Abstract
Recently, there has been a substantial gain in our understanding of the role that natural killer (NK) cells play in mediating innate host immune responses against viruses and cancer. Although NK cells have long been known to be capable of killing cancer cells independently of antigen recognition, the full therapeutic potential of NK cell–based immunotherapy has yet to be realized. Here we review novel methods to activate and expand human NK cells ex vivo for adoptive transfer in humans, focusing on the important phenotypic and functional differences observed among freshly isolated, cytokine activated, and ex vivo–expanded NK populations.
Natural killer cell therapy for cancer: a new hope
The ability of natural killer (NK) cells to kill tumor cells without the need to recognize a tumor-specific antigen provides advantages over T cells and makes them appealing for investigation as effectors for immunotherapy.1,2 There has recently been a substantial gain in our understanding of the role that NK cells play in mediating innate host immune responses against viruses and cancer. Although NK cells have long been known to be capable of killing cancer cells independently of antigen recognition, the full therapeutic potential of NK cell–based immunotherapy has yet to be realized. Here we review novel methods to activate and expand human NK cells ex vivo for adoptive transfer in humans, focusing on the important phenotypic and functional differences observed among freshly isolated, cytokine activated, and ex vivo–expanded NK populations.
Ex vivo NK cell activation and expansion
Unlike T cells, NK cells represent only a minor fraction of human lymphocytes. NK cells are defined by their CD3−/CD56+ phenotype, comprising 5% to 15% of circulating lymphocytes, and are commonly divided into CD56dimCD16+ (90%) and CD56brightCD16− (10%) subpopulations with distinct effector functions. Recently, investigators have shown that NK cell tumor cytotoxicity can be enhanced by disrupting NK cell signaling through inhibitory receptors such as KIR, PD-1, and NKG2A.3,4 Furthermore, exposing tumors to drugs that down-regulate ligands for NK cell inhibitory receptors or up-regulate death receptors for NK cell effector molecules or ligands that bind NK cell–activating receptors can be used as a strategy to sensitize tumors to NK cell–mediated apoptosis. Recently, anti-PD-1 and anti-PD-L1 monoclonal antibodies and the immunomodulatory drug lenalidomide were shown to enhance both NK cell tumor trafficking and NK cell–mediated antibody-dependent cell-mediated cytotoxicity, and cytokine release against tumors while simultaneously suppressing regulatory T cell function.5-8 Similarly, anti-CTLA-4 monoclonal antibodies have been shown to augment NK cell antibody-dependent cell-mediated cytotoxicity and TNFα release against melanoma cells by FcγRIII (CD16) binding to antibody-bound tumor cells, as well as through regulatory T cell inactivation.9-11 These data suggest these agents could be used in conjunction with autologous NK cell infusions as a method to potentiate NK cell–mediated antitumor effects in patients with advanced cancers. Further, recent advances in our ability to expand NK cells ex vivo have fueled translational research investigating a variety of novel methods to bolster immunity against cancer through the use of adoptive NK cell infusions.12-16
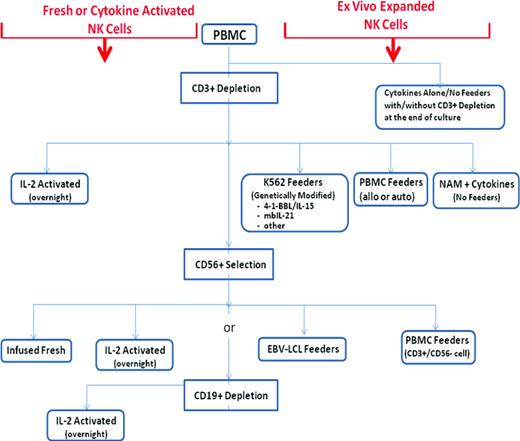
Until recently, the therapeutic potential of NK cell–based immunotherapy remained largely unexplored because the small number of NK cells isolated after a typical apheresis procedure and the inability to reliably expand large numbers of NK cells ex vivo precluded investigators from pursuing phase 1 trials evaluating for an NK cell dose-response relationship. At present, it is not at all clear what threshold of NK cell numbers is needed to achieve an antitumor effect after adoptive NK cell transfer. Short- and long-term cell cultures containing cytokines without feeder cells, such as IL-15 and IL-2, given alone or in combination with other growth factors, typically result in relatively small ex vivo NK cell expansions.17-21 Although some investigators have observed differences in their growth rate, in our experience, NK cells obtained from cancer patients proliferate ex vivo similarly to those obtained from healthy donors.22,23 Because adoptive NK infusions as tumor immunotherapy remain in a proof-of-concept phase, with allogeneic infusions often being given after immunosuppressive chemotherapy or after an HLA-mismatched transplantation, most investigators have pursued methods to expand highly purified NK cells so that both the efficacy and any toxicities of the infused product can be directly attributable to NK cells themselves.12,18,24-29 In general, to prevent T-cell and NKT-cell contamination and overgrowth, T cells are usually depleted from PBMCs either before the initiation of NK cell cultures or at the end of the expansion culture.30 Strategies to activate and/or expand NK cells for adoptive infusion in humans with cancer are summarized in Figure 1.
Methods to activate and/or ex vivo expand human NK cells for infusion in patients with cancer.
Methods to activate and/or ex vivo expand human NK cells for infusion in patients with cancer.
