Abstract
High-risk acute myelogenous leukemia (AML) constitutes a distinct subset of disease based on clinical and biological characteristics and comprises a significant percentage of all cases of adult AML. Biologic features such as distinct clonal cytogenetic and molecular abnormalities identify a subgroup of AML patients characterized by poor response to induction chemotherapy and poor long-term survival after treatment with consolidation chemotherapy. Clinical variables that predict for poor response include AML relapsed after less than 1 year of remission and AML characterized by resistance to conventional agents. We review here our understanding of the defining biologic subtypes of AML and discuss how adequate initial evaluation can be used to inform the choice of treatment. By defining high-risk biologic and clinical variables, a strong case can be made for treating patients with investigational agents, with treatment directed at distinct cytogenetic or molecular abnormalities. Allogeneic transplantation is the only form of therapy available outside of the setting of a clinical trial that may offer a chance for long-term survival for patients with high-risk AML.
Introduction
High-risk acute myelogenous leukemia (AML) constitutes a biologically distinct subset of disease and comprises a sizeable percentage of cases of adult AML.1-3 Based on retrospective review of large registries and given the lack of progress or variation in treatment over the past 25 years, a distinct profile of cytogenetic and molecular features can be used to assign risk.4,5 Although high-risk disease features cluster among clinical phenotypes, such as among patients over age 60, those with antecedent hematological disorders, and those who have received prior treatment with cytotoxic chemotherapy, high-risk karyotype or the expression of mutated flt3, kit, and other molecular markers explain both the poor response to induction chemotherapy and the high relapse rate previously attributed to clinical variables alone.6-9 Unfortunately, there has been no regulatory pathway to approval of novel agents based on adverse disease biology.10-12 Transplantation of allogeneic histocompatible hematopoietic progenitor cells has been the only approach that seems to improve what would be a dismal outcome after conventional induction and consolidation chemotherapy.13-15 Improvements in supportive care, experience with alternative hematopoietic stem cell donors, and the ability to deliver dose-adjusted preparative conditioning to a more diverse group of patients have made transplantation the preferred postremission strategy for patients with high-risk AML. Unfortunately, failure of induction chemotherapy and early relapse still make allogeneic transplantation an intervention feasible only for a fraction of patients with high-risk disease. We offer here a disease assessment and treatment algorithm for patients with adverse cytogenetics or high-risk molecular abnormalities based on approved therapies and make recommendations regarding the off-label use of the limited therapies available for clinicians managing patients with adverse-prognosis AML.16-18
Nature of the problem: why is high-risk AML high risk?
A stable treatment paradigm for the management of AML in adults has allowed for analysis of discrete clinical and biological variables as they predict for distinct response and survival.6-9 Clinical variables long recognized to impact response include therapy-related AML, AML arising out of an antecedent hematologic disturbance, and AML in the elderly.16 Based in large measure on retrospective studies, a pattern of recurrent leukemia-specific cytogenetic abnormalities was first identified as important in differentiating AML with respect to response to induction chemotherapy and survival after consolidation chemotherapy or even allogeneic hematopoietic cell transplantation.17-19 Patients understand distinct biologic subtypes of AML as they would understand that genetic difference leads to phenotypic difference when visiting a rose garden: all of the flowers are called by the same name, “rose,” but are distinctly different in terms of look, smell, and tolerance to differing environmental conditions. So too are leukemias different, but the implications are more ominous.
Distinguishing AML by the dominant clonal cytogenetic pattern does not address how these abnormalities occur or why they define disease behavior better than histochemical stains or immunophenotype.20 AML characterized by t(8;21)(q22;q22), inv (16)(p13;q22), t(16;16)(p13;q22), and even t(15;17) respond favorably to cytarabine/anthracycline-based therapy (hereafter referred to as 7&3).21-23 Furthermore, disease-free survival for those who achieve remission is typically prolonged, although a subgroup often sustains early relapse, those whose disease is also characterized by a mutation in kit.24,25 At the other end of the AML spectrum are leukemias characterized by complex (greater than 3) abnormalities, monosomies of any chromosome (typically chromosome 5 and/or 7), inv(3), t(3;3), t(6;9), the rare t(9;22), and 17p abnormalities. There is some disagreement about chromosome 11 abnormalities, but it appears that 11q23 abnormalities other than t(9;11) should be grouped among the high-risk cytogenetic abnormalities.26 Patients with AML characterized by any of the high-risk cytogenetic features have disease that is less likely to remit with induction chemotherapy and those whose disease does respond are far more likely to sustain relapse despite even the most aggressive consolidation treatment, including allogeneic hematopoietic cell transplantation. Among the favorable-risk and adverse-risk AML subtypes, only the t(15;17) is characterized by distinct clinical and morphological features. Cytogenetics adds significant value to the understanding of a patient's disease, at least as long as there is no change in the current management.
