Abstract
Perioperative management of antithrombotic therapy is a situation that occurs frequently and requires consideration of the patient, the procedure, and an expanding array of anticoagulant and antiplatelet agents. Preoperative assessment must address each patient's risk for thromboembolic events balanced against the risk for perioperative bleeding. Procedures can be separated into those with a low bleeding risk, which generally do not require complete reversal of the antithrombotic therapy, and those associated with an intermediate or high bleeding risk. For patients who are receiving warfarin who need interruption of the anticoagulant, consideration must be given to whether simply withholding the anticoagulant is the optimal approach or whether a perioperative “bridge” with an alternative agent, typically a low-molecular-weight heparin, should be used. The new oral anticoagulants dabigatran and rivaroxaban have shorter effective half-lives, but they introduce other concerns for perioperative management, including prolonged drug effect in patients with renal insufficiency, limited experience with clinical laboratory testing to confirm lack of residual anticoagulant effect, and lack of a reversal agent. Antiplatelet agents must also be considered in the perioperative setting, with particular consideration given to the potential risk for thrombotic complications in patients with coronary artery stents who have antiplatelet therapy withheld.
Introduction
Hematologists frequently are consulted for management recommendations regarding patients receiving long-term oral antithrombotic therapy who require a temporary interruption of their anticoagulation therapy before surgery or an invasive procedure. It is currently estimated that approximately 250 000 patients in North America will require interruption of oral anticoagulant therapy each year.1 The management of these patients, however, is problematic. On the one hand, disruption of oral anticoagulant therapy will expose the patient to an increased risk for thromboembolism, but the degree of risk clearly varies among such patients. On the other hand, there is an increased risk for hemorrhagic complications if anticoagulant therapy is continued, which is affected by the type of surgery or invasive procedure in which the patient will undergo. In addition, maintaining full anticoagulant therapy in the perioperative setting has the potential to inadvertently lead to an increased risk for thromboembolism in those patients who sustain a hemorrhagic event and subsequently have their anticoagulant therapy withheld or even reversed. For each patient, therefore, these issues must be considered before management recommendations are formulated.
Although this clinical situation is not uncommon, there is relatively limited evidence available to guide therapeutic recommendations. In addition, we are entering a rapidly changing landscape, one in which 2 novel oral anticoagulants recently have been approved for use in patients with atrial fibrillation and in which several other agents currently are either under review or being studied (Table 1).2–5 These agents introduce new issues and concerns with perioperative management and will be discussed later in the article. In addition, there is an expanding array of antiplatelet agents in use that also need to be considered in the perioperative period (Table 2).6–9 This article will focus on the preoperative assessment of patients taking these various antithrombotic therapies and discuss management strategies for this frequently encountered clinical situation.
Anticoagulant agents: relevant information for perioperative management of anticoagulant agents2–5
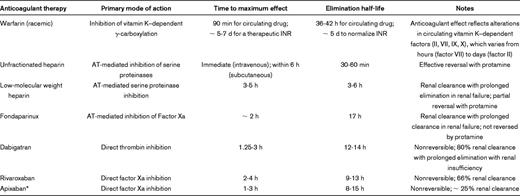
AT indicates antithrombin; INR, international normalized ratio.
*Apixaban currently is not available for clinical use in the United States.
Antiplatelet agents: relevant information for perioperative management of antiplatelet agents6–9

COX indicates cyclooxygenase.
*Peak plasma levels shown for nonenteric-coated aspirin; peak levers are delayed up to 3-4 h with enteric-coated aspirin.
†Cangrelor currently is being studied in clinical trials and is not available for clinical use.
Preoperative assessment
The perioperative management of patients who are receiving antithrombotic therapy, whether anticoagulant and/or antiplatelet, is determined by the assessment of the patient's risk for thromboembolic events considered against the risk for perioperative bleeding. These issues will determine whether antithrombotic therapy can be safely withheld around the time of the surgery or procedure or whether bridging therapy would need to be considered. Bridging therapy has been defined as the administration of a short-acting anticoagulant, such as a low-molecular-weight heparin (LMWH) or unfractionated heparin (Table 1), during the time when a long-acting anticoagulant, such as warfarin, is being withheld before the surgery, and subsequently after the surgery or procedure until the long-acting anticoagulant is again within the target therapeutic range. Although this sounds relatively straightforward, the lack of high-quality data has led to the empiric use of a great variety of strategies for various clinical indications.
