Abstract
Although enormous progress in therapeutic research has improved the lives of patients with hematologic malignancies, these earlier achievements resulted from strategic combinations of agents with unique mechanisms of action and nonoverlapping toxicities. Continued investment in the modern era of drug discovery and development will focus on targeted therapies. Targeting of specific molecular pathways is expected to achieve effective tumor cell reduction with less overall toxicity. The translational processes involved in moving novel therapeutic strategies from the laboratory toward the clinic require close monitoring. The efforts in both cancer drug discovery and development will require extensive collaboration among basic scientists, clinical investigators, and regulatory scientists. The transition from older methods of therapeutic research will require laboratory support to define eligible patients based upon their pretreatment profile. The principles of preclinical drug development based upon decades of experience in predicting toxicity and designing therapeutic strategies are still needed to insure that safety is a high priority. The opportunities for developing novel targeted combination therapies in uniquely profiled patients will hopefully enable successful breakthroughs. Several concrete examples of exciting new agents are discussed here. Defining the predicted mechanism of resistance to these new targeted agents will enable investigators to subsequently design strategies to circumvent resistance with effective combinations. Drug discovery and development are complex and expensive, so efficiency and cooperation in task completion must be tracked.
Introduction
The processes of drug discovery and development are both complex and critically important for cancer patients. Although substantial progress has been made in changing the natural history of previously fatal hematologic malignancies, continued success relies on identifying novel agents that are both safe and effective.1,2 Several eras in cancer drug development evolved encompassing themes from modulating biochemical pharmacology to, more recently, using molecular pharmacology to selectively target oncogenic events. Most forms of malignant disease require combinations of agents with different mechanisms of action and nonoverlapping toxicities.3,4 The remarkable successes in treating hematologic malignancy were achieved with the incorporation of multiple agents in the effective therapeutic regimen. Although achieving prolongation of survival often entails securing a complete remission, therapeutic agents that result in malignant cell reduction may yield a long-lasting remission.5 Disease relapse heralds the emergence of resistant cells with the likelihood that a second remission is less apt to occur.6 Second remissions may also be shorter in duration with progressively increasing challenges to identify effective therapy.7 Understanding the mechanism of resistance may provide important clues to developing a salvage therapy.8 The processes of novel drug discovery and development are increasingly linked to identifying a molecular target that can be pursued with the intention of modifying tumor cell survival. The processes of discovery and preclinical development require time and resources if safety is to be a high consideration.
Preclinical discovery and developmental tasks
Designing a novel agent involves knowledge of structural biology coupled with expertise in organic chemistry to optimize the chemical structure of a “lead” compound.9 Lead optimization is the process whereby the medicinal chemist modifies the therapeutic agent to interface with the molecular target to produce the desired effect on the malignant cells while minimizing toxicity to normal tissues. Selective toxicity spares normal tissue while enhancing the interaction of the agent and the desired molecular target within the cancer cell, the tumor microenvironment, and the normal tissue. The medicinal chemist will make strategic changes in the structure of the agent followed by biologic studies to evaluate the impact of these modifications on “hitting the target.” This is an iterative process requiring careful evaluation and adequate time to select the final lead agent. Consultation with an expert in pharmaceutical chemistry insures that the ultimate design incorporates understanding of plasma protein binding and stability and an appropriate formulation for administration.
Once the optimized lead is identified, in vivo evaluation in animal models permits a better estimate of the therapeutic index along with refinement of the pharmacokinetic profile and validation of the pharmacodynamic effects. Achieving a plasma concentration of the therapeutic agent(s) capable of interacting in a positive sustained manner with the intended target without inducing unacceptable toxicity is another important goal. Small animal models are often evaluated for estimation of the potential therapeutic index. Defining the optimal route and proposed schedule of administration in the appropriate formulation is equally important.
Analytical methods must be developed and validated to measure quantitatively the plasma concentrations of the agent itself and the major metabolites. The time investment in preclinical pharmacology is important to insure that the targeted levels of the agent are achievable in vivo and whether an effective schedule of administration can be designed. The time and effort in developing the analytical methods will benefit the subsequent studies in patients by providing “near real-time” analysis that will be useful.