Refinements in risk stratification
The inability to find a regulatory pathway for treatment that targets biologically distinct AML outside of acute promyelocytic leukemia is the driver that has led to further refinement in our concepts of biologically distinct AML subtypes. The World Health Organization has recognized this refinement in the diagnostic evaluation of AML by refining the classification of AML to include biphenotypic leukemia, therapy-related leukemia, cytogenetics, and molecular genetics.27,28 Recurrent single-gene mutations, not really in isolation but dominant in the clone, have been used to further refine our concepts of disease. Therefore, AML characterized by t(8;21), inv(16), or t(16;16) with the kit mutation may respond well to induction chemotherapy, but seems to be associated with a much higher risk of relapse after high-dose cytarabine-based consolidation chemotherapy than would otherwise be expected and is responsible for a change in designation; these leukemias are now considered AML of intermediate risk. The influence of kit in inv(16;16) may not be the same as the adverse impact of the mutation in t(8;21), but may warrant consideration of alternative postremission therapy, especially allogeneic transplantation in first remission. In this same category of intermediate risk are leukemias characterized by normal karyotype but often with mutation in flt3, a transmembrane tyrosine kinase with either a single amino acid substitution in the kinase domain or, more commonly, with internal tandem duplication (ITD). Flt3 ITD is clearly associated with adverse prognosis. Alternatively, isolated mutation in the nucleophosmin1 gene (the most common mutation identified in normal-karyotype AML) is associated with favorable prognosis. In the South German AML96 trial of 909 elderly patients entered prospectively, patients received 2 courses of daunorubicin/cytarabine induction and, according to protocol, patients in remission received 1 cycle of consolidation with cytarabine (1000 mg/m2 twice daily for 5 days) and amsacrine.27 With a median follow-up for all patients of 5.7 years, the authors found that karyotype, NPM1 and flt3 mutation status, CD34 expression, age, leukocyte count, lactate dehydrogenase, and BM blasts at day 15 were all independent prognostic factors for remission induction. The combination of NPM1 and flt3 mutational status was important in the multivariate analysis, and NPM1 mutation status was independent in its influence on the rate of remission. In their analysis of disease-free survival among the 299 patients who achieved remission, and of the entire group for survival, multivariate analysis again showed that cytogenetic risk group, NPM1/flt3 mutational status, lactate dehydrogenase, and WBC at diagnosis were independent risk factors. In this study, the presence of flt3 ITD was not of prognostic importance for survival, although it was for remission and disease-free survival. These findings have been corroborated in other settings: among younger patients and among patients with AML undergoing allogeneic transplantation in first remission.
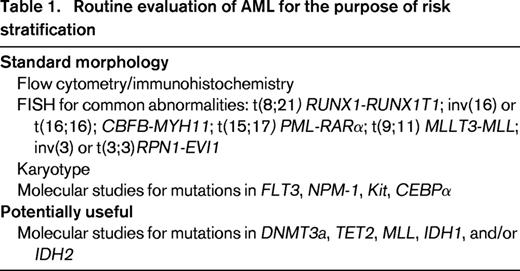
The prognostic value of both pretreatment cytogenetics and molecular analysis, at least for NPM1 and flt3, is of sufficient importance to recommend that these studies be done for all patients at diagnosis28 ; anything below this could be considered an incomplete evaluation, yet most laboratories only offer molecular testing upon physician request at the time of initial BM biopsy (Table 1). Reflex testing, as done for other findings in the clinical laboratory such as abnormal thyroid-stimulating hormone, has not, in general, been incorporated into the hematopathology workup of newly diagnosed AML.