Ex vivo cytokine-activated NK cells
Although culturing NK cells in cytokine-containing medium alone is less effective in expanding NK cells compared with cultures containing feeder cells, such culture conditions are capable of activating NK cells quickly, even after a short overnight incubation, substantially enhancing NK cell cytotoxicity against tumor targets ex vivo. Miller et al used a strategy of CD3 depletion of mononuclear cells (using the Miltenyi CliniMACS system) collected by apheresis from haploidentical donors, followed by a brief 8- to 16-hour culture in X-VIVO15 medium containing IL-2 (1000 U/mL), with activated cells being infused into patients after haploidentical stem cell transplantation or after no transplantation31 in patients with relapsed hematological malignancies and solid tumors including breast cancer and ovarian cancer.32 Products such as these, although devoid of T cells, only contain on average ∼ 40% NK cells and may have substantial numbers of contaminating monocytes and B cells. The addition of a CD56+ selection after CD3+ T-cell depletion typically improves NK cell purity to the 90% range and reduces B-cell contamination to less than 1%.33 This approach is more effective in avoiding T-cell contamination and is more commonly used in studies exploring adoptive transfer of NK cells after allogeneic haploidentical transplantation, where T-cell contamination could lead to lethal GVHD.12,28,34,35 It is important to consider that when CD3 depletion alone is used to enrich for overnight-activated NK cells, B-cell contamination can lead to passenger B-lymphocyte-mediated complications such as EBV-lymphoproliferative disorder and, when products are infused in the context of minor ABO incompatibility, acute hemolytic anemia. To avoid this complication, the University of Minnesota, which has the most experience with the use of IL-2–activated NK cells, now incorporates both CD3+ T-cell and CD19+ B-cell depletion on overnight IL-2–activated NK cell products before their infusion.33
Ex vivo NK cell expansion without feeder cells
Some clinical studies of adoptive NK cell transfer have used short-term (12-18 hours) IL-2–activated NK cells.32 IL-2 alone expands small numbers of NK cells, typically 10- to 20-fold after 14 days of cell culture, far less than murine studies predict would be needed to mediate antitumor effects in humans with cancer. Sutlu et al recently developed a unique method to expand moderate numbers of clinical-grade NK cells in an automated bioreactor without the need for feeder cells.36 When this technique was used, human NK cells expanded a median 77-fold after 21 days of cell culture from unsorted PBMCs. These cells were activated ex vivo, being more cytotoxic against K562 target cells than those that were expanded in flasks, and expressed higher levels of the natural cytotoxicity receptor NKp44. Expanded cell cultures contained 10% to 80% (average 38%) CD3−/CD56+ NK cells with significant numbers of CD3+/CD56− T cells and/or CD3+/CD56+ NK cell–like T cells, potentially obligating the use of T-cell depletion on these expansion products before they could be used in the allogeneic setting.25 Alici et al similarly showed that highly activated NK cells could be expanded from unseparated PBMCs of myeloma patients using media containing cytokines and the anti-CD3 antibody OKT3 (removed from cultures after day 5).37 Day 20 cultures contained an average of 65% NK cells, which expanded a median 1625-fold (range 502-2658), although T-cell contamination in cultures averaged 22% with this approach.
Recently, Frei et al reported on a method to expand clinical-grade NK cells from CD3-depleted apheresis products in feeder cell-free medium containing nicotinamide (NAM), a specific inhibitor of NAD(+)-dependent enzymes.38 In MEMα medium supplemented with IL-2, IL-15, and NAM (at concentrations of 2.5-7.5 mM), NK cells cultured in G-Rex flasks (Wilson Wolf) expanded by 60- to 80-fold in 2 weeks. Remarkably, no medium changes or manipulation of the cell cultures were required during the 2-week expansion process, with expanded cells containing a highly pure population of activated NK cells (> 95% CD3−/CD56+). NK cells expanded using this approach underwent typical phenotypical and functional changes observed with cytokine-induced NK cell activation, including up-regulation of TRAIL and enhanced cytotoxicity against K562 and other tumor cell lines, compared with fresh NK cells. Remarkably, NK cells expanded with NAM-containing media were found to substantially up-regulate surface expression of the homing receptor CD62L (L-selectin), which typically decreases during ex vivo NK cell activation with cytokine-alone-containing medium. Adoptive infusion of human NK cells expanded using this technique into irradiated NOD/SCID mice had improved homing to the BM compared with NK cells expanded in medium without NAM, perhaps the consequence of CD62L up-regulation.
Ex vivo NK cell expansion using feeder cells
Several different methods (Figure 1) using feeder cells or APCs have been developed recently to expand large numbers of highly activated NK cells ex vivo, providing the opportunity to study the full potential of adoptive NK cell immunotherapy in humans. Irradiated PBMCs, Epstein-Barr virus-transformed lymphoblastoid cell lines (EBV-LCL), gene-modified K562 cells expressing NK cell–stimulatory molecules such as 4-1BB ligand and membrane-bound IL-15, and other irradiated tumor cell lines are most commonly used as feeder cells in NK cell expansion cultures (Table 1).2,26,39-44 Typically, these cultures are initiated using an enriched NK cell population isolated from apheresis products that have undergone CD3+ T-cell depletion with or without CD56+ selection to maximize NK cell purity. Utilization of feeder cells in NK cell cultures can dramatically enhance NK cell expansion numbers ex vivo, and this technique has been used recently for the large-scale expansion of clinical-grade NK cells.27,30,41,45 Substantial phenotypic and functional differences are observed between freshly isolated, IL-2–activated, and ex vivo–expanded NK populations, which theoretically could have an impact on their therapeutic efficacy after adoptive infusion.30,41,45,46 Criteria for the release of cytokine-activated and ex vivo–expanded NK cell products vary among institutions and depend upon the clinical setting in which they are used. For example, protocols using allogeneic NK cell infusions given from HLA-mismatched donors will often restrict contaminating total T cells/kg to < 1-5 × 105 to prevent the possibility of severe GVHD,28,47 whereas NK cells given in the context of an autologous infusion may be more permissive of T-cell contamination. With expanded NK cell products, many centers will perform sterility cultures 24 hours before and the day of product release, as well as a gram stain, PCR for mycoplasma, testing for endotoxin, and flow cytometry. At the National Heart, Lung, and Blood Institute (NHLBI), NK cells expanded using EBV-LCL are required on the day of release to contain at least 90% NK cells (CD3−/CD56+), have less than 5% contaminating CD3+ T cells and CD19+ B cells, and a viability of at least 70% as measured by 7-amino-actinomycin D (7-AAD) staining.48
Methods to isolate, activate, and expand NK cells for clinical applications

HSCT indicates hematopoietic stem cell transplantation; UCB, umbilical cord blood; N/A, not applicable; HD, Hodgkin disease; and MM, multiple myeloma.