If the agent under investigation is a synthetic compound, then plans for scale-up synthesis and production of a stable product should be secured before extensive preclinical testing is initiated. If a natural product, or a derivative thereof, is selected as the optimal agent, then a plan for securing a sufficient supply of this purified material must be determined. The chemical characteristics including feasibility of scale-up synthesis or isolation need to be defined. Toxicology studies are conducted on the proposed schedule of administration that is likely to be used in the initial clinical trials in humans.10 These studies are essential in predicting the dose-limiting toxicity that may be observed in patients and in setting the safe starting dose for a truly novel agent. The necessary studies are often conducted on 2 animal species. If there is general agreement regarding the dose and toxicity studies in the animal models, then the initial dose level recommended for patients can be determined as being in the range of 1/10 the dose that caused serious toxicities. If there is a difference in tolerance between the 2 species, a conservative approach is required in establishing the initial dose projected for human trials. Projections for a safe starting dose will take into consideration the more sensitive of the 2 species.
The entire process for preclinical evaluation of a new agent is expensive, labor intensive, and time consuming.11 The total cost of each project is variable, but may readily exceed multiple millions of dollars depending upon the class of agent. The total estimated cost of developing a new antineoplastic agent from preclinical design through completion of approval for marketing can exceed one billion dollars. Acquisition and assembly of the entire preclinical data package and compiling the Investigational New Drug application for submission to the US Food and Drug Administration (FDA), along with designing a clinical protocol, entails years of work. Project coordination and management are absolutely essential because the time for patent protection is declining throughout this required period. From the initiation of submission of a patent for a novel agent through the period of preclinical and early clinical evaluation, the entire protected patent life may be reduced in half. Although protected time for marketing may ultimately be extended after drug approval for patients with diseases that qualify for “orphan disease status,” it is imperative that development be coordinated and tracked to insure success.
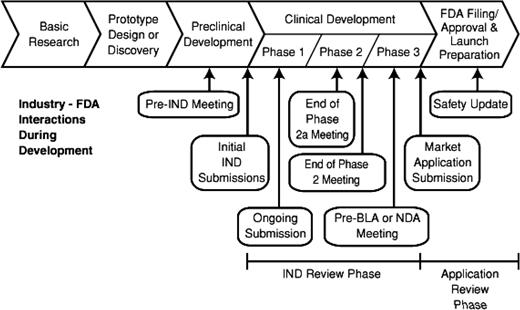
Despite the profound impact of the length of time needed for developmental on the potential return on investment, failure to establish the necessary preclinical information may result in an unsafe initial trial in patients. The time and effort in preclinical studies can determine the fate of the project. In Figure 1, the entire process of drug development encompassing preclinical studies followed by early trials in patients shows the strategic points where consultation may be useful.
In an effort to improve the efficiency of preclinical safety evaluations, toxicologists are pursuing alternative strategies to gain critical information on tissue tolerance. Within the pharmaceutical industry, the FDA, and the National Cancer Institute's (NCI's) Developmental Therapeutics Program, studies exploring modern ways of evaluating toxicity remain a high priority. Several of the proposed novel strategies (eg, ex vivo assays to predict toxicities) are still in development.10 The NCI has conducted numerous studies to define the preclinical toxicology of novel anticancer agents. The use of small rodents and dogs provides data that supports a safe starting dose in humans.11 Every rule, however, has an exception.
Historical example: phase 1 trial of fludarabine monophosphate, a novel antileukemic agent
Fludarabine monophosphate was synthesized by Dr John Montgomery as a halogenated purine analog of adenosine.12 This agent was thought to be resistant to deamination because of the insertion of the halogen in the purine base. In 1979, preclinical studies on this promising antileukemic agent were initiated. Despite differences in species tolerance, the dog data were used to establish the initial dose recommended for phase 1 study in humans (ie, 260 mg/m2 administered as a single intravenous dose over a short infusion: more than 10-fold higher than currently advised). The initial patients on this phase 1 trial developed profound neutropenia, but recovered.13,14 There were differences in metabolism of fludarabine in the preclinical toxicology studies between these 2 species (rodent and dog). Subsequent investigation confirmed that the dog was able to metabolize this agent differently than either man or rodent. Therefore, the safe starting dose based upon the dog's tolerance required substantial readjustment from the initial doses of the drug to complete the study in humans. In addition to dose adjustment, the schedule of administration was changed to a multiple-day dosing regimen, ultimately leading to the maximum tolerable dose being established at 25 mg/m2/d for 5 days.