Assessment of thromboembolic risk
Although in several recent observational studies investigators would suggest that simply interrupting warfarin therapy for a procedure is associated with an overall very low rate of postoperative thromboembolism,10,11 a patient's risk of thromboembolism during a brief interruption in anticoagulant therapy depends on specific characteristics related to the clinical indication for anticoagulation. A suggested approach for stratifying thromboembolic risk according to the indication for anticoagulation is provided in Table 3.1,12–14 For patients with atrial fibrillation, the CHADS2 score (see definition and scoring in Table 3), although not prospectively validated for use in the perioperative setting, typically is used for stratification.13 For patients with venous thromboembolism, important considerations include the timing of the most recent thromboembolic event15 and the presence (or absence) of underlying prothrombotic risk factors.1,16 For patients with prosthetic cardiac valves, considerations include the location of the valve, type of valve, and the occurrence of a previous stroke or transient ischemic attack.1,17
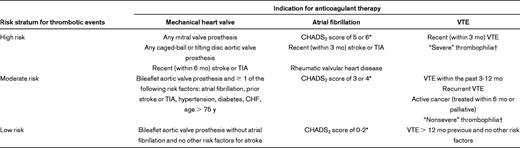
CHF indicates congestive heart failure; TIA, transient ischemic attack and VTE, venous thromboembolism.
*The CHADS2 score is calculated by the cumulative score for congestive heart failure (1 point), hypertension (1 point), age > 75 y (1 point), diabetes mellitus (1 point), and previous stroke or TIA (2 points).13
†“Severe” thrombophilias include deficiency of protein C, protein S, or antithrombin, antiphospholipid antibodies, or multiple abnormalities (eg, compound heterozygosity for factor V Leiden and prothrombin G20210A). “Nonsevere” thrombophilias include heterozygosity for factor V Leiden or prothrombin G20210A. The clinical relevance for these inherited risk factors in predicting risk of recurrent VTE, however, is unclear.14
When one uses this approach, patients with a > 10% annual risk for thromboembolism are classified as “high risk” for a thromboembolic event, patients with a 5%-10% annual risk are classified as “moderate risk,” and patients with a < 5% annual risk for thromboembolism are classified as “low risk”1 ; however, a patient's individual characteristics may modify this risk stratification. For example, a patient with atrial fibrillation and a stroke that occurred several years previously might be considered a greater-risk patient, even if the CHADS2 score is < 5, compared with a patient with a similar CHADS2 score but without a previous stroke.1
In addition to the risk of thromboembolism present when anticoagulation is withheld, one must also consider the potential risk for thrombus formation in relation to the specific procedure or surgery that is being performed. For example, neurologic and vascular surgical procedures are associated with a greater risk for stroke in patients with atrial fibrillation than other types of procedures (eg, urologic or orthopedic surgery).18
Assessment of bleeding risk
As with the evaluation of the thromboembolic risk for the individual patient, an assessment of the bleeding risk similarly needs to take into consideration patient-specific as well as surgery/procedure-specific variables. In a recent cohort study, patient-specific variables that were associated with an increased risk for bleeding included a history of bleeding, a mechanical heart valve in the mitral position, the presence of active cancer, and thrombocytopenia.19 A bleeding risk score that used these 4 parameters, referred to as “BleedMAP,” was shown to correlate with an increased risk for periprocedural bleeding.19 The HAS-BLED score, ie, Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (> 65 years), Drugs/alcohol concomitantly, which originally was developed for use in the nonperioperative setting, recently has been shown to predict bleeds during the bridging of patients on chronic anticoagulation.20 This scoring system uses the presence or absence of hypertension, abnormal renal or liver function, previous stroke, bleeding history or predisposition, labile international normalized ratio (INR), older age (> 65 years), or drug/alcohol use concomitantly to assess bleeding risk.