*All feeder cells were gamma irradiated.
†K562-CD64-CD84-41BBL-truncated CD19-mbIL21.
CD3−/CD56+ indicates a 2-step purification of NK cells: CD3 depletion and a subsequent CD56 positive selection.
NK cells expanded using irradiated EBV-LCL feeder cells.
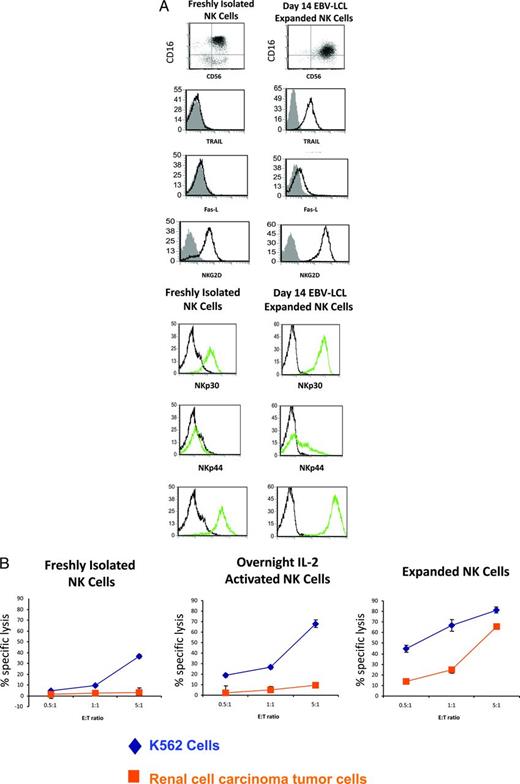
EBV-LCL cells have been known for decades to induce NK cell proliferation in vitro.39,49,50 Preclinical data from our laboratory established that large numbers of highly pure NK cells (99% CD3−/CD56+) could be expanded from PBMCs enriched for NK cells using Miltenyi immune-magnetic beads in which cells first underwent CD3+ T-cell depletion followed by CD56+ NK cell selection.41 Optimal NK cell expansions were obtained by coculturing enriched NK cells with a clinical-grade 100 Gy-irradiated EBV-LCL feeder cell line (TM-LCL 20:1 feeder-to-NK cell ratio) in X-VIVO 20 medium containing 10% human AB serum and 500 IU/mL of IL-2, with NK cells continuing to expand in cultures for up to 28 days. Using this technique, expansions of NK cells in the range of 800- to 1000-fold could be achieved in 2 weeks in a closed system using Baxter PL732 bags. EBV-LCL feeder cell eradication from 2-week cell cultures was confirmed by absence of detectable EBV-encoded early small RNAs. Ex vivo expansion led to a significant increase in NK cell surface expression of CD56, TRAIL, FasL, NKG2D, NKp30, NKp44, and NKp46 compared with resting NK cells (Figure 2A). Expression of perforin did not change, although there was a small but consistent increase in the intracellular expression of granzymes A and B and surface expression of LFA-1, NKG2C, CD244, and CD158b. Compared with nonexpanded NK cells, expanded NK cells also secreted (either spontaneously or after coculture with tumor targets K562 and RCC cells) higher levels of IFNγ, IL-2, FasL, and TRAIL. The net effect of changes in NK cell phenotype and cytokine secretion resulted in expanded NK cells having markedly higher levels of cytotoxicity against K562 and various other tumor cell lines compared with resting or overnight IL-2–activated NK cells (Figure 2B).46 Whether enhanced cytotoxicity occurred due to an increase in expression of NK cell activating receptors or was more the consequence of expanded NK cells having increased levels of molecules that induce tumor apoptosis (ie, TRAIL, FasL, granzymes, etc) is unclear.
Phenotype and function of freshly-isolated, IL-2-activated and expanded NK cells. (A) Phenotype of freshly-isolated versus expanded NK cells. (B) Cytotoxicity of NK cells against tumor cells. Used with permission from Berg et al.41
Phenotype and function of freshly-isolated, IL-2-activated and expanded NK cells. (A) Phenotype of freshly-isolated versus expanded NK cells. (B) Cytotoxicity of NK cells against tumor cells. Used with permission from Berg et al.41
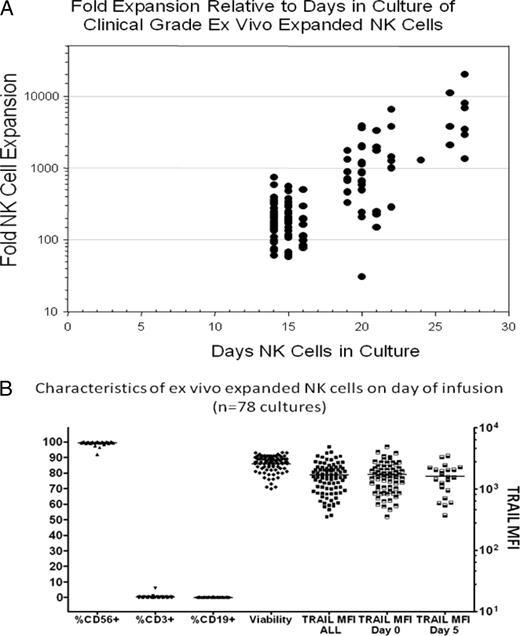
Based on these preclinical data, we scaled this method up for a clinical trial with an expansion approach that used Good Manufacturing Practice (GMP) conditions and a third-party irradiated allogeneic EBV-LCL feeder line (initially TM-LCL produced by the Beckman Research Institute at City of Hope, and then later SMI-LCL produced by the PACT group for the NHLBI) with IL-2–containing medium to expand large numbers of highly purified, highly activated NK cells from patients with cancer using a “closed bag” culture system. We are currently performing a clinical phase 1 trial investigating the safety and efficacy of infusing large numbers of these ex vivo–expanded autologous NK cells after treatment with bortezomib to sensitize tumors to NK cell cytotoxicity.14,51,52 In this study, patients undergo a 15 L apheresis to isolate NK cells that were enriched using the Miltenyi CliniMACS to deplete CD3+ T cells followed by CD56+ selection. These enriched NK cells are then frozen in multiple aliquots and can be used for subsequent thawing and ex vivo expansion. First, 5-12 × 107 enriched NK cells are placed in culture and are then expanded ex vivo in Baxter bags over 14-27 days using the SMI-EBV-LCL feeder cell line as above. After treatment with bortezomib, cohorts 1 through 4 received a single infusion of ex vivo–expanded NK cells on day 0 in a dose-escalating fashion (up to a dose of 1 × 108 NK cells/kg). Cohorts 5-7 received 1 × 108 NK cells/kg on day 0 and a second escalating dose of NK cells (from the same NK cell culture) infused on day +5 (up to a dose of 1 × 109 NK cells/kg, respectively). Patients with stable disease or regression were eligible to receive additional cycles of therapy. A total of 78 adoptive NK cell infusions have been given to 26 patients with a variety of different cancers.48 Among the 82 NK cell cultures initiated, 78 (95%) expanded successfully to achieve the target NK cell dose, 2 cultures failed to expand sufficiently to meet the targeted NK cell number, and 2 cultures were discarded due to contamination (one each with fungal and bacterial contamination). NK cells on the day of harvest expanded a median 198-fold (range 58-750), 895-fold (range 149-6647), and 3637-fold (range 1298-20 196) on culture days 14-16, 19-22, and 24-27, respectively (Figure 3A). NK cells at harvest contained a median 99.7% (range 92%-100%) CD3−/CD56+ NK cells and had a median 87% (range 71%-93%) viability assessed by 7-AAD on the day of harvest (Figure 3B). This study has established that large numbers of highly pure