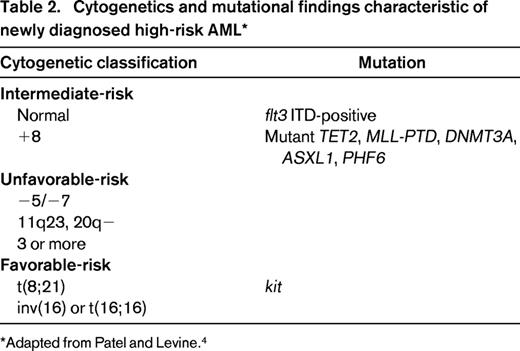
Other molecular abnormalities may contribute to refinements in prognostic variables, particularly in cytogenetically normal AML that constitutes 30% to 40% of leukemias in the clinical setting.29 The CCAAT/enhancer binding protein alpha (CEBPα) gene mutation confers favorable prognosis, at least in the absence of unfavorable markers. Recurrent mutations in IDH1, a Krebs cycle protein, affect the active site of the enzyme and inhibit the activity of the wild-type allele.30-33 IDH2 is a mitochondrial protein. Mutations in both IDH1 and IDH2 have been identified in approximately 20% of patients with normal-karyotype AML and are associated with a poor prognosis. MLL is a gene on chromosome 11q23; a partial tandem duplication has been identified in fewer than 10% of patients with cytogenetically normal AML and may confer an increased risk of relapse.34 Mutations in the TET2 oncogene family, often associated with chromosome 4q24 abnormalities, have been identified during evolution of myelodysplasia or myeloproliferative disease to AML and have been associated with diminished survival.35,36 Mutations in TET2 have been associated with shorter survival, typically in older patients with normal-karyotype AML or in younger patients with favorable-risk cytogenetics. TET2 mutations seem to function as epigenetic modifiers and may serve to alter the methylation pattern of DNA; loss of TET2 function may result in impaired hematopoietic differentiation. Mutations in IDH1 or IDH2 do not coexist with TET mutations, but also lead to impaired hematopoietic differentiation and expansion of cells with stem-like function. RUNX1 mutations have also been identified as independent prognostic markers for shorter survival in a retrospective study of intermediate-risk cytogenetic AML.37 ASXL1 and PHF6 mutations are seen in a variety of diseases, the former in myeloproliferative disorders, the latter in T-cell acute lymphoblastic leukemia (ALL).4,37,38 Both mutations may be seen in AML. ASXL1 mutations are seen in older patients with AML. Whether these latter abnormalities will add prognostic value in prospective studies remains to be determined (Table 2).39
In clinical practice, we recommend routine testing at diagnosis of cytogenetic abnormalities by both FISH and cytogenetic analysis and evaluation for a manageable set of gene-based molecular abnormalities to identify disease associated with high risk of treatment failure. These typically include flt3, NPM1, CEBPA, and kit. In a large randomized study of dose-intensive induction of daunorubicin and cytarabine conducted by the Eastern Cooperative Oncology Group (ECOG), the recently identified IDH2 R140Q mutations ASXL1 and PHF6 contributed to adverse survival.39 It is unknown whether these additional molecular abnormalities can contribute to the further risk-stratification of patients with intermediate-risk AML. Among patients with intermediate-risk, flt3-negative AML, patients with disease characterized by NPM1/IDH mutation have disease associated with a favorable outcome. The absence of IDH mutation adversely affects the otherwise favorable population. Therefore, the combination of potentially favorable or adverse alleles determines relapse risk, particularly in normal-karyotype, flt3-wild type AML. For those with disease characterized by flt3 mutation, the addition of mutations in TET2, MLL, and DNMT3 confer an even worse prognosis on this group with high-risk disease.40
Mutational profiling may provide prognostic information to the treating physician. The question is whether this prognostic information can contribute to treatment decisions that might favorably affect an otherwise dismal outcome with conventional chemotherapy or might suggest less intense therapy for those with favorable disease features.
