction therapy in ALL, comes only after these (inefficient) drugs have already been applied. Therefore, the patient may have been exposed to a toxic regimen without any benefit or with only minor benefit. The search for markers predictive of nonresponse at time of diagnosis is thus highly relevant.46–48 Quite recently, it was also found that new genetic markers for ALL may have additional prognostic power alone or in combination with MRD response assessment.49,50 High expression of CRLF2 was shown to predict a high relapse rate, even though the MRD response of this subgroup was considered rather favorable.51 In addition, certain germline genetic variants were demonstrated to be associated with MRD response.42,52
In summary, MRD has evolved as one of the most powerful diagnostic tools in the clinical management of ALL. At this time, it cannot yet be replaced by upfront diagnostic markers to predict response and relapse with the same level of sensitivity and specificity. Clinical intervention based on MRD results must rely on robust quality assurance of any given MRD detection method, because phenomena such as regenerating immature (normal) lymphocytes or clonal evolution of the leukemia may interfere with the interpretation of MRD results.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from EUSAPharma, Medac, and Novartis. Off-label drug use: None disclosed.
Correspondence
Martin Schrappe, MD, Department of Pediatrics, Christian-Albrechts-University of Kiel, University Medical Center Schleswig-Holstein, Schwanenweg 20, 24105 Kiel, Germany; Phone: +49-431-5971621; Fax: +49-431-5973966; e-mail: m.schrappe@pediatrics.uni-kiel.de.