clinical-grade NK cells can reproducibly be expanded ex vivo using irradiated EBV-LCL feeder cells with NK cells expanding a median 3637-fold after 19-22 days of ex vivo culture. With the exception of thyroiditis and one patient who developed transient hypoxia after the infusion of 2.5 × 108 NK cells/kg on day +5, infusions of these ex vivo–expanded NK cells have been well tolerated. This study continues to dose escalate, with additional cohorts intended to establish whether expansions up to a dose of 1 × 109 NK cells/kg are technically feasible and can be infused safely into patients. However, because the Baxter PL732 bag is no longer being produced and culture volumes at these higher NK cell–expansion numbers require large volumes of medium (> 20 L), the ability of G-Rex100 containers to support these expansions at higher NK cell concentrations is currently being evaluated.
Fold expansion of ex vivo–expanded clinical grade NK cells and their characteristics on day of infusion. (A) NK cells were adoptively infused into cancer patients on the day of harvest. A total of 78 NK cell cultures expanded using irradiated EBV-LCL feeder cells were infused 14-27 days after culture initiation. (B) Phenotype/purity and viability of 78 clinical-grade NK cell products expanded over 14-27 days from cancer patients using EBV-LCL feeders. Used with permission from Reger et al.48
Fold expansion of ex vivo–expanded clinical grade NK cells and their characteristics on day of infusion. (A) NK cells were adoptively infused into cancer patients on the day of harvest. A total of 78 NK cell cultures expanded using irradiated EBV-LCL feeder cells were infused 14-27 days after culture initiation. (B) Phenotype/purity and viability of 78 clinical-grade NK cell products expanded over 14-27 days from cancer patients using EBV-LCL feeders. Used with permission from Reger et al.48
NK cells expanded using irradiated PBMCs.
Irradiated allogeneic PBMCs have been used for years to expand T cells for adoptive infusion in humans, including tumor-infiltrating lymphocytes and antigen-specific T-cell lines and clones. Irradiated allogeneic PBMCs can likewise be used as feeder cells to expand NK cells ex vivo, although expansion numbers achieved with PBMCs are lower than those achieved using EBV-LCL or genetically modified K562 cells.26,41 We found that when allogeneic PBMCs were used as feeder cells, NK cells isolated from PBMCs using CD3+ depletion followed by CD56+ selection were most efficiently expanded when 25-Gy-irradiated feeder cells were added to cultures at a 20:1 ratio in culture medium containing 500 IU/mL IL-2 and 10% single donor or pooled AB plasma in upright culture flasks or Baxter bags at a starting density of 1 × 106 NK cells/mL.41 Under these conditions, up to a 100-fold increase in cell number was achieved in 15 days and, after a second round of expansion for an additional 14 days, increases of up to 200- to 400-fold could be achieved, although results varied depending on the NK cell donor. Autologous irradiated PBMCs can likewise be used as feeder cells to stimulate ex vivo NK cell expansion.24,27,53 Kim et al used an approach in which NK cells were isolated using the Miltenyi 2-step CD3+ depletion followed by CD56+ selection, with the non-NK cell fraction of cells being irradiated with 25 Gy and then used as feeder cells in NK cell cultures.27 Using this approach, a highly purified population of NK cells could be expanded in AIM-V medium in 14 days, with NK cells expanding 300- and 169-fold when feeder PBMCs were obtained from healthy donors and cancer patients, respectively.
NK cells expanded using irradiated gene-modified K562 cells.
The MHC class I deficient chronic myelogenous leukemia cell line K562 can also be used as a feeder cell to induce NK cell proliferation ex vivo.29,30,44,45,54 Imai et al retrovirally transduced this line with a construct containing 2 NK cell–stimulatory molecules, the ligand for the costimulatory surface molecule 4-1BB (CD137), and the human IL-15 gene fused to GFP and a gene encoding the CD8α transmembrane domain, leading to surface-bound IL-15 expression. These genetically modified K562 cells coexpress surface-bound IL-15 and 4-1BB ligand and are highly efficient at inducing NK cell proliferation in vivo. A 7-day coculture of purified CD3-depleted and CD56-enriched NK cells with 100-Gy-irradiated K562-mb15-41BBL in RPMI 1640 or SCGM medium induced a median 21.6-fold expansion of CD3−/CD56+ NK cells from peripheral blood (range 5.1- to 86.6-fold) without inducing T-cell proliferation, with NK cells expanding more than 277-fold after a 21-day cell culture.45 Complete elimination of leukemic feeder cells was confirmed by a Click-iT EdU Alexa Fluor 647 flow cytometry assay and staining of the final product for GFP expression.30 When unfractionated PBMCs (in contrast to purified NK cells) were stimulated with irradiated K562-mb15-41BBL feeder cells using GMP conditions, NK cells expanded a median 90.5-fold (range 33-141) in 7 days and contained a median 83.1% (range 72.9%-85.9%) CD3−/CD56+ NK cells. Similar to EBV-LCL–expanded NK cells, K562-mb15-41BBL expanded NK cells with up-regulated surface expression of the natural cytotoxicity receptors NKp30, NKp44, and NKp46, and activating receptors such as 2B4, DNAM-1, and NKG2D were significantly more cytotoxic to K562 targets than freshly stimulated or IL-2–activated NK cells.