Clinical factors associated with high-risk AML
In addition to the disease-specific cytogenetic and molecular features associated with a high risk of resistance to induction therapy, there are many clinical factors associated with poor prognosis.41 Comorbid medical conditions and advanced age are often cited as risk factors for treatment-related mortality, but resistance to therapy is the major cause of treatment failure and is also more common among patients over age 70, suggesting that even in the absence of known biologic risk factors, advanced age confers resistance to leukemia treatment. Of course, regardless of risk, relapsed leukemia, leukemia refractory to induction chemotherapy, and leukemia characterized by the persistence of minimal residual disease after induction chemotherapy define high-risk AML. Of these, residual disease defined by multiparameter flow cytometry or positive PCR for disease-specific genes describes a group of patients with significant risk of early recurrence after consolidation therapy, including consolidation in the form of allogeneic transplantation.42 Patients with disease characterized by adverse biologic or clinical features are prime candidates for alternative therapies either during (re)induction or to consolidate remission.
Treatment of high-risk AML
The major question for physicians managing acute leukemia is to define effective alternative therapy based on a risk-adapted model. Another question is whether currently available risk-adapted therapy offers an advantage over standard treatment or if disease models merely identify prognostic variables that cannot be addressed clinically. Supportive of this somewhat pessimistic perspective is a recent report from the German AML Intergroup that prospectively compared different treatment strategies for a diverse group of AML patients comparing 5 different treatment strategies against a common standard treatment of cytarabine at 100 mg/m2 daily by continuous infusion and daunorubicin 60 mg/m2 by IV infusion over 2 hours on days 3, 4, and 5. Standard consolidation consisted of cytarabine 3 g/m2 every 12 hours by infusion over 3 hours on days 1, 3,and 5.27 The studies did not enroll patients on the basis of specific disease-related or clinical features, but regardless of the investigational treatment, which included high-dose cytarabine induction, intermediate-dose cytarabine with daunorubicin, the addition of etoposide to induction, or double induction with thioguanine, cytarabine, and daunorubicin followed by high-dose cytarabine and mitoxantrone, no survival advantage could be detected in favor of any of the investigational regimens. Because the studies did not enroll on the basis of risk profiles, retrospective analysis of age, secondary AML, cytogenetic risk, and NPM1 mutational status could not identify an advantage for one treatment over the other. The investigators came to the sober conclusion that the uniform results regardless of induction may represent the limit of current antileukemia approaches. Strictly speaking, however, none of the studies attempted to “build a better mousetrap” on the basis of randomization at diagnosis of distinct AML subtypes.
When is investigational therapy warranted?
Investigational induction would be an appropriate response when treating a patient with high-risk disease features. This would require waiting to identify biologic risk factors at diagnosis or enrolling based on clinical factors known to be associated with a high risk of induction failure, such as secondary AML or AML relapsed or refractory after standard treatment. High-dose cytarabine is the standard against which all other treatments must be compared for the management of relapsed or refractory AML.43 Based on a theoretical model established in the late 1970s, higher doses of cytarabine than were conventionally used were recommended to maximize araCTP incorporation into leukemia cells. The benefit of dose escalation was demonstrated in small studies in refractory and relapsed AML, with a dose limit of 3 g/m2 given every 12 hours for 6 days. Ophthalmologic, skin, and central nervous system toxicity limited the dose. The variation in response rates from 20% to 80% can be attributed to the significant biological and clinical heterogeneity of patients enrolled, but this makes comparative analysis of salvage regimens difficult. Few randomized studies stratified for significant variables that are available to guide therapy and, in the absence of effective postremission strategies for the majority of patients, it is difficult to demonstrate a superior survival of combination regimens over single-agent high-dose cytarabine.44,45