Surgical procedures differ in their risk for bleeding, although there are limited data identifying the relative risk for bleeding associated with different procedures.1 Procedures involving highly vascular organs, such as the liver, kidney, and spleen, have a greater risk for bleeding even in the absence of perioperative antithrombotic drug administration. Other procedures associated with an increased risk for bleeding include urologic surgery, bowel resection, and colonic polyp resection, especially for large, sessile polyps, and major surgery associated with extensive tissue injury (for example, cancer surgery, joint arthroplasty). Certain procedures, such as implantation of a pacemaker or cardioverter/defibrillator, or intracranial or spinal surgery, may not intrinsically exhibit an increased risk of hemorrhage, but even a small amount of excess bleeding in these confined locations can be associated with an adverse outcome.12
Perioperative management of patients on chronic vitamin K antagonist therapy
Procedures associated with a low bleeding risk
Interruption of oral anticoagulant therapy may not be necessary in patients undergoing certain procedures with a low bleeding risk. In general, interruption of warfarin is not required for minor dental procedures, including tooth extractions and endodontal procedures.12 Continuing warfarin with concomitant administration of a prohemostatic agent (eg, an antifibrinolytic agent, such as tranexamic acid or aminocaproic acid used as a mouthwash before and after the dental work), or interrupting warfarin for only 2-3 days, resulting in an INR that is slightly subtherapeutic at the time of the procedure, are reasonable management strategies.1 Similarly, interruption of warfarin therapy generally is not necessary for patients undergoing minor skin excisions, such as treatment of basal and squamous cell skin cancers and actinic keratosis. Although a greater incidence of minor skin bleeding was reported in patients who continued warfarin compared with those whose warfarin therapy was interrupted, most bleeds were self-limited.1,21 In contrast to most dental and dermatologic procedures, cataract removal is generally an avascular procedure. In a recent systematic review and meta-analysis, patients who underwent cataract surgery while on vitamin K antagonist therapy exhibited an increased risk for bleeding (odds ratio 3.26; 95% confidence interval [CI] 1.73-6.16), but almost all bleeding events were self-limited, and none of the patients sustained compromised visual acuity because of hemorrhage.22
Procedures associated with a moderate-to-high bleeding risk
In this clinical setting, warfarin will need to be interrupted to safely conduct the surgery or invasive procedure. For patients assessed as having a low risk for thromboembolic complications (Table 3), warfarin can simply be stopped before the procedure, typically beginning 5 days before, and then resumed once the procedure has been completed. The overall time that the patient will be subtherapeutic on warfarin may range from several days to a week or more, but if the risk for thromboembolism is considered low enough during this relatively brief period of time, then this approach represents the safest strategy.
Patients assessed as having a moderate-to-high risk for thromboembolic complications raise greater concern for the possibility of a thromboembolic event during the period of time when the INR is subtherapeutic. For these patients, a short-acting agent such as unfractionated heparin or LMWH may be used as a “bridge” during the time when the INR is subtherapeutic to decrease the period of time when the patient is at risk for thromboembolism. The risk with this approach, however, is the potential for hemorrhage when anticoagulants are used too close to the time of the surgery or procedure, and care must be taken with the planned timing of stopping and restarting the short-acting agent. Although conceptually this process may sound relatively simple, the devil lies in the details, given the various combinations of when to stop and when to restart each agent, as well as the doses used, and the potential for adverse outcomes that may occur with each of these strategies.
Interruption of vitamin K antagonist before surgery
The current recommendation for patients taking warfarin is that it should be discontinued approximately 5 days before surgery or a procedure.1 The elimination half-life of warfarin is 36-42 hours, and withholding warfarin for 5 days would allow sufficient time for the regeneration of functional vitamin K–dependent coagulation factors to achieve normal hemostasis (assuming normal diet and an INR that was not markedly supratherapeutic on the day warfarin was stopped). The authors of a prospective cohort study in a nonoperative setting demonstrated that a 5-day period of warfarin interruption in patients with therapeutic INRs was sufficient to allow normalization or near-normalization of the INR.23 In a prospective, randomized trial, authors compared withholding warfarin beginning on day −5 before surgery with withholding warfarin beginning on day −2 before surgery and administering 1 mg of vitamin K on day −1.24 For patients in whom warfarin was withheld beginning on day −5, the mean INR on the day of surgery was 1.24 (95% CI 1.19-1.29), but for patients in whom warfarin was withheld beginning on day −2, the mean INR was 1.61 (95% CI 1.50-1.71).24 There are no randomized trials in which investigators have compared the effects of withholding warfarin for 5 days before surgery with the withholding of warfarin for < 5 days on perioperative bleeding outcomes.1
The elimination half-lives of vitamin K antagonists other than warfarin differ, with acenocoumarol having a shorter elimination half-life (10 hours) and phenprocoumon a longer elimination half-life (3-5 days).25,26 However, most investigators studying the perioperative management of vitamin K antagonist therapy have studied warfarin, and limited data are available relevant to these other vitamin K antagonists.