Lapteva et al developed a large-scale system to expand clinical-grade NK cells using irradiated K562-mb15-41BBL feeder cells in gas-permeable static cell culture flasks (G-Rex).30 Using unfractionated PBMCs, NK cells expanded in large-scale experiments by up to 128-fold (range 70-128) after an 8-day culture in SCGM medium and contained a median 77% (range 52%-88%) pure CD3−/CD56+ NK cells. This method did result in moderate T-cell contamination, with NK cell cultures containing up to 34% (range 4%-34%) CD3+ T cells. These investigators showed that supernatants taken from NK cell expansion cultures induced substantial MHC class I up-regulation on K562-mb15-41BBL cells, which stimulated CD8+ T cells ex vivo, including T cells that were found to have alloreactivity. However, contaminating T cells could be completely removed from NK cell cultures using CliniMACS CD3+ depletion, and this step would be necessary if this method were used to expand allogeneic donor NK cell products. This system required no manipulation after setup of the initial cell culture and achieved higher fold increases in NK cell numbers in G-Rex flasks than was observed with the use of gas-permeable bags.
Several investigators have shown that K562-mb15-41BBL feeder cells are capable of expanding NK cells isolated from allogeneic donors and patients who have received heavy prior treatment with chemotherapy for cancers such as acute myelogenous leukemia, gastric cancer, and multiple myeloma.22,37,45,55 Adoptive transfer of NK cells expanded from myeloma patients using K562-mb15-41BBL feeder cells into myeloma-bearing immune-deficient mice has been shown to result in NK cell in vivo proliferation for up to a month and inhibition of myeloma tumor growth, suggesting a potential therapeutic application for these cells in humans with myeloma.
Investigators have recently reported that additional genetic modification to K562 cells can be used to further enhance their potential to expand NK cells. Denman and Lee developed a K562 cell line that was genetically modified to express CD64 (FcγRI), CD84 (B7-2), CD137L (4-1BBL), truncated CD19, and membrane-bound IL-21 (mbIL-21) rather than mbIL-15.56,57 Although NK cells stimulated with K562 cells expressing either mbIL-15 or mbIL-21 had similar phenotypes, mRNA expression profiles, and tumor cytotoxicity profiles, mbIL-21–expanded NK cells had longer telomeres, increased expression of CD160, superior cytokine secretion of IFN-gamma and TNF-alpha, and better ex vivo expansion. By day 21, NK cells expanded from unseparated PBMCs increased a median 31 747-fold using mbIL-21 K562 cells compared with only a 325-fold expansion using mbIL-15 K562 feeders. Remarkably, NK cell cultures stimulated weekly × 3 using mbIL-21–expressing K562 cells in IL-2–containing medium continued to expand for up to 6 weeks in culture, whereas NK cells expanded using mbIL-15 K562 typically became senescent at 4 weeks.
NK cells can also be expanded ex vivo from primitive stem cell populations including hematopoietic progenitor cells58,59 and human embryonic stem cells.60 Spanholtz et al recently developed a fully closed large-scale system to expand clinical-grade NK cells from CD34+ cells enriched from umbilical cord blood61 using a stromal-cell–free method. NK cells expanded from CD34+ cells using this technique were >90% pure (CD3−/CD56+) and were highly activated, displaying high expression of activating receptors and high degrees of cytotoxicity against K562 cells. Despite these findings, the use of stromal cells in expansion cultures may offer advantages to stromal-free conditions. Dezell et al have shown that NK cell expansion from hematopoietic progenitors using stromal cells appears to increase the yield of NK cells compared with stromal-free conditions, perhaps by differentiating less committed progenitors into the NK cell lineage.62 Despite this potential advantage, stromal-based expansions are logistically more challenging to use under GMP conditions, potentially limiting their application in the clinical setting.