Against this background of high-dose cytarabine salvage, a variety of agents have been added, including anthracyclines, etoposide, and purine analogs. The addition of anthracyclines has some basis in reason given the higher rate of remission achieved for patients in the ECOG 1900 trial randomized to higher-dose daunorubicin during initial induction, at least compared with a control arm dose of 45 mg/μ2.46 Whether this translates to management later in the disease course is subject to question. Prior infusion with fludarabine or cladribine has been shown to increase the araCTP accumulation in leukemia blasts induced by high-dose cytarabine, prompting a variety of combination regimens in the relapsed/refractory setting. Clofarabine is a second-generation purine analog that combines the cytotoxic characteristics of both fludarabine and cladribine and studies demonstrate that, like the parent compounds, synergistic cytotoxicity can be achieved with cytarabine. The largest trial of salvage therapy in older patients with high-risk AML was the CLASSIC I study.47 The was a phase 3, randomized, double-blind, placebo-controlled trial conducted in 326 patients age 55 and older with relapsed or refractory AML comparing treatment with high-dose cytarabine alone against the combination of cytarabine and clofarabine. The cytarabine dose administered was 1 g/m2 as a 2-hour infusion daily for 5 days given 3 hours after completion of clofarabine or placebo. The choice of the cytarabine dose was based on the saturable kinetics of araCTP incorporation and the advanced age of the population under study. Given the advanced age of the subjects, the postremission strategies consisted, in general, of a single, optional consolidation cycle of unproven efficacy; few patients underwent allogeneic transplantation. The combination therapy worked, at least in terms of inducing remission. Response was significantly higher for the combination arm (46.9% vs 22.9%), as was event-free survival. However, the combination arm produced significantly more treatment-related adverse events in this high-risk, older population, including deaths as a result of these adverse events (14.3% vs 5.2%, respectively). The causes of deaths in the combination arm included cerebral hemorrhage, pneumonia, pulmonary and subdural hemorrhage, renal failure, hepatic venoocclusive disease, myocardial infarction, and epidermal necrosis. Disease progression contributed to deaths in both groups. Because the study was designed with survival as the primary end point, the effect on survival of the addition of clofarabine to induction did not achieve statistical significance either for the patients with refractory AML or for those with relapsed disease, although event-free survival at 4 months favored the combination arm.
A major flaw in the methodological design of any registration trial of novel agents is the requirement to demonstrate a survival advantage. As demonstrated in the clofarabine/cytarabine trial, uncertainty about the best postremission strategy can render an advantage in rate of remission meaningless. Without specifying postremission therapy, the impact of a better induction regimen is diluted by inconsistencies in consolidation management in a 2-armed study. Furthermore, anything that may enhance the cytotoxic effect of cytarabine could, in theory, prolong myelosuppression or contribute to treatment-related toxicity and mortality. Combinations of cytotoxic agents may affect response rates, but are unlikely to make an impact on survival unless there is a plan for postremission consolidation with allogeneic transplantation, the only proven form of postremission therapy for patients with relapsed/refractory AML in advanced remission.
Other approaches to improving remission induction by combining novel agents with conventional cytotoxic drugs have targeted high-risk disease earlier in the treatment paradigm. These include gemtuzumab; farnesyl transferase inhibitors; inhibitors of flt3 or histone deacetylase, and CXCR4. Subsequent to its withdrawal from the United States market, the Acute Leukemia French Association presented its results of a phase 3 open-label study of gemtuzumab given for 3 doses of 3 mg/m2 on days 1, 4, and 7 during conventional 7&3 induction and included the drug in consolidation for older patients with previously untreated AML.48 The daunorubicin dose used in the trial was 60 mg/m2. Complete response was similar in both groups; however, the 2-year event-free survival was 40.8% for patients allocated to the combination arm compared with 17.1% for the control group. Relapse-free survival and overall survival were both significantly better for the combination group without an associated increase in treatment-related mortality, although persistent thrombocytopenia was more common in the gemtuzumab-treated group (16% vs 4%). The UK National Cancer Research Institute AML Working Group reported a favorable increase in remission rate when gemtuzumab was added to low-dose cytarabine, although this did not translate into a survival advantage, again suggesting that a successful postremission strategy is needed for a novel regimen to demonstrated improved survival.49