Resumption of vitamin K antagonist after surgery
For most types of surgery, vitamin K antagonist therapy can be resumed on the evening of or the day after surgery, once postoperative bleeding has been controlled and oral intake has resumed. For patients undergoing a surgical procedure associated with a high risk of postoperative bleeding, anticoagulant therapy can be delayed for a day or 2 before being restarted. In general, early resumption of warfarin after surgery or a procedure is unlikely to incur an increased risk of bleeding in the postoperative setting, given the expected delay before a therapeutic anticoagulant effect is achieved, typically ∼ 5-7 days after restarting the warfarin.27,28
Laboratory monitoring of the INR in the perioperative setting
Measuring the INR the day before surgery can be used to ensure that it will reach a target value that the surgeon or proceduralist considers safe to proceed on the day of the procedure. For patients with INR values above the target, a low-dose of oral vitamin K (1 mg) is generally sufficient to achieve the target INR and not require cancellation of the procedure.29
Use of bridging anticoagulation
Patients at greater risk for thromboembolism are not infrequently treated with unfractionated heparin or LMWH during the period of time that warfarin is being withheld. In a patient with normal renal function, an outpatient strategy with a LMWH generally is used. The LMWH typically is started ∼ 36-48 hours after the last dose of warfarin (3 days before surgery) and typically is stopped ∼ 24 hours before the surgery. For patients undergoing a procedure associated with a greater risk for bleeding, it would be reasonable to withhold the LMWH for longer than 1 day before surgery. Similarly, for patients with impaired renal function, it would be reasonable to decrease the last dose and/or withhold the LMWH for > 24 hours before surgery or consider whether to simply avoid use of a bridging anticoagulant in the preoperative setting. An alternative strategy for patients with significant renal insufficiency (creatinine clearance < 30 mL/min) who are at high risk for thromboembolism would be to consider the use of unfractionated heparin for bridging.
Resumption of therapeutic dose LMWH after surgery should generally be delayed for at least 24 hours after most procedures and potentially longer after major surgery.12 In 1 prospective cohort study in which all patients received enoxaparin 1.5 mg/kg daily, beginning within 12-24 hours after surgery, major bleeding occurred in 8 of 40 patients undergoing major surgery (20%) and 1 of 148 patients undergoing minor surgery (0.7%).30 In other studies that allowed more flexibility in either the timing of when postoperative anticoagulation was initiated, or the initial dose administered, the authors reported a lower incidence of major bleeding (< 5%).27 In general, these findings would support delaying resumption of therapeutic LMWH for at least 24 hours after surgery and possibly longer for major procedures.
For those patients who are being converted back to warfarin, typically the first dose can be administered in the early postoperative setting, as noted previously. Once the INR is within the target therapeutic range, preferably on 2 separate measurements, the LMWH “bridge” can be discontinued. For patients with a history of a previous reaction to LMWH, fondaparinux may be substituted for the LMWH, but the longer half-life of fondaparinux needs to be considered when providing perioperative therapeutic recommendations (Table 1).
Perioperative management of patients on new oral anticoagulants
The new oral anticoagulants that currently are available in North America include dabigatran, a direct thrombin inhibitor, and rivaroxaban, a direct inhibitor of factor Xa (Table 1). Other factor Xa inhibitors that are in development include apixaban and edoxaban. These agents have several important differences from warfarin and other vitamin K antagonists, primarily through their mechanisms of action on normal hemostasis.3 In particular, the new oral agents exert their anticoagulant effect by directly inhibiting the function of individual coagulation factors, with no impact on the synthesis of new zymogens. Consequently, when stopping or starting the new oral agents, the anticoagulant effect is lost and regained much more quickly than with warfarin (Table 1), which can impact bleeding risk in the perioperative setting.
Interruption of the new oral anticoagulants before surgery
The timing of discontinuation of dabigatran before surgery is impacted on by the patient's renal function and the risk of bleeding associated with the surgical procedure.31 With normal renal function, dabigatran should be stopped ∼ 24 hours before the procedure. In surgical procedures with a greater risk of bleeding (eg, neurosurgery, cardiovascular surgery), or with spinal anesthesia, consideration should be given to stopping the drug 2-4 days before the procedure. As renal function declines, the half-life of the drug prolongs, and it will need to be held for at least 2 days (with a creatinine clearance between 30 and 50 mL/min) or longer (if creatinine clearance is ≤ 30 mL/min), even for low-risk procedures. Rivaroxaban is cleared to a lesser extent by the kidney (66%)3 and in most patients can be safely stopped within 1-2 days before the procedure.32 As with dabigatran, however, a more conservative approach would be to consider withholding the drug for a longer period of time in patients with significant renal impairment.