Differences among fresh, IL-2–activated, and ex vivo–expanded NK cells
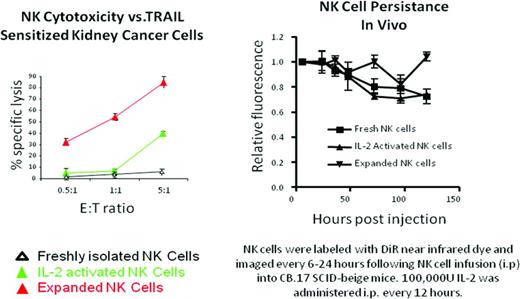
Although cultures using irradiated EBV-LCL or genetically modified K562 feeder cells substantially increase the number of NK cells that can be expanded compared with cytokine alone containing cultures, concerns exist that extensive ex vivo expansion might significantly reduce the in vivo proliferative potential and long-term viability of these populations after adoptive transfer in humans. We investigated for differences in phenotype, tumor cytotoxicity and in vivo persistence between short-term IL-2–activated and long-term–expanded NK cells.41,46 CD3−/CD56+ NK cells that were isolated from donors by immunomagnetic bead selection were either activated with IL-2 (500 U/mL) for 12-16 hours or were expanded ex vivo over 14 days using irradiated EBV-LCL feeder cells in IL-2–containing medium (500 U/mL). Short-term IL-2–activated NK cells did not expand in number, in contrast to EBV-LCL–stimulated NK cells, which expanded up to 1000-fold by culture day 14. FACS analysis revealed that expanded NK cells had significantly higher expression of TRAIL, NKG2D, and the natural cytotoxicity receptors NKp30, NKp44, and NKp46 compared with freshly isolated and IL-2–activated NK cells, with no differences in telomere lengths between the 3 populations as assessed by Flow-FISH. Expanded NK cells were significantly more cytotoxic against K562 cells compared with overnight IL-2–activated NK cells (Figure 2B) and had increased cytotoxicity against a TRAIL-sensitized kidney cancer tumor cell line (Figure 4). Using bioluminescent imaging, the 5-day in vivo longevity was found to be similar among freshly isolated, IL-2–activated, and expanded NK cells labeled with DiR and then infused into immunodeficient CB.17 SCID-beige mice receiving twice daily IL-2 (100 000 units ip).46 Although NK cells expanded for 14 days using EBV-LCL maintained similar telomere lengths and longevity in vivo as nonexpanded short-term IL-2–activated NK cells, these data should be interpreted with caution because results may vary depending on the methods used to expand NK cells and the culture duration. Extensive culturing of NK cells beyond 4 weeks typically leads to their senescence when using either EBV-LCL feeders or K562-mb15-41BBL feeder cells. Although transduction of the hTERT gene into expanded NK cells can overcome this limitation,63 its application in the clinical setting is impractical. Remarkably, Denman et al found NK cells expanded with mbIL-21–expressing K562 cells for 21 days had longer mean telomere lengths than NK cells expanded with mbIL-15 K562 cells, even though the mbIL-21–stimulated NK cells had the greatest amount of ex vivo proliferation.56 Therefore, altering methods used to expand NK cells could potentially overcome problems related to NK cell senescence associated with long-term culturing.
Cytotoxicity of human NK cells against tumor cells and persistence in vivo following adoptive transfer in immunodeficient mice. Left: NK cytoxicity versus TRAIL-sensitized kidney cancer cells. Right: NK cell persistence in vivo. NK cells were labeled with DiR near infrared dye and imaged every 6-12 hours after intraperitoneal NK cell infusion into CB.17 SCID-beige mice. IL-2 (100 000 U) was administered intraperitoneally every 12 hours. Used with permission from Berg et al.46
Cytotoxicity of human NK cells against tumor cells and persistence in vivo following adoptive transfer in immunodeficient mice. Left: NK cytoxicity versus TRAIL-sensitized kidney cancer cells. Right: NK cell persistence in vivo. NK cells were labeled with DiR near infrared dye and imaged every 6-12 hours after intraperitoneal NK cell infusion into CB.17 SCID-beige mice. IL-2 (100 000 U) was administered intraperitoneally every 12 hours. Used with permission from Berg et al.46
Pitfalls of NK cell expansion
Up-regulation of FAS
Cellular activation increases cell susceptibility to apoptosis and activation-induced cell death. The effects of ex vivo expansion of NK cells in terms of their susceptibility to programmed cell death mediated through Fas and other cellular death receptors has been poorly characterized. Fas expression appears to be similar on overnight IL-2–activated NK cells compared with fresh NK cells. In contrast, Fas expression increases when NK cells are maintained in IL-2 for more than 2 days or when NK cells are expanded with either EBV-LCL or K562 cells, increasing their susceptibility to rhFasL-mediated apoptosis.64 Culturing with rhFasL increased NK cell apoptosis a median 5.6-fold (P = .0001) with 10- to 14-day–expanded NK cells compared with a 1.2-fold increase (P = .32) with fresh NK cells and no increase with overnight IL-2–activated NK cells. In contrast to Fas, surface expression of the TRAIL death receptors DR4 and DR5 do not appear to be affected by NK cell activation or in vitro expansion. These data suggest that expanded NK cells are more susceptible to Fas-mediated apoptosis compared with fresh and overnight IL-2–activated NK cells, potentially enhancing their susceptibility in vivo to apoptosis. Methods to expand activated NK cells while avoiding up-regulation in Fas surface expression remain an active area of investigation.
Homing
Whether adoptively infused NK cells are able to mediate clinically meaningful antitumor effects will ultimately be determined by their ability to home and traffic to the microenvironment where the tumor resides. Therefore, maintaining and/or enforcing the expression of homing receptors in expanded NK cell populations is required to achieve cell delivery via the vasculature to any target tissue. To move from the bloodstream into inflamed tissue sites, leukocytes must attach to the vascular endothelium, migrate between adjacent endothelial cells, and penetrate the basement membrane.65 The molecular mechanism underlying these events involves a series of sequential adhesive interactions between activated leukocytes that are chemoattracted to endothelial cells that are activated by inflammatory mediators such as IL-1 and TNF-α. The initial step in emigration from postcapillary venules is a low-affinity interaction between leukocyte ligands with selectins expressed on endothelium cells referred to as “rolling” or “tethering.” Furthermore, the interaction of chemokine receptors expressed on lymphocytes with chemokines secreted by the tumor or infected cells play a critical role in their ability to home to their target cells.66
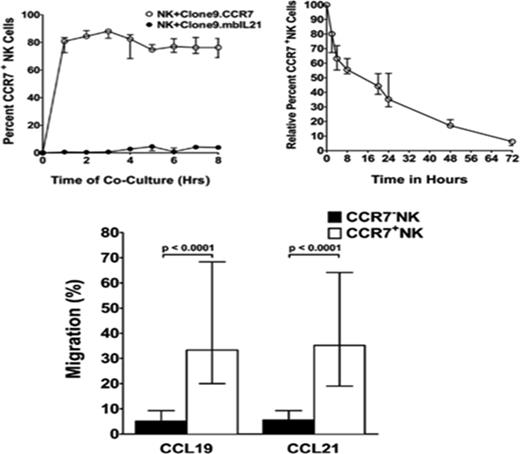
At present, little is known regarding the ability of ex vivo–expanded NK cells to migrate to lymph nodes, the BM, and other environments where tumors reside. The expression of chemokine receptors on NK cells may be critical to this process. NK cells expanded with genetically modified K562 cells contain predominantly CD56+/16+ bright NK cell populations, which do not express CCR7, a chemokine receptor that is known to facilitate NK cell homing to lymph nodes.67 Although IL-18 can up-regulate CCR7, it does so in only a minority of NK cells.68 Somanchi et al recently demonstrated that mbIL-21–expressing K562 feeder cells can be further genetically modified to express other transgenes, the products of which can be rapidly and transiently expressed in NK cells via trogocytosis by coculturing with expanded NK cells. K562 cells expressing mbIL-21 and CCR7 (clone9.CCR7) rapidly transferred CCR7 to expanded NK after a brief 1-hour culture, with up to 80% of NK cells acquiring CCR7 surface expression (Figure 5).69 Although surface expression was transient, declining to baseline by 72 hours, NK cells that became positive for CCR7 had improved NK cell migration toward the CCR7 ligands CCL19 and CCL21 in transwell experiments and had increased homing into the lymph nodes of athymic mice. The relative ease of this approach to modify NK cells potentially makes it a viable strategy for scale-up under GMP conditions to explore its ability to improve ex vivo–expanded NK cell homing in humans.