Several agents directed at kinase mutations have been incorporated into therapy for AML characterized by flt3 ITD. Originally developed as antagonists for use in the relapsed and refractory settings, it is conceivable that one of these agents may make it to initial management. Midostaurin, lestaurtinib, quizartinib, and sorafenib have all shown single-agent activity in the relapsed setting.50-54 The most promising among these for the treatment of relapsed disease, quizartinib, may induce or select for unique mutations that confer resistance and may require further manipulation with other drugs such as ponatinib. Midostaurin has been studied in combination with induction chemotherapy for newly diagnosed AML characterized by flt3 mutation, but results have not yet been published. A phase 1/2 study of sorafenib combined with high-dose cytarabine (1.5 g/m2 by continuous IV infusion daily for 4 days in patients under 60 or for 3 days in patients over age 60) and idarubicin yielded complete remission in 14 of 15 patients with flt3-mutated AML, suggesting that the additional agent may have made an impact on response rate.55 These results have not yet been confirmed in a randomized trial. Results of sorafenib combined with low-dose cytarabine in elderly patients with AML, irrespective of flt3 mutational status, were not encouraging based on both toxicity and a low (10%) response rate.56
Other agents that hold promise in the relapsed setting deliver chemotherapy in a different way. CPX-351 is a liposomal formulation of cytarabine and daunorubicin in a fixed molar ratio of 5:1. It has shown activity in the relapsed setting, particularly in the setting of secondary leukemia, a target for a current phase 3 clinical trial in patients with untreated high-risk AML.57 The study is randomized against conventional 7&3, so, again, postremission therapy may be important if demonstrating a survival advantage remains the outcome demanded for regulatory approval.
Several novel agents, built on a cytotoxic model, have been evaluated in advanced acute leukemia. Elacytarabine is a novel nucleoside analog that is cytotoxic and independent of the transporter hENT1 for cellular uptake and activity.58 It has demonstrated activity in the phase 2 setting, and the phase 3 trial against a dealer's choice of combination cytotoxic regimens has met its accrual target and results are awaiting analysis but do not appear favorable. Hypomethylating agents and histone deacetylase inhibitors, both alone and in combination with agents such as lenalidomide, are also under study, although responses in the relapsed setting seem low.59,60 Sensitization to cytotoxic agents may also represent a way to avoid drug resistance. Originally studied with G-CSF given as a priming agent, cytarabine alone or with other cytotoxic agents has been studied for increased efficacy and toxicity after mobilization of blasts from the BM niche.61 A current trial with the CXCR4 antagonist plerixafor has targeted relapsed and refractory AML primed with the agent, and then treated with a combination of mitoxantrone, etoposide, and cytarabine after an initial phase 1/2 study in 52 patients that demonstrated a response rate of 46%. These agents could provide a chemotherapy bridge to the more-definitive allogeneic transplantation (Table 3).
The development of novel agents in the relapsed/refractory AML setting or, for that matter, moving these agents to initial therapy based on approval for distinct biologic or clinical subtypes depends on showing an improvement in survival. Right now, the only approach that seems to work is allogeneic transplantation. Allotransplantation as initial management for disease refractory to induction or chemotherapy-resistant relapse does not seem to be an encouraging option. Survival in fewer than 10% to 20% of selected patients does not provide convincing evidence that allogeneic transplantation is an effective strategy in the setting of active disease. No center has convincingly demonstrated that an alternative conditioning regimen exists that would simultaneously address chemotherapy-resistant leukemia and induce long-term survival. The Center for International Blood and Marrow Transplant Research reviewed the results of allogeneic transplantation in 2255 patients with acute leukemia in relapse or with primary induction failure between 1995 and 2004.62 The 3-year survival was 19% among patients with AML and 16% for patients with ALL. The morality at 100 days after transplantation was 39%. As expected, first complete remission duration less than 6 months, the presence of circulating blasts, an unrelated/alternative donor, and poor performance status predicted for a dismal outcome. More importantly, the presence of poor-risk cytogenetics also predicted for poor outcome. The poor outcome was most frequently progression of leukemia. A combination of all of the risk factors made it virtually impossible to achieve long-term survival. The statistics have led some to suggest that delaying allogeneic transplantation by administering salvage chemotherapy may not be warranted,63 but the poor survival rate for those with multiple risk factors suggests that planting new sod when the existing lawn is full of weeds is not likely to yield a weed-free turf.