Resumption of the new oral anticoagulants after surgery
For patients undergoing major abdominal procedures or urologic surgery, the new oral anticoagulants should not be restarted until after all postoperative bleeding has stopped, given their rapid onset of action (Table 1). One strategy that has been suggested is to use a lower dose of dabigatran (eg, 75 mg) or rivaroxaban (eg, 10 mg) for the initial dose after surgery, followed by resumption of the usual maintenance dose if no bleeding is encountered.32
Laboratory testing in the perioperative setting
Although routine laboratory monitoring is not necessary for dabigatran and rivaroxaban, some individuals may wish to use laboratory testing to confirm the lack of any residual anticoagulant effect before surgery. For patients taking dabigatran, a thrombin time before surgery can be helpful because this test is particularly sensitive to the presence of dabigatran.31 The thrombin time is extremely sensitive to dabigatran, and in some patients, a dilute thrombin time that has been calibrated to clinically relevant concentrations of dabigatran may be preferable, if available.31 For patients taking rivaroxaban, the sensitivities of various prothrombin time and activated partial thromboplastin time reagents vary in their ability to detect low levels of circulating drug.33 Antifactor Xa chromogenic assays, similar to those used to measure heparin levels, are being evaluated and may prove useful for patients taking rivaroxaban.34
Use of bridging anticoagulation
Bridging therapy with a LMWH or unfractionated heparin is not necessary with the new oral anticoagulants, given the relatively rapid clearance of the drug from the circulation and the rapid onset of action when reintroduced (Table 1).
Perioperative management of patients on chronic antiplatelet therapy
Antiplatelet drugs that irreversibly inhibit platelet function include aspirin and the thienopyridines clopidogrel, ticlopidine, and prasugrel (Table 2). Ticagrelor is a direct-acting, reversibly binding, oral P2Y12 receptor antagonist that exhibits a rapid onset and offset of antiplatelet effect.7 Other reversible antiplatelet drugs include dipyridamole and cilostazol, and the nonsteroidal antiinflammatory agents have also a transient antiplatelet effect. Newer antiplatelet agents that are currently being studied in clinical trials include cangrelor and elinogrel. Cangrelor is an intravenous agent that has an excellent acute profile, with a rapid onset of action, a rapid offset, and a half-life of just a few minutes (Table 2).8 Perioperative management of these agents presents some additional challenges not encountered with the anticoagulant therapies.
Assessment of thromboembolic risk
Antiplatelet medications are used for the primary and secondary management of atherosclerotic thrombotic disease, particularly in the management of patients with stroke, acute myocardial infarction or acute coronary syndromes, or peripheral vascular disease, as well as patients undergoing percutaneous coronary intervention or cardiac surgery.35 Dual antiplatelet therapy with a thienopyridine in combination with aspirin has been shown to dramatically reduce the incidence of adverse events in patients receiving coronary artery stents, and premature discontinuation of antiplatelet therapy is associated with an increased risk of stent thrombosis, myocardial infarction, and death. Consequently, a recent science advisory from the American Heart Association, the American College of Cardiology, and other organizations stressed the importance of 12 months of dual antiplatelet therapy after placement of a drug-eluting stent and that elective surgery should be postponed until after this duration of time, if possible.36
Assessment of bleeding risk
The assessment of bleeding risk for perioperative management of antiplatelet therapy is similar to the concerns raised previously for anticoagulant therapies.
Stopping oral antiplatelet therapy before surgery
In general, for procedures associated with a low bleeding risk, antiplatelet therapies do not need to be withheld, similar to the recommendations for anticoagulant therapies.1 In contrast, for procedures associated with a moderate-to-high bleeding risk, a critical variable that must be considered is the individual risk for cardiovascular events. For patients at greater risk for cardiovascular complications, consideration should be given to either continuing aspirin around the time of the procedure or delaying the procedure until the patient has a lower risk. For patients at low risk for cardiovascular events, on the other hand, stopping antiplatelet therapy before the procedure is reasonable.1 For patients undergoing coronary artery bypass grafting (CABG), continuing aspirin would be appropriate, although the most recent guidelines from the American College of Chest Physicians suggest that thienopyridine therapy should be held before CABG.1
Although these therapeutic agents have relatively short half-lives, the most frequently used agents (aspirin, clopidogrel) irreversibly inhibit platelet function, necessitating withholding their administration for 7-10 days before surgery or an invasive procedure that requires complete elimination of the antiplatelet effect (Table 2). No randomized trials have assessed whether withholding antiplatelet therapy for a shorter period of time would provide a safer approach.