Clinically, observations were made in the phase 1/2 trials in patients that established low-dose fludarabine as an active antileukemic agent.13,15 With subsequent dose escalation as a part of the comprehensive phase 1 investigation in hematologic malignancies, high daily doses (eg, 96 mg/m2/d for 5 days) in patients with refractory acute leukemia were found to have profound delayed severe neurologic toxicity.13 One major pharmaceutical sponsor discontinued exploration based upon this toxicity, but fortunately another sponsor enabled the successful development of this drug for use in patients with chronic lymphocytic leukemia (CLL) and low-grade lymphoma. Fludarabine was approved by the FDA in 1991 and has formed the backbone of therapeutic regimens in CLL for more than 2 decades.
Once approved, additional studies were conducted in other patients with a hematologic malignancy. Fludarabine has subsequently been incorporated into many preparative regimens to facilitate nonmyeloablative BM transplantation.16-19 This agent has actually revolutionized the field of nonmyeloablative stem cell transplantation. Incorporation of purine analogs into the preparative regimen has enabled older individuals to tolerate this procedure. Therefore, this agent plays a major role in treating patients with low-grade B-cell malignancies.
Although the neurologic toxicity characterized as a demyelinating injury was not initially predicted by the preclinical studies in rodents and dogs, the relationship to dose intensity coupled with the delayed nature of onset eluded early detection. Many preclinical toxicology protocols routinely euthanize the animals by day 30. Therefore, delayed onset of neurotoxicity or other serious toxicities would not be detected if the animals were euthanized too early. In retrospect, the protocol for evaluating preclinical toxicity of new agents might benefit from observing a small cohort of animals for longer than 30 days. Careful analysis of the preclinical toxicology is critically important both for establishing the safe starting dose and for defining potential qualitative types of dose-limiting toxicities. Although we continuously search for opportunities to expedite preclinical studies, a meticulous approach to evaluating toxicology is warranted.
Current example of an exceptionally promising antileukemia agent: ibrutinib
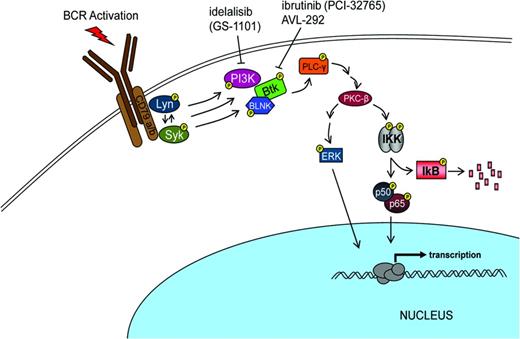
Over the past 3 years, Bruton tyrosine kinase (BTK) has been recognized as a rational target for treating B-cell malignancies.20 BTK is involved in proliferation and survival of both normal and malignant B cells (Figure 2). In addition to an impact on B-cell signaling, this target can effect B-cell migration and adhesion. Substantial evidence suggests that malignant B cells are protected from cell death by interacting with the microenvironment in both lymph nodes and BM.
The search for a small molecule that could selectively inhibit B-cell signaling identified ibrutinib (PCI-32765) as such a candidate.21,22 In 2007, Celera Genomics identified agents that irreversibly inactivate BTK by binding to Cys-481. Although the initial interest focused upon finding an agent that would be targeted for rheumatoid arthritis, the recognition that such an agent might have benefit in other autoimmune diseases and lymphoma progressed rapidly to evaluating the concept in lymphoid malignancy. In vivo demonstration of ibrutinib's activity in B-cell lymphoma occurring in a dog model prompted further development of this agent for patients with B-cell malignancies. Investigators showed that this agent interfered with CLL proliferation, survival, and migration. In 2012, Advani et al reported that Ibrutinib had promising clinical activity in patients with relapsed and refractory B-cell malignancies.23-25 Several investigators reported impressive results in patients with previously untreated CLL and in those with advanced disease. Overall results showed that responses were observed in 67% to 74% of patients. The responses were characterized as durable and were associated with reduction in nodes and spleen size. Patients responded despite poor prognostic parameters including chromosomal abnormality (eg, del 17p) or IgVH unmutated disease. In addition, an unusual peripheral mobilization of the leukemic cells accompanied the subsequent reduction in adenopathy.