Allogeneic transplantation for patients whose AML was characterized by a first remission of greater than 6 months, intermediate-risk cytogenetics, and no circulating blasts produces a favorable long-term outcome in approximately 40%, suggesting that survival rates for patients with lower disease-risk would be comparable to those whose disease was in complete remission before transplantation. Identifying a high risk of relapse and allogeneic transplantation earlier in leukemia management is the better strategy. Still, adverse disease biology confers increased risk. Although there are no prospective trials of donor versus no donor for the management of high-risk AML in first remission, retrospective studies suggest that the adoptive immune therapy achieved with allogeneic transplantation offers a survival advantage for patients with AML characterized by flt3 ITD or adverse cytogenetics. Relapse is no doubt higher among these patients, but leukemia-free survival in the range of 50% to 60% has been reported. Notwithstanding an early recommendation to the contrary by a group in the United Kingdom,64 a relapse incidence for flt3-mutated AML of 30% reported by the European Group for Blood and Marrow Transplantation after allogeneic transplantation seems much lower that what has been reported after multiple cycles of consolidation chemotherapy.15 Allogeneic transplantation is now considered to be indicated for the management of this type of normal-karyotype AML in first remission.
Allogeneic transplantation is also recommended as postremission management for patients whose disease is characterized by adverse cytogenetics, leukemia arising from antecedent myelodysplasia, or for leukemia in second or greater remission. Although these variables also predict for a high risk of relapse after allogeneic transplantation,65-67 transplantation is simply the best or only option offering potential long-term survival in patients with AML characterized by adverse disease biology and even among those with adverse clinical features such as older age. Newer strategies to deliver the adoptive immunotherapy potential of the allograft with dose-reduced preparative conditioning may be associated with a higher risk of leukemia relapse than what has been seen with conventional myeloablative conditioning, but offers transplantation to a more representative population of older patients: those who have been heavily treated in the past and those with comorbid medical conditions but whose disease warrants transplantation. There are presently no alternatives available to improve the results of consolidation therapy for those patients with high-risk AML and there are no drugs in development that target postremission management as a pathway to regulatory approval.
Concluding points
Identifying high-risk features of AML at diagnosis, outside of a clinical trial, is mandatory both for prognosis and for recommendations regarding postremission therapy for the majority of adults under age 70. Although identifying adverse disease biology at diagnosis has not yet led to a change in remission-induction strategies using approved agents, features such as monosomal karyotype, flt3 mutational status, and leukemia characterized by short first remission will very likely influence the choice of treatment in the near future. For now, it is advisable to initiate, at the minimum, a complete karyotypic profile by FISH and conventional cytogenetics, as well as molecular assessment for mutations in flt3, NPM1, kit, and CEBP-α, with careful attention to additional markers such as IDH2 and DNMT3 that may become important in the near future. Patients should also be evaluated at their first visit with histocompatibility testing and evaluation for potential family or alternative donors should be initiated as soon as authorized. For patients with AML characterized by adverse disease features and for those whose disease has recurred after short first remission or has been refractory to conventional induction, I strongly recommend referral to a center capable of entering the patient into a clinical trial of novel chemotherapy or disease-specific therapy targeting molecular weaknesses identified in the initial evaluation. To continue to administer 7&3 or single-agent high-dose cytarabine to patients with AML characterized by adverse disease biology or chemotherapy resistance, respectively, is to continue to settle for a wholly inadequate standard. For those of us committed to changing the outcome of therapy for the sizeable percentage of leukemia patients who have high-risk AML, we need to demand from the regulatory authorities the same degree of latitude in drug approval that they have granted to serve the unmet medical needs in the management of resistant lymphoma, multiple myeloma, and ALL, for which successful phase 2 trials have led to approvals and have expanded the tools available for the practicing hematologist.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Ozmosis, Astellas, Clavis, Sunesis, Cyclacel, and BMS. Off-label drug use: Novel agents under study for the management of AML including elacytarabine, voreloxin, plerixafor, and the approved agent clofarabine.
Correspondence
Gary J. Schiller, MD, CHS 42-121, UCLA David Geffen School of Medicine, Los Angeles, CA 90095; Phone: (310) 825-5513; e-mail: gschiller@mednet.ucla.edu.