Resumption of antiplatelet therapy after surgery
The maximal antiplatelet effect occurs within minutes after resumption of aspirin (Table 2). In contrast, the maximal antiplatelet effect of clopidogrel may not be reached until after 7 days of daily administration of a standard dose (75 mg/d), but this period can be shortened by the administration of an initial loading dose.35
Laboratory testing of antiplatelet therapy
Several platelet function assays are available for measuring the antiplatelet effect of aspirin, clopidogrel, and the other P2Y12 ADP receptor–blocking agents. The clinical significance of these assays is uncertain, however, and the assay results have not been shown to correlate with clinical outcomes.37 Consequently, laboratory testing generally is not recommended for the management of these patients.
Use of bridging antiplatelet therapy
Similar to the strategies that have been developed for the perioperative management of warfarin in patients at high risk for thromboembolic complications, strategies also have been investigated with the use of short-acting antiplatelet agents in patients on chronic therapy with clopidogrel, given its longer onset and offset of action. The short-acting intravenous glycoprotein IIb/IIIa receptor inhibitors eptifibatide38 and tirofiban39 have been used in small case series as “bridging” antiplatelet therapy in patients requiring temporary withdrawal of clopidogrel. More recently, the nonthienopyridine adenosine triphosphate analog cangrelor was investigated as a bridging antiplatelet agent in a prospective, randomized, double-blind, placebo-controlled multicenter trial involving patients receiving a thienopyridine who underwent CABG.40 A greater rate of maintenance platelet inhibition in patients treated with cangrelor compared with placebo was observed with this approach without an increase in major bleeding before surgery or an increase in CABG-related bleeding.40 An alternative strategy that allows for “transient” reversal of dual antiplatelet therapy in high-risk patients involves the use of specifically timed platelet transfusions before the procedure, based on the elimination half-lives of aspirin and clopidogrel (Table 2).41 Additional clinical trials are needed to document the efficacy in decreasing thromboembolic complications using this strategy.
Inferior vena cava filters
For patients with a recent venous thromboembolism, it is preferable to defer all surgical procedures until at least 1 month, and preferably 3 months, of anticoagulant therapy have been administered.42 If this is not feasible, and major surgery needs to be performed within several weeks of an acute episode of proximal deep-vein thrombosis or pulmonary embolism, a retrievable inferior vena cava filter can be considered for use during the procedure.42,43 The filter should be removed in the postoperative setting as soon as anticoagulation can be reestablished to minimize the potential for any complications related to the device.
Future directions
Several clinical trials are currently open and enrolling patients into studies designed to address some of the deficiencies noted in this work. For example, although bridging anticoagulation with LMWH frequently is used in patients on chronic warfarin therapy who need their anticoagulation held for a procedure, there is no firm evidence that this approach actually prevents perioperative thromboembolic events. BRIDGE (Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery) is a prospective randomized trial supported by the National Heart, Lung, and Blood Institute that is enrolling patients with atrial fibrillation or flutter who require temporary interruption of warfarin for a surgery or procedure. The study compares bridging with therapeutic-dose LMWH with placebo (www.clinicaltrials.gov no. NCT00786474). PERI-OP2 is a second trial that is also designed to study the safety and efficacy of LMWH as a bridge for patients with atrial fibrillation or prosthetic aortic valves who need anticoagulation withheld for a procedure (www.clinicaltrials.gov no. NCT00432796). These 2 studies will address the issue of efficacy and safety of bridging therapy in patients on chronic warfarin anticoagulant therapy.
This article was selected by the Blood and Hematology 2012 American Society of Hematology Education Program editors for concurrent submission to Blood and Hematology 2012. This article is reprinted with permission from Blood. 2012; Volume 120.
Acknowledgments
This work was supported by the Centers for Disease Control and Prevention (DD000014; DD000897) and the National Heart, Lung, and Blood Institute (HL087229 and HL072289).
Disclosures
Conflict-of-interest disclosure: T.L.O. has received research support from Eisai, Pfizer, GlaxoSmithKline, Daiichi Sankyo, Stago, and Instrumentation Laboratory Inc. and has acted as a consultant for Sanofi Aventis, Boehringer Ingelheim, Bristol Myers/Squibb, and Instrumentation Laboratory Inc. Off-label drug use: None disclosed.
Correspondence
Thomas L. Ortel, MD, PhD, Duke University Medical Center, Box 3422, Rm 0563 Stead Bldg, Durham, NC 27710; Phone: 919-684-5350; Fax: 919-681-6160; e-mail: thomas.ortel@duke.edu.