NK cell trogocytosis as a method to increase NK cell surface expression of CCR7. Used with permission from Somanchi et al.69
NK cell trogocytosis as a method to increase NK cell surface expression of CCR7. Used with permission from Somanchi et al.69
Although NK cells cultured with irradiated EBV-LCL can be expanded by more than 1000-fold and are more cytotoxic to tumor cells compared with resting or IL-2–activated NK cells, we have observed these expanded cells undergo a substantial reduction in surface expression of CD62L, which could hinder their ability to be recruited from the circulation into the BM and secondary lymphoid tissues, where hematological malignancies reside. As discussed previously, investigators have reported an NK cell expansion technique that uses NAM in the medium, which appears to substantially increase CD62L expression on NK cells, leading to their improved homing into the spleens and BM of immune-deficient mice.38 Furthermore, work conducted in our laboratory has shown that treatment of NK cell cultures using EBV-LCL feeders with NAM added to culture medium on day +7 resulted in the expansion of NK cells that contained substantially higher CD62L surface expression compared with cultures without NAM.70 These NAM-cultured NK cells showed improved homing to mouse BM 24 hours after infusion into immune-deficient NSG mice compared with mice receiving expanded NK cells that were not cultured in NAM. These data suggest that NK cell expansion techniques that incorporate NAM-containing media could be used as a method to improve NK cell trafficking to the BM, potentially enhancing the antitumor effects of adoptively transferred NK cells against a variety of hematological malignancies.
Dependence on cytokines
Methods used to activate and/or expand NK cells ex vivo may affect the need for exogenous cytokine administration with IL-2 or IL-15 to support in vivo NK cell proliferation and cytotoxicity. Ex vivo–expanded NK cells have enhanced cytokine secretion profiles and are significantly more cytotoxic to tumor cells, although maintenance of this activated state is often dependent on the persistence of IL-15 or IL-2. We found that day 14 EBV-LCL–expanded NK cells had a rapid decline in both the percentage of NK cells expressing TRAIL and NKG2D within 16-24 hours of IL-2 removal from the medium. TRAIL and NKG2D expression was restored by the subsequent addition of IL-2 back into the medium and was IL-2 dose dependent.41 Reductions and subsequent increases in TRAIL and NKG2D surface expression that occurred with the removal and addition of IL-2 were directly correlated with NK cell cytotoxicity against tumor cells. Culturing previously expanded NK cells in medium containing no or low doses of IL-2 (0-5 IU/mL) for 24 hours resulted in a substantial decline in NK cell cytotoxicity against K562 and other tumor target cells compared with cultures containing 50-500 IU/mL of IL-2, where cytotoxicity was maintained. Likewise, spontaneous secretion of FasL and TRAIL and multiple cytokines, including GM-CSF, TNF-α, and IFN-γ was also IL-2 dose dependent, declining rapidly in cultures in which the concentration of IL-2 was decreased or where IL-2 was removed. These data suggest that cytokine dependence may occur with extensive ex vivo NK cell expansion, although these effects too may be dependent on the method used to expand NK cells and the degree of ex vivo–induced NK cell proliferation.
Cryopreservation/thawing
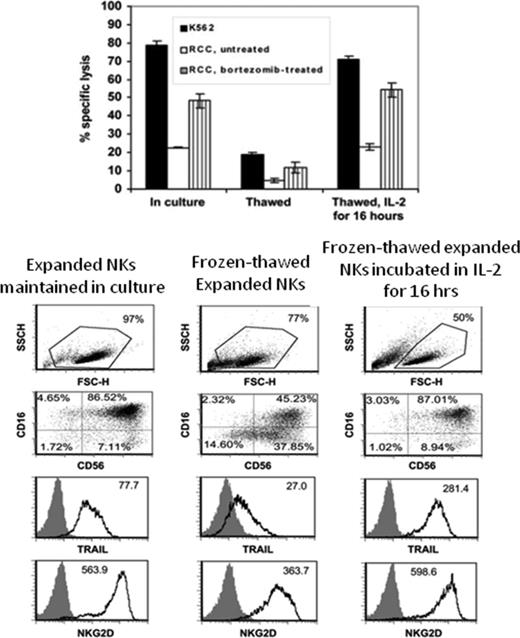
The ability to cryopreserve and subsequently thaw NK cells while maintaining their cytolytic activity could logistically facilitate clinical trials evaluating multiple rounds of adoptive NK cell infusions. NK cells isolated from fresh PBMCs can be cryopreserved, have viability in the range of 80% to 90% upon thawing, can be activated with overnight culture in IL-2, and expand well ex vivo with the use of various feeder-cell–based expansion methods. Lapteva et al found that NK cells expanded with IL-15- and 4-1BBL–expressing K562 feeder cells maintained excellent stability after cryopreservation, with an average 91% viability (range 85%-94%) upon thawing; stability data were confirmed for up to 12 months on expanded NK cells maintained in liquid nitrogen.30 However, subsequent studies by the same group have shown low recovery (∼ 30% in some cases) of cryopreserved cells after thawing and overnight culture and no in vivo expansion after infusion into patients.23,47 Furthermore, despite their excellent post-thaw viability, potency assays showed that freshly thawed NK cells failed to lyse K562 cells, with overnight culturing in IL-2–containing media (10 ug/mL) being required to rescue NK cell cytotoxic function. Similarly, we found that NK cells expanded using EBV-LCL feeders that were frozen and then thawed maintained excellent viability (>90%),41 although the cytolytic capacity of these cells to kill K562 and renal cell carcinoma cells was substantially lower than that of expanded NK cells that had never undergone cryopreservation. Thawed NK cells were found to have lower surface expression of TRAIL and NKG2D and were more likely to contain populations that were dim or negative for CD16. The cytotoxicity of expanded NK cells that were frozen and then thawed could be rescued by culturing in IL-2–containing medium, although (Figure 6) the viability of thawed NK cells (assessed by 7-AAD staining) declined from 93% to 97% immediately after thawing to 38% to 50% at 16 hours. These results suggest that expanded NK cells that have been cryopreserved may require reculturing in IL-2–containing medium after thawing to restore function before infusion in patients.