Ibrutinib is orally bioavailable and well tolerated. Pharmacodynamic assays confirmed that the administration of this agent in vivo resulted in highly effective binding of the BTK target. The extremely promising and durable early responses were primarily partial remissions. Two recent multi-institutional studies reported extraordinary results in patients with either CLL or mantle cell lymphoma.26,27 Strategic combinations have also been explored with ibrutinib and chemoimmunotherapy.28,29 In February 2013 the FDA announced that ibrutinib had qualified for the designation of “breakthrough therapy” based upon preliminary evidence in patients with CLL associated with del 17p.
Breakthrough therapy status was enacted in 2012 by the FDA to identify that an agent in the clinic showing compelling data may be “a potential new medicine for patients with serious or life-threatening disease.” Therapy for several B-cell malignancies (including Waldenstrom macroglobulinemia, relapsed/refractory mantle cell lymphoma, and CLL with deletion of 17p) was included in the list of diseases being considered for FDA review under the designation for “breakthrough therapy.” The data with ibrutinib in those trials will be reviewed by the FDA to consider making the drug available to appropriate patients. The creation of this “breakthrough” pathway for review represents a major commitment by FDA to facilitate access for safe and effective therapeutic advances for patients with serious and fatal diseases.
The success with the development of ibrutinib as targeted therapy involved having an excellent agent with outstanding pharmacologic properties using a validated pharmacodynamics assay coupled with focused teams of basic and clinical investigators working toward a well-defined common goal. Note that this agent has progressed rapidly and safely toward a regulatory review in less than 4 years. The potential for the evolution of drug resistance resulting from the use of targeted monotherapy illustrates the necessity for continued exploration of other agents targeting additional key enzymes in malignant cells or the use of strategic combinations of agents to circumvent the evolution of resistant disease. Several other inhibitors of BCR signaling under investigation include GS1101 directed at PI3 kinase p110delta and AVL-292, another BTK inhibitor.30
Identification of novel targets in modern phase 1 studies
In the past, heavily pretreated patients with advanced cancer on phase 1 trials had limited responses. With the advent of personalized oncology, participation of patients in molecularly targeted therapeutic trials may improve recognition of those likely to respond by requiring genomic characterization as a condition for eligibility. Genomic sequencing may facilitate enrolling the right patient on the correct trial. Furthermore, these studies may elucidate new understanding of drug resistance and treatment failure. The strategic pursuit of the BRAF V600E mutation as a therapeutic target provides an interesting case in point.
BRAF mutations in malignant melanoma were described in 2002, leading to searches for agents that would inhibit this target.31 Vemurafenib was the first BRAF inhibitor to be approved for the treatment of human cancer. Although different BRAF mutations occur, BRAFV600E is found in approximately 50% of patients with melanoma. In early-phase clinical trials, patients genotyped for this mutation showed an impressive response rate. In a randomized phase 3 trial in patients with metastatic malignant melanoma who demonstrated the characteristic mutation, vemurafenib improved survival and overall response compared with dacarbazine.32 Based upon compelling clinical data, the FDA approved this agent. However, the duration of response to vemurafenib is limited. There is well-recognized evolution of resistant disease and the increased risk of additional nonmelanoma skin cancer and keratoacanthomas.
An “unexpected” observation that BRAFV600E mutations are observed in essentially all patients with the classic form of hairy cell leukemia (HCL) was published in 2011.33 Not surprisingly, clinical investigators quickly translated this observation in an effort to treat patients with nonresponsive classic HCL. Three remarkable cases have identified that vemurafenib is successful in achieving responses in patients with HCL who failed multiple attempts with standard therapy.34-36 Although the overall rate and durations of response have not yet been evaluated and the optimal dose and schedule have not been established, there is substantial interest in these leads. An ongoing clinical trial of vemurafenib in relapsed patients with classic HCL expressing BRAF V600E is currently under way.
Safe and accelerated drug approval for fatal diseases
Many promising drugs have encroached upon the end of the protected “patent life” before entering definitive trials in patients designed to support drug approval. Therefore, every effort must be made to keep drug development on time by tracking accountability for necessary preclinical and clinical task completion. Once the novel agent has entered early phase 1 and 2 clinical trials, exploration for safe and expedient completion of early-phase clinical trials will incorporate correlative pharmacologic studies. Various proposals for expediting the actual dose escalation strategies have not dramatically shortened the time required to complete the careful early evaluations. In general, fewer patients are treated at ineffective lower doses with these newer dose escalation protocols. Hopefully, the selection of predictive biomarkers coupled with identifying the appropriate patient for the targeted therapeutic agent will improve our results. Many pitfalls en route to new drug approval can be avoided by coordinated team work involving both preclinical scientists and clinical investigators.
The prolonged development of Taxol (approaching 30 years) was complicated by preclinical challenges in formulation essential for administration. After the solution to a formulation was identified, the intravenous administration of the agent was no longer a barrier. The next unanticipated serious hurdle related to an inadequate supply of this critically important natural product. The NCI had to establish a Taxol Supply Task Force to solve this problem of securing a natural product before the agent could be approved. Securing a reliable supply of effective agents is a necessity for insuring access for patients after approval. Collaboration with a pharmaceutical sponsor was necessary to address the supply issues with Taxol. FDA granted “orphan drug status” to the supplier and this made it possible to engage a commercial supplier to make this promising agent available to patients with many malignancies.37
The FDA has established specific mechanisms for improving access to promising new drugs. The experts at FDA meet with sponsors throughout the drug development process (Figure 1). For drugs under development that require early review, the new “breakthrough” category has been established. Early approval based upon outstanding clinical results has occurred when appropriate. Furthermore, the FDA has a proven track record of approval of promising new drugs at a more efficient pace than regulatory agencies in either Europe or Canada.38 The responsibility of the FDA to insure both the safety and effectiveness of new therapeutic agents is governed by law. Assembling the exhaustive preclinical data to support a novel trial and then conducting the early definitive trials in patients requires meticulous attention to detail. Selecting the appropriate trial design in consultation with a pharmaceutical sponsor, the NCI, and the FDA provides assurance that the extensive effort required for product approval will be optimally productive. In addition, the NCI has enacted new guidelines to monitor progress in early clinical trials. Trials that fail to meet their benchmark accrual are now closed rather than extending beyond clinical interest. In consideration of patients who volunteer for experimental early trials, it is our obligation to insure that excellent clinical trials will be prioritized for timely completion. Finally, novel clinical trial designs can be explored with effective targeted therapies to enhance access to promising agents at the earliest possible time.39
Summary
The process of drug development is complex and expensive, and myriad challenges and obstacles will be encountered. However, the examples provided here and elsewhere tell us that these issues can frequently be overcome with careful assessment of preclinical pharmacology and toxicology, identification of meaningful pharmacodynamic and clinical end points, and extensive communication and collaboration between sponsor, investigators, and the regulatory agencies. Opportunities exist to explore predictive biomarkers and novel trial design to improve this critically important process.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: Investigational agents under development are used as examples of the process required to gain FDA approval. In addition, targeted agents that have been approved for use in one cancer will be explored in other malignant conditions expressing the same target (eg, the BRAF inhibitor vemurafenib approved in melanoma has been used in relapsed HCL). The investigational nature of therapies under discussion is emphasized and supported by peer-reviewed literature.
Correspondence
Michael R. Grever, Division of Hematology, OSU Medical Center, 395 W 12th Ave, Columbus, OH 43210; Phone: 614-293-8724; Fax: 614-293-6656; e-mail: michael.grever@osumc.edu.