Effects of freeze/thawing on the cytotoxicity and phenotype of expanded NK cells. Used with permission from Berg et al.41
Effects of freeze/thawing on the cytotoxicity and phenotype of expanded NK cells. Used with permission from Berg et al.41
Future directions: engineering a better NK cell
The development of an efficient method that results in stable genetic modifications to NK cells could be used to characterize NK cell differentiation, acquisition of self-tolerance, and tumor trafficking in vivo, as well as to manipulate NK cells to improve their homing and enhance their cytotoxicity against infectious diseases and tumors. For example, retroviral transduction of primary NK cells to express endogeneous IL-15 has been proposed as a method to avoid the need for exogenous cytokine administration after the adoptive infusion of expanded NK cells.71 The genetic disruption of expression of inhibitory receptors on NK cells such as KIR or NKG2A could potentially be used as a method to overcome the tumor evasion that occurs as a consequence of MHC class I expression. Lentiviral vectors (LVs) encoding shRNA targeting various regions of the NKG2A transcript have been used to silence NKG2A expression in the NK cell line NKL.4,72 NKG2A-silenced NK cells had enhanced killing of HLA-E–expressing tumors ex vivo and in vivo after their infusion into tumor-bearing mice. Although LVs have been used to efficiently transfer genes into human T cells and the NK cell lines such as NK92 and NKL, LV transduction of fresh and ex vivo–expanded human NK cells has been more challenging.73 We used an LV-expressing enhanced green fluorescence protein driven by a murine stem cell virus long terminal repeat promoter to transduce CD3− and CD56+ and/or CD16+ human NK cells that were either resting, IL-2 activated, or expanded ex vivo using an irradiated EBV-LCL feeder cells.4 Resting NK cells were difficult to transduce with LVs, even at high multiplicities of infection, with transduction efficiencies in the range of only 3% to 14%. The efficiency of LV transduction was improved when the NK cells were prestimulated ex vivo with IL-2, IL-15, or IL-21. Transduction efficiencies improved to 21% ± 0.2% in NK cells cultured for 24 hours in medium containing IL-2 (200 U/mL) and to 28.7% ± 12.9% when NK cells underwent ex vivo expansion over 10 to 14 days using irradiated EBV-LCL feeder cells and medium containing IL-2 (500 U/mL). Transduced NK cells maintained stable enhanced green fluorescent protein transgene expression ex vivo, which peaked 5 days after LV transduction and remained stable for an additional 9 days. The phenotype, cytokine production, and cytotoxicity of NK cells compared with tumor targets were not altered by LV transduction.4 Sutlu et al have shown that the efficiency of lentiviral transduction of NK cells can be improved using both IL-2 and IL-21 in the culture medium. Furthermore, these investigators found that the transduction efficiency of freshly isolated NK cells cultured in IL-2- or IL-21–containing medium could be increased to the 50% range by inhibiting NK cell innate immune receptor signaling by adding BX795 to the medium, an inhibitor of TBK1/IKKε that acts as a common mediator in the signaling pathways of the receptors RIG-I, MDA-5, and TLR3.74
Similar to T cells, transduction of chimeric antigen receptors (CARs) into NK cells has been explored recently as a method to induce tumor-specific NK cell killing. Shimasaki et al used a novel method of electroporation to introduce anti-CD19-4–1BBL-CD3ζ mRNA into nonexpanded and expanded NK cells, obtaining efficiencies of 40.3% and 61.3%, respectively, and have optimized this method for future clinical application.75 CARs specific for antigens expressed on B-cell malignancies such as CD19 and CD20, HER2/ErbB2GD2 on breast tumors, and GD2 on neuroblastoma tumors, when transduced into primary or expanded NK cells, have been shown to overcome tumor resistance to killing by autologous NK cells. These data, as well as recent data showing the efficacy of T-cell–based CAR therapy targeting CD19 in B-cell malignancies, suggest that NK cells modified to express CARs after mRNA or viral transduction represent a therapeutic tool worthy of exploration in the clinical setting.43,44,57,73,76-79 The ability of CAR-expressing NK cells to home to the tumor may be a critical determinant of their efficacy in humans. As discussed previously, expanded NK cells may lack or down-regulate molecules that are critical for NK cell homing from the circulation.70 Therefore, the use of expansion techniques that modify the phenotype of NK cells to improve their ability to home to the BM and lymph nodes may be necessary to optimize the therapeutic potential of CAR-expressing NK cells.38,67 The genetic manipulation of NK cells is technically challenging, expensive, and remains relatively inefficient despite the above described advances. Expansion techniques that engineer NK cells to express desired surface molecules via the trogocytosis method pioneered by Somanchi et al might offer a more efficient, safer, and more practical approach than genetic modification of NK cells via viral transduction.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Richard W. Childs, MD, Section of Transplantation Immunotherapy, National Institutes of Health, Bldg 10-CRC, Rm 3-5330, 10 Center Dr, Bethesda, MD 20892; Phone: 301-451-7128; Fax: 301-480-2664; e-mail: childsr@nhlbi.nih